Abstract
Intranasal administration has been widely used to investigate effects of the neuropeptides vasopressin and oxytocin on human behaviors and neurological disorders, but exactly what happens when these neuropeptides are administered intranasally is far from clear. In particular, it is not clear whether a physiological significant amount of peptide enters the brain to account for the observed effects. Here, we investigated whether intranasal administration of vasopressin and oxytocin to rats induces expression of the immediate-early gene product Fos in brain areas that are sensitive to centrally administered peptide, whether it alters neuronal activity in the way that centrally administered peptide does, and whether it affects behavior in ways expected from studies of centrally administered peptide. We found that, whereas intracerebroventricular (icv) injection of very low doses of vasopressin or oxytocin increased Fos expression in several distinct brain regions, intranasal administration of large doses of the peptides had no significant effect. In contrast to the effects of vasopressin applied topically to the main olfactory bulb, we saw no changes in the electrical activity of olfactory bulb mitral cells after intranasal vasopressin administration. In addition, vasopressin given intranasally had no significant effects on social recognition or short-term recognition memory. Finally, intranasal infusions of vasopressin had no significant effects on the parameters monitored on the elevated plus maze, a rodent model of anxiety. Our data in rats suggest that, after intranasal administration, significant amounts of vasopressin and oxytocin do not reach areas in the brain at levels sufficient to change immediate early gene expression, neural activity or behavior in the ways described for central administration of the peptides.
Keywords: vasopressin, oxytocin, olfactory bulb intranasal, mitral cells, c-Fos, blood pressure, behavior
Introduction
In mammals, oxytocin and vasopressin have been implicated in the regulation of complex social behaviors, including attachment, social recognition, aggression, as well as anxiety-related behavior (1). Accordingly, many recent studies have addressed the putative roles of these neuropeptides in human social behavior (2). The peptides are now seen as potential targets for novel treatment approaches for human mental disorders characterized by social dysfunction, such as autism, social anxiety disorder, borderline personality disorder and schizophrenia (3, 4).
To investigate whether the effects of these neuropeptides on behavior is the result of a direct action on the CNS, the established experimental approach in laboratory rodents is to administer the substance either directly into brain tissue, or into the cerebral ventricular system. In humans, different ways of administration must be used, but systemic administration is often problematical either because of poor penetration of the two neuropeptides across the blood-brain barrier, and because of their hormonal actions at peripheral targets (5). This has led researchers to experiment with intranasal administration of peptides, under the supposition that this route of administration might allow rapid access to adjacent brain regions while avoiding side effects.
Intranasal application of peptides has long been used as a way of administering them into the bloodstream (6). Intranasal administration of oxytocin was first used more than fifty years ago to evoke milk-let down in women during lactation (7), and it has been used in obstetrics to facilitate delivery for more than 80 years (8), exploiting the hormonal actions of oxytocin at the mammary gland and uterus respectively. An extended form of arginine vasopressin, DDAVP, has a long history of use by intranasal administration for the treatment of diabetes insipidus in children (9, 10).
Recent publications have reported many behavioral effects of intranasal application of vasopressin and oxytocin in humans, from studies using a bewildering diversity of protocols, (3, 11). However, there is no direct evidence that any oxytocin or vasopressin reaches the brain from any of the human studies. Only one study has measured oxytocin in CSF (12), they report a very small rise after a large intranasal application – a rise so small that it could reflect release of endogenous oxytocin. Accordingly, it is important to resolve whether any of the effects of the two intranasally administered peptides are really mediated by activation of central vasopressin and/or oxytocin receptors, or by receptors in peripheral tissue which then impacts on brain activity (13).
While there is an extensive literature on the effects of vasopressin and oxytocin on a number of brain functions after intracerebroventricular (icv) injection in in rats and mice (14), to our knowledge, there is no published evidence of any such effects of intranasally administered vasopressin and oxytocin. For example, icv injection or local brain application of either peptide triggers the expression of early immediate genes including c-fos in specific brain areas, including especially areas where specific receptors for vasopressin and/or oxytocin are expressed (15-20). Changes in the electrical activity of neurons in several of these brain regions have been reported by local and icv administration of the two peptides in vivo (21-24). Finally, central administration of oxytocin or vasopressin modulates several social behaviors including social recognition, memory for peers, development of partner preference and bonding (14, 25-27). One recent study in voles has reported some effects of intranasal oxytocin on social behavior and anxiety (28).
The present experiments were designed to obtain an understanding of the central actions of vasopressin and oxytocin in rats after intranasal administration. We report a lack of effect of vasopressin and oxytocin after intranasal application in a variety of experimental designs including studies on immediate early gene expression, blood pressure and heart rate, in vivo electrophysiology and behavior testing.
Materials and Methods
All reagents were purchased from Sigma-Aldrich unless otherwise stated.
Animals
Experiments were performed on adult male Sprague-Dawley rats (250-350g), housed under controlled conditions (12 h light: 12 h dark, 21°C) with free access to food and water. Experimental manipulations conducted in Germany were approved by the Committee on Animal Health and Care of the local governmental body and performed in strict compliance with the EEC recommendations for the care and the use of laboratory animals (86/609/CEE). Procedures conducted in the UK were approved by the local Ethics Committee and the UK Home Office under the Animals Scientific Procedures Act 1986. Experiments conducted in the USA were performed according to institutional guidelines and approval by the Animal Care and Use Committees of the Wake Forest University. Only the minimum number of rats necessary to produce reliable scientific data was used.
Fos expression
Intranasal peptide administration
Rats were housed singly three days prior to experiment and handled for 10-15 min each day to familiarize rats with the handler, to minimize any stress-induced Fos response on the days on the experiment. On the morning of the experiment, the rats were briefly anesthetized with isoflurane, and placed in a supine position, with the head supported at 45° to the body (29, 30). Rats were given one of the following: artificial cerebral spinal fluid (aCSF, 10 μl), vasopressin (1 μg dissolved in 10 μl aCSF) or oxytocin (1 μg dissolved in 10 μl aCSF) by slowly pipetting a 5 μl volume into each nostril while the rats were prone on their backs (n=6 per treatment group). The doses of oxytocin and vasopressin given intranasally are equivalent to the total rat pituitary content of oxytocin and vasopressin, and exceed by 500 fold the amounts needed to elicit effects by icv administration. After 5 min, rats were returned to their cages where they recovered from anesthetic until terminally anesthetized for tissue fixation (see below).
Intracerebroventricular (icv) administration of peptide or vehicle
Rats were implanted with a left lateral ventricular cannula under isoflurane anesthesia via a burr hole in the skull drilled 0.6 mm posterior to and 1.6 mm lateral to bregma. A guide cannula (4.5 mm long, Bilaney Consultants Ltd, Sevenoaks, UK) was secured in place using dental cement glued to two stainless steel screws driven into the skull. At this time, dummy caps were attached to the guide cannula to keep the guide cannula patent, and rats were singly housed. Rats were allowed three days to recover, and were gently handled for 10-15 min each day. On the day of experiment, each rat was briefly anesthetized with isoflurane, the dummy cap removed and replaced with an injection cannula (5 mm long, Bilaney Consultants Ltd, Sevenoaks, UK) connected to a Hamilton syringe with fine tubing which had been pre-loaded with the peptide or vehicle solution. Each rat was injected with either 2 μl aCSF or 2 ng of either vasopressin or oxytocin dissolved in 2 μl aCSF (n=6 per treatment group). The injection cannula was removed after 3 min and rats were returned to cages where they recovered from anesthetic until terminally anesthetized for tissue fixation (see below).
Tissue preparation
Previous studies with fluorescently-tagged peptide S showed fluorescent labeling in different brain regions at 15-30 min after intranasal administration (30), and expression of Fos (the protein product of c-fos) is typically maximal by 90 min after an acute stimulus and plateaus for at least an hour. Thus, 2 h after treatment (icv injection or intranasal treatment), rats were terminally anesthetized for brain fixation by cardiac perfusion with 4% paraformaldehyde solution. Rats were deeply anesthetized (pentobarbital, 120 mg/100 g body weight by intraperitoneal injection) then perfused through the ascending aorta first with heparin (5000 U/ml; 300 ml) in 0.9% saline solution followed by 300 ml of a 4% paraformaldehyde in 0.1M phosphate buffer (PB) pH 7.4 solution. The brains were removed and immersed overnight in a solution of 0.2% paraformaldehyde and 15% sucrose in 0.1 M PB at 4°C. The tissue was then placed in a solution of 30% sucrose in 0.1M PB and left until the tissue had sunk (usually 48 h). The rat brains were snap-frozen and cut at 40 μm thickness using either a cryostat or freezing microtome. Sections were cut in 0.1 M phosphate buffer (PB) and stored in a cryoprotectant solution (30% ethylene glycol + 20% glycerol in 0.05 M sodium phosphate buffer, pH 7.3) at -20°C until used for immunohistochemistry. For each of the olfactory bulbs and hypothalami of each brain, every third section was collected together and processed by free-floating immunocytochemistry to detect Fos immunoreactivity as described below.
Immunocytochemistry
After washing in 0.1M PB to remove all cryoprotectant, sections were incubated for 20 min in 0.3% H2O2 in 0.1M PB to quench endogenous peroxidase activity. Sections were washed at least 4 times for 10 min between each of the following steps and unless otherwise indicated washed and incubated at room temperature. To block non-specific interaction of secondary antibodies with the tissue, sections were then incubated for 60 min in a blocking buffer consisting of 3% normal horse serum + 1% BSA + 0.2% Triton X-100 in 0.1 M PB. Sections were then incubated for 60 min at room temperature then 48h at 4°C with Fos primary antibody (PC38, polyclonal raised in rabbit, used at 1:20000 or 100000, Calbiochem) which was diluted in the blocking buffer. After extensive washing in 0.1 PB, the sections were incubated for 60 min with Biotinylated-anti-rabbit IgG (1:500, raised in horse, Vector Laboratories) and washed again. Sections were next incubated for 60 min in ABC complex diluted as detailed by manufacturer (Vectorstain elite ABC kit, Vector Laboratories, UK). After washing in 0.1 M PB, the sections were transferred to 0.1M Tris solution (pH 7.4) for 5 min. Fos immunoreactivity was then visualized using a solution of 0.025% diaminobenzidine + 2.5% Nickel II sulphate + 0.08% ammonium chloride + 0.015% H2O2 in 0.1 M Tris. A test section was first incubated in this solution to determine the optimal time to develop the diaminobenzidine (DAB) stain, in this case it was found to be 6.5 min. After visualizing, the sections were rapidly transferred back to 0.1 M Tris to stop further development of the DAB stain. After 5 min, sections were washed extensively in 0.1M PB, finally in 0.05M PB and mounted on gelatinized slides. After air-drying, the slide-mounted sections were counterstained (Nuclear Fast Red, Vector Laboratories, 5 min then washed in tap water for 3 min), dehydrated in ascending concentrations of ethanol (70 and 80% for 5 min each then 90 and 100% for 10 min each) and cover slipped using DPX mountant. No labeling was detected when primary antibodies were omitted (negative control).
Quantification of Fos positive cells
Four investigators independently quantified the number of Fos positive cells in a number of brain regions, including the main olfactory bulb, accessory olfactory bulb, anterior olfactory nucleus (each with a number of sub-regions), the piriform cortex, lateral septum (divided into dorsal and ventral), supraoptic nucleus, paraventricular nucleus, paraventricular thalamic nucleus, suprachiasmatic nucleus and amygdala. Another investigator repeated counting of selected regions to confirm consistency. All investigators were blinded to the treatments at the time of counting. Images of the regions were acquired using a Leica digital camera, controlled by Leica acquisition software (AIS), and attached to an upright Leica microscope and x10 objective. Images from at least 6 regions from every rat in each treatment group were acquired. Using ImageJ, these images were converted to 8 bit, thresholded using the same parameters, and Fos-positive cells counted using the Analyze Particles macro. The conditions for thresholding and for the macro were determined in part by comparing count results with manually counted images and ensuring that counts made manually and via the software matched for 20 regions. As the size of the olfactory areas and piriform cortex exceeded the field of view or differed widely in size in the sections, non-overlapping, multiple regions of interest (ROI) were used. The number of Fos-positive cells within each ROI were normalized by the surface area of that ROI to allow comparison. Thus the number of Fos-positive cells in these regions are expressed as mean ± S.E.M. per 104 μm2 (corresponding to an area of 100×100 μm). As all the other areas described above were encompassed within the field of view, the total number of Fos-positive cells was counted for each region and are expressed as mean ± S.E.M. per section.
In vivo electrophysiology
In urethane-anesthetized Sprague-Dawley rats (250-300g), extracellular recordings were made using glass microelectrodes filled with 0.9% NaCl (tip diameter ~1 μm). Recordings were made from single mitral cells in the main olfactory bulb (~7 mm anterior to bregma, ~1.3 mm lateral) identified antidromically (collision test) by electrical stimulation of the ipsilateral lateral olfactory tract (1.4 mm posterior to bregma, 3.2 mm lateral, 9.5 mm deep, latencies to antidromic activation in range 1.0-5.8 ms (24, 31). Vasopressin (4 μg in 4 μl saline) or saline alone was given intranasally via a microsyringe connected to a thin polyethylene tubing (PE10) inserted in the nostril ipsilateral to the recording side after a stable period of recording of basal electrical activity. Activity quotients (proportion of time active relative to total time) were calculated as described previously (24).
Behavior
Drug administration
30 min before being tested in each behavioral test, rats were lightly anaesthetized with isoflurane (Baxter, Deutschland GmbH, Unterschleißheim, Germany). For the social discrimination test rats were given bilateral intranasal infusions (5 or 10 μl per nostril; of either aCSF or vasopressin diluted in aCSF (50 ng or 10 μg) using a micropipette. For the elevated plus maze test and open field test, animals received 0.1 μg in 10 μl (5 μl per nostril). After infusion, rats were returned to their home cages and their quick re-awakening was recorded (all signs of drowsiness and ataxia due to the anesthesia vanished within 5 min).
Social discrimination
Olfactory-cued, short-term social recognition memory was tested during the light phase between 9:00 am and 3:00 pm using the social discrimination test. Singly housed adult male rats were tested in their home cages (37 × 21 × 15 cm). Stimulus animals (juveniles, 22-26 days old of both sexes) were isolated and kept individually in a fresh cage with food and water ad libitum 2.5 h before starting the test. The test consists of two 4-min exposures of the juvenile to the adult. A mirror behind the experimental subjects’ cage facilitated the observation of the animals. During the first exposure, the adult investigates a juvenile rat to acquire its olfactory signature (Juvenile 1, J1). J1 is then removed and returned to its cage. After an interval of either 30 or 120 min, J1 is re-exposed to the adult, together with a second, novel juvenile (Juvenile 2, J2; Fig. 3A). The time that the adult spends investigating each juvenile (direct sniffing and/or licking at the surface of the body of the juvenile) is measured by a trained observer blind to the animal's treatment using a computer keyboard by pressing pre-set keys. This allows for an easy detection of the investigatory behavior even in freely moving animals. A significantly longer investigation duration of J2 vs J1 is taken as evidence for an intact recognition memory (32). The procedure, including the age and sex of the juveniles as well as the critical criteria and the appropriate analysis and presentation of the results obtained are described in detail elsewhere (32).
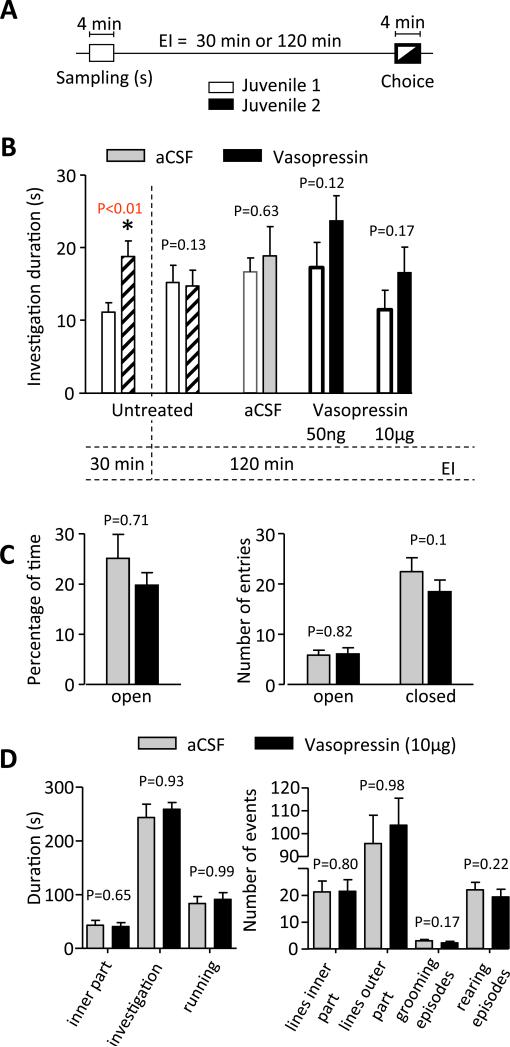
Figure 3.
A: Experimental protocol used for testing the olfactory recognition memory as a function of the exposure interval (EI). During the sampling session (s) a given conspecific juvenile (Juvenile 1) was exposed for 4 min to the adult experimental subject. After an exposure interval of 30 min or 120 min, the same juvenile was re-exposed to the adult during a 4 min choice session together with a second, unfamiliar juvenile (Juvenile 2). B: Investigation duration of adult male rats (control n = 12; vasopressin n = 11) during sampling and choice. None of the treatments was able to produce a discrimination at the exposure interval of 120 min between both juveniles indicated by the significantly longer investigation duration of Juvenile 2 versus Juvenile 1 as observed at the exposure interval of 30 min under untreated conditions. No impact of intranasal infusion of vasopressin on anxiety-related behavior as measured in (C) the elevated plus maze or (D) open field test. Means + S.E.M., * P = 0.01, paired Student's t-test.
Elevated plus maze
The elevated plus maze is widely used to monitor anxiety-related behavior (33). In our labs, this is made of black polyvinyl chloride and has two open and two closed arms (50 × 10 × 40 cm) interconnected by a 10 × 10 cm central platform, and is mounted 50 cm above the floor. The brightness was set at 30 lux. For testing, a rat is placed on the central platform of the apparatus facing a closed arm, and its behavior recorded on video camera (mounted to the ceiling of the test room) and monitored from an adjacent room for 7 min. The number of entries into open/closed arms, and the time spent in open/closed arms are measured. An entry is defined as placing both forepaws into the given compartment of the maze. The maze is cleaned after each trial.
Open field
General behavioural activity including anxiety-related behaviour (34) was monitored in the open field. The apparatus existed of a white square open field (80 × 80 cm) with 25 cm high walls. The field was divided by 10 × 10 cm squares marked on the floor. Testing was done under uniform bright illumination (315 ± 40 lux). On the testing day, a given experimental subject was placed in the centre of the field and the behaviour was recorded for 10 min using a video camera located above the centre of the arena. The recorded digital videotapes were analyzed offline by a trained observer unaware of the animal's treatment. The following behaviours were scored: time spent in the (unprotected) inner part of the field, time spend investigating the field (sniffing), time spend in running (fast locomotion), number of lines (marking the 10 × 10 cm squares on the floor of the field) crossed in both the inner and the outer part of the field; number of episodes spent in rearing (posture sustained with the hind paws on the floor) and grooming (including washing or mouthing of forelimbs, hind paws, face, body and genital). Before testing each rat, the open field was carefully wiped with an alcohol solution and dried.
Blood sampling
Male rats were anaesthetized with urethane (1.25 g/kg i.p., Sigma) and the left femoral artery cannulated. Blood samples (0.3 ml) were withdrawn into heparinized syringes, centrifuged and the plasma separated and stored at -20°C for subsequent vasopressin radioimmunoassay. The remaining blood cells were suspended in an equivalent volume of 0.9% sterile saline and returned to the rat via the cannula. Blood samples were taken 10 min before, then 15 and 45 min after intranasal vasopressin (1 μg in 10 μl aCSF) or vehicle administration. The vasopressin content of samples was measured by specific radioimmunoassay after extraction as described previously (35).
Blood pressure monitoring
Rats were anesthetized with urethane (1.2 g/kg, intraperitoneal), placed on a warmed surgical platform and catheters filled with heparinized saline (50 U/mL) were inserted into the left femoral artery and vein for blood pressure measurement and drug administration respectively. The arterial catheter was connected to a pressure transducer (model 156 PC 15GWL, Microswitch, Freeport, IL), connected to TS430 blood pressure amplifier (Transonic Systems, Ithaca NY) and cardiovascular parameters were measured with a computerized data collection system (IOX, EMKA Technologies, Falls Church VA). Arterial blood pressure and heart rate were recorded for 20 min (5 min before and 15 min after drug administration). Vasopressin (0.2 or 20 μg/10 μl), oxytocin (0.2 or 20 μg/10 μl) or (aCSF, 10 μl), by pipetting 5 μl into each nostril while the rats were prone on their backs. Vasopressin (0.05 μg/ 100 μl) and oxytocin (10 μg/ 100 μl) were also given intravenously.
Data analysis
All statistical analysis was performed using SigmaStat® (Systat Software Inc., Richmond, CA, USA) or Graphpad Prism (V 4.03; Graphpad Software, San Diego, California, U.S.A.) software. Immunohistochemistry data were analyzed by Mann-Whitney Rank Sum Test or Kruskal-Wallis one-way analysis of variance (ANOVA) on ranks followed by Dunn's post-hoc tests (to compare anesthetic control with intranasal vehicle and vasopressin). Student's t-tests were used otherwise (after verifying that data tested did not deviate from normality assumptions). Arterial pressure and heart rate were averaged in 10-s blocks. Baseline values were taken 1 min before drug administration and peak responses were compared to that baseline. Statistical analysis was conducted using a two-way repeated measures ANOVA with factors of drug administration (before and peak after administration) and dose (saline, low and high dose of peptide). Behavioral data were tested using Student's t-test either paired (investigation durations for J1 vs J2 for each treatment separately) or unpaired for all parameters monitored in the open field. All values are expressed as mean + S.E.M., and differences were considered significant when P < 0.05.
Results
Fos expression
Fos, the protein product of the immediate-early gene c-fos is widely used marker of neuronal activation and several studies have shown that icv injection of vasopressin or oxytocin triggers the expression of Fos in a variety of brain regions (15-19). Vasopressin given icv at a dose of 2 ng induced significant increases in Fos expression in many areas of the olfactory system and in several hypothalamic nuclei (Figure 1, Table 1 and 2). As expected, the amygdala, paraventricular nucleus, supraoptic nucleus and the lateral septum all showed significant increases in Fos expression in response to icv injection of either peptide compared to icv vehicle. The suprachiasmatic nucleus, the paraventricular thalamic nucleus and the piriform cortex responded significantly only to vasopressin, indicating peptide specificity (Table 1 and 2).
Figure 1.
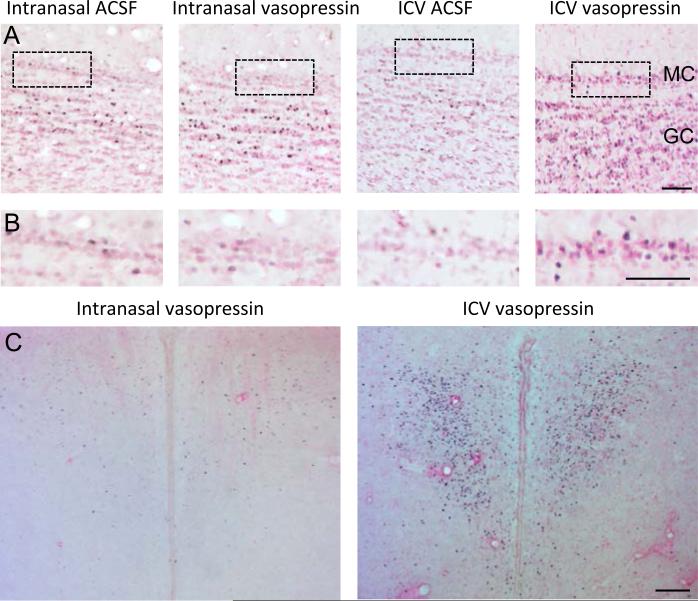
Representative examples showing immunohistochemistry for the c-Fos protein in (A,B) the mitral and granule cell layer of the main olfactory bulb and (C) the paraventricular nucleus of the hypothalamus in response to intranasal or icv administration of vasopressin. MC - mitral cell layer, GC = granule cell layer. All scale bars 100 μm.
Table 1.
Number of Fos positive nuclei in areas of the olfactory system (per 104 μm2) and the hypothalamus (per area) in response to intranasal and intracerebroventricular (icv) administered vasopressin. Mean ± S.E.M.
| anesthetic control | intranasal aCSF | intranasal vasopressin | icv aCSF | icv vasopressin | |
|---|---|---|---|---|---|
| Main olfactory bulb | |||||
| Granule cells | 0.4±0.1 | 1.1±0.1* | 1.2±0.1* | 0.9±0.2 | 18±2# |
| Mitral cells | 3.2±0.5 | 10.2±1.1* | 9.6±0.7* | 1.2±0.4 | 19.5±2.7# |
| Epiform plexus | 0.22±0.06 | 0.29±0.04* | 0.3±0.03* | 0.4±0.1 | 0.3±0.1 |
| Glomerular layer | 1.1±0.2 | 1.4±0.2* | 1.1±0.1* | 0.8±0.01 | 0.99±0.18 |
| Accessory olfactory bulb | |||||
| Granule cells | 0.09±0.02 | 0.09±0.04 | 0.08±0.03 | 0.11±0.04 | 0.08±0.03 |
| Mitral cells | 0.82±0.3 | 0.28±0.07 | 0.25±0.05 | 0.44±0.28 | 1.2±0.4# |
| Glomerular layer | 0.5±0.1 | 0.25±0.09 | 0.2±0.08 | 0.15±0.06 | 0.12±0.03 |
| Anterior olfactory nucleus | |||||
| Pars interna (dorsal) | 1.4±0.4 | 11.5±2.3 | 9.6±1.7 | 0.8±0.16 | 7.1±1.1# |
| Pars interna (ventral) | 0.1±0.01 | 0.45±0.05* | 0.5±0.05* | 0.28±0.06 | 4.8±0.5# |
| Pars interna (lateral) | 0.26±0.06 | 3.54±1.5* | 2.7±1.2* | 0.13±0.03 | 4.49±0.45# |
| Pars interna (medial) | 0.2±0.04 | 1.1±0.3* | 0.75±0.13* | 0.06±0.02 | 3.63±0.26# |
| Piriform cortex | 0.36±0.04 | 0.38±0.04 | 0.39±0.04* | 0.9±0.14 | 2.7±0.85# |
| Lateral septum | |||||
| - dorsal | 4.3±1.4 | 4.2±0.6 | 3.1±0.7 | 2.4±0.4 | 21.7±4.6# |
| - ventral | 3.9±1.1 | 5.5±1 | 4.7±0.7 | 4.5±0.6 | 21.7±3.8# |
| Supraoptic Nucleus | 1.4±0.4 | 2.1±0.6 | 2.1±0.7 | 1.3±0.3 | 4.1±0.3# |
| Paraventricular nucleus | 2.8±0.4 | 2.1±0.6 | 3±0.6 | 1.44±0.3 | 11.6±1.3# |
| Paraventricular thalamic nucleus | 2.4±0.7 | 6.5±1.5 | 5.6±1.1 | 1.8±0.5 | 51.4±8.9# |
| Amygdala | 6.2±1.2 | 15.9±2.4* | 9±1.2 | 14.1±3.5 | 48.6±8.7# |
| Suprachiasmatic nucleus | 0.7±0.2 | 1.3±0.3 | 1.6±0.7 | 0.7±0.2 | 20.1±2.2# |
anesthetic control compared to intranasal aCSF and vasopressin
P < 0.05 compared to icv vehicle.
Table 2.
Number of Fos positive nuclei in areas of the olfactory system (per 104 μm2) and the hypothalamus (per area) in response to intranasal and intracerebroventricular (icv) administered oxytocin.
| intranasal aCSF | intranasal oxytocin | icv aCSF | icv oxytocin | |
|---|---|---|---|---|
| Main olfactory bulb | ||||
| Granule cells | 0.5±0.1 | 0.5±0.1 | 1.3±0.5 | 3.7±1# |
| Mitral cells | 1.4±0.3 | 1.4±0.4 | 2.7±0.9 | 6.1±2.2 |
| Epiform plexus | 0.2±0.1 | 0.2±0.1 | 0.26±0.1 | 0.16±0.04 |
| Glomerular layer | 0.01±0.003 | 0.03±0.01 | 0.008±0.006 | 0.03±0.01# |
| Accessory olfactory bulb | ||||
| Granule cells | 0.03±0.02 | 0.03±0.01 | 0.02±0.01 | 0.02±0.01 |
| Mitral cells | 0.2±0.04 | 0.2±0.03 | 0.2±0.1 | 0.7±0.2# |
| Glomerular layer | 0.0±0.0 | 0.01±0.01 | 0.0±0.0 | 0.01±0.01 |
| Anterior olfactory nucleus | ||||
| Pars interna (dorsal) | 1.2±0.2 | 1.9±0.4 | 0.96±0.17 | 1.1±0.23# |
| Pars interna (ventral) | 0.77±0.14 | 0.87±0.17 | 0.8±0.2 | 2.1±0.31# |
| Piriform cortex | 0.27±0.05 | 0.14±0.03 | 0.19±0.05 | 0.2±0.04 |
| Lateral septum | ||||
| - dorsal | 1.5±0.6 | 1.7±0.5 | 2.4±0.7 | 20.9±3.1# |
| - ventral | 2.6±0.8 | 3.3±0.9 | 2.3±0.5 | 21±2.7# |
| Supraoptic nucleus | 0.1±0.07 | 0.1±0.04 | 2±0.3 | 2.8±0.3# |
| Paraventricular nucleus | 4.4±1.2 | 3.3±0.7 | 1.4±0.2 | 9.2±0.8# |
| Paraventricular thalamic nucleus | 0.6±0.2 | 0.7±0.2 | 2±0.4 | 0.9±0.2 |
| Amygdala | 1.5±0.4 | 1.1±0.3 | 0.9±0.3 | 41.3±5.5# |
| Suprachiasmatic nucleus | 0.5±0.2 | 0.5±0.2 | 1.3±0.3 | 0.8±0.2 |
Mean ± S.E.M.
P < 0.05 compared to icv aCSF.
In marked contrast, there was no significant difference in any brain region after intranasal administration of vasopressin when compared to intranasal administration of vehicle (Table 1), even though these used a dose 500 times higher than the dose shown to be effective by icv administration.
However, application of both vehicle and vasopressin increased Fos expression in regions the olfactory system when compared to anesthetic control (Table 1). Thus, Fos expression in the olfactory bulb is sensitive to nasal disturbances (even under anesthesia), but there was not a specific response to intranasal vasopressin.
Electrophysiology
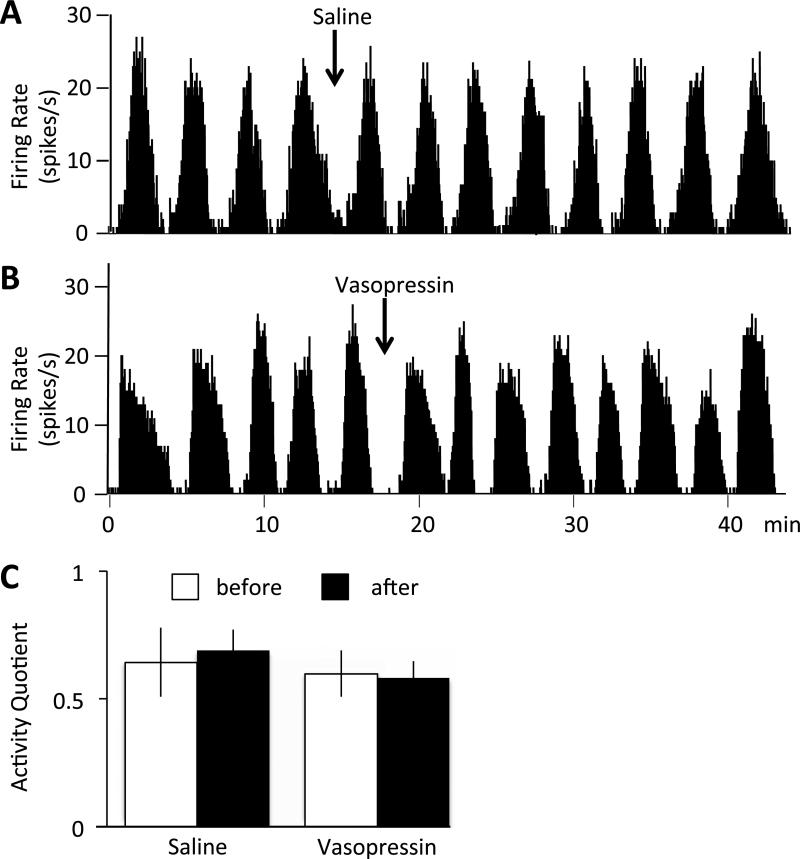
The main olfactory bulb contains receptors for vasopressin and oxytocin and intrinsic vasopressin cells that process olfactory signals relevant to social discrimination. We have previously shown that topical administration of vasopressin onto the exposed bulb dorsal to the recording site (in doses of 4 ng and 40 ng) significantly reduced the activity quotient of mitral cells (24).
We tested whether intranasally administered vasopressin had a similar effect on mitral cell activity. Recordings in freely-breathing anaesthetized rats showed as patterned discharge comprising intermittent long bursts of action potentials, and the activity quotient measures the proportion of time active. However, intranasal administration of 1μg vasopressin had no significant effect (activity quotient 0.60±0.08 before and 0.58±0.06 after vasopressin, n=6; 0.64±0.13 before and 0.69±0.09 after vehicle, n=3; Fig. 2).
Figure 2.
Firing rate of identified mitral cells before and after intranasal administration of saline (A) or vasopressin (B). C: The activity quotient (proportion of time active relative to total time) did not change in response to intranasal saline (n=3) or vasopressin (n=6) administration. Means ± S.E.M.
Behaviour
Social discrimination
In the social discrimination test, the time that rats spend investigating a juvenile to which they have been previously exposed (“familiar” juvenile) is compared with the time they spend investigating a novel juvenile. Normal, untreated rats spend longer investigating novel juveniles than juveniles they encountered 30 min previously, but juveniles encountered 120 min previously are investigated as much as novel juveniles (Fig. 3A; (32). Le Moal et al. (1087) first showed that icv infusions of 0.5-2 ng vasopressin improve short-term social recognition memory in rats (36), in line with the memory-improving effect of local treatment with vasopressin in this task (37, 38). It has been shown previously that bilateral injection of 0.5 ng vasopressin into the olfactory bulbs preserves social recognition responses (39) while blocking the actions of vasopressin in the olfactory bulbs (using antagonists or small interference RNA against the vasopressin V1a receptor, or local selective destruction of vasopressin cells with diphtheria toxin in transgenic rats) impairs the social recognition abilities of rats (24).
Here we gave vasopressin at doses of 50ng and 10μg; these doses exceed the doses that have been shown to be effective centrally by 100-2000 fold. Neither Ringers’ solution nor vasopressin given intranasally had any significant effect on recognition performance, failing to affect either general social curiosity or to improve short-term recognition memory (Fig. 3B).
Elevated plus maze
In rats, vasopressin is implicated in the modulation of anxiety-related behavior, for example, administration of ~200 pg vasopressin into the septum increases open-arm entries and open-arm duration in the elevated plus maze (40). Here we found no significant effects of intranasal infusions of vasopressin at a dose 5000 times higher (0.1 μg) on any of the parameters monitored on the elevated plus maze (Fig. 3C).
Open field
A recent study reported that, in the open field test, female voles cross fewer lines after long-term treatment with intranasal oxytocin than voles treated with saline (28). Previous studies in rats have shown that icv injection of vasopressin does not affect open field behaviour (41). In the present study we found no significant effect of intranasal infusions of vasopressin compared to vehicle on any of the parameters monitored on the open field test (time spent in the unprotected inner part of the field, time spent investigating the field (sniffing), time spend in running (fast locomotion), number of lines crossed in both the inner and the outer part of the field; number of episodes spent in rearing (posture sustained with the hind paws on the floor) and grooming; P values between 0.17 and 0.99; Fig. 3D).
Blood pressure
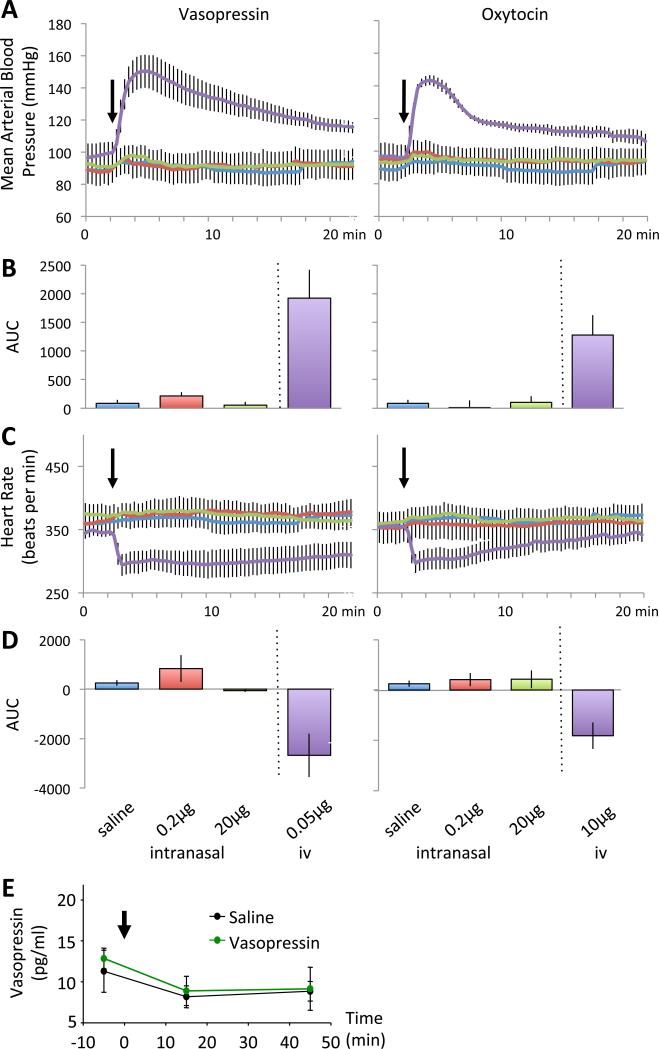
Injection of 1-10 ng vasopressin into the lateral ventricles (ICV) or various brain regions increases blood pressure and heart rate in rats, whereas icv injections of 0.3 μg oxytocin reduce blood pressure, but heart rate remains unchanged (42-44).
In the present study, intranasal administration of either 0.2 μg or 20 μg doses of vasopressin had a modest effect on arterial pressure and heart rate, but these effects were not significantly different to those which accompanied vehicle administration (Fig. 4). After intranasal administration of saline or vasopressin there was a small increase in mean arterial pressure (F1,4=25.4, p<0.01), but no effect of dose (p<0.18) and no interaction between administration of test solutions and dose (p<0.3) indicating that the increase was a non-specific response to administering a solution intranasally. Intranasal administration of 0.2 μg vasopressin increased arterial pressure by 8±2 mmHg (from 88±8 to 96±7 mmHg) while a 100 fold higher dose (20 μg) likewise increased arterial pressure by 8±1 mmHg (91± 6 to 99±6 mmHg). Intranasal administration of saline increased mean arterial pressure by 5±1 mmHg (89±7 to 94±7 mmHg). By contrast, intravenous administration of 0.05 μg vasopressin produced a pronounced (52±12 mmHg) and long lasting pressor response. Intranasally administered saline or vasopressin also increased heart rate (F1,4=7.8, p<0.05) but again this response was not dose-related (p<0.1) and there was no interaction between dose and administration of test solution (p<0.3). As expected, intravenous administration of vasopressin resulted in a pronounced bradycardia (346±19 to 300±17 bpm).
Figure 4.
Measurements of mean arterial blood pressure (A) and heart rate (C) in response to intranasal or icv administration of saline, vasopressin or oxytocin. B) and D) area under the curve for the treatments. E: Vasopressin content in plasma samples before and after intranasal administration of vasopressin or saline (n=6 each group). Means ± S.E.M.
Intranasal administration of oxytocin or a placebo also had only modest effects on arterial pressure (F1,5=637, P<0.001) and heart rate (Fig. 4). Administration of 0.2 μg increased arterial pressure by 5±1 mmHg (from 95±17 to a peak of 101±6 mmHg.) Likewise, the higher dose of oxytocin had a similar effect, increasing arterial pressure by 7±1 mm Hg from 93±8 to 100±7 mmHg. Again there was no significant effect of dose (p<0.09), nor was there a dose-by-administration interaction (p<0.8), suggesting that any effect was due to administration of any solution via this route. In a similar manner, intranasal administration of solutions was associated with an increase in heart rate (F1,5=11.2, p<0.02) but again, there was no effect of dose (p<0.6) and no interaction effect (p<0.4). By contrast, intravenous administration of 10 μg oxytocin increased arterial pressure by 47±9 mmHg (from 97±8 to 144±3). As with vasopressin, this pressor response was associated with a bradycardia (355±20 to 303±17 bpm). Intravenous administration of oxytocin can exert vasodilatory effects mediated through a nitric oxide mechanism (45), but at high concentrations oxytocin has pressor actions (46), through stimulation of vasopressin receptors (47-49); at the dose given (10 μg), this was as expected (this dose was chosen to be 200 times higher than the dose of vasopressin given intravenously).
Plasma sampling
There were no significant effects of intranasal infusions of vasopressin on plasma vasopressin concentrations at any sampling point (Fig. 4E) compared to vehicle.
Discussion
The present study was designed to unravel, in the laboratory rat, the intracerebral targets and physiological consequences of intranasally delivered vasopressin and oxytocin – previously shown to be highly effective in modulating the behavioral response in humans.
When delivering doses of vasopressin or oxytocin this large into the nasal cavity in any study, careful consideration needs to be given as to exactly where it goes. Why it should ever be assumed that drugs delivered intranasally do not enter the bloodstream is not clear. In fact, intranasal application of peptides has long been used a way of administering them into the bloodstream; deep inhalation of aerosols delivers substances into the lungs, where they can readily cross the permeable membranes of bronchial capillaries. As mentioned, an extended form of arginine vasopressin, DDAVP, has long been used by intranasal administration for the treatment of diabetes insipidus in children (9, 10) – the effectiveness of which requires that the administered peptide reaches the kidneys. Intranasal administration of oxytocin was first used to evoke milk-let down in women during lactation (7) and it has been used in obstetrics to facilitate delivery (8), exploiting the actions of oxytocin at the mammary gland and uterus respectively. In 1961, the first placebo-controlled clinical trial of intranasal oxytocin for milk let-down appeared (50), reporting that administration of 4-5 IU (8-10 μg) oxytocin intranasally to lactating mothers before each feeding session led to increased weight gain of the babies. In 1971, a study of its use in 1800 patients in two community hospitals concluded that, when given as doses of 0.4-0.8 IU (0.8-1.6 μg) every 15-20 min, it was safe and effective in augmenting delivery (51). Thus inhaled oxytocin and vasopressin clearly can enter the bloodstream in humans in sufficient levels to exert clinically meaningful hormonal effects. However, in the present experiments we found no evidence of entry of vasopressin into the blood following intranasal administration of 1μg – there was no significant increase in plasma concentration, nor any effect on blood pressure or heart rate (effects which would be expected from both central and peripheral administration of the peptides). It seems likely therefore that the present method of administration – direct topical application of drops of vasopressin-containing solution to the nasal cavity of anesthetized rats – does not favor access into the lungs in the way that deep inhalation does. It seems most likely that the eventual fate of vasopressin administered in this way is to be incorporated into mucus and broken down in the gastro-intestinal tract after swallowing; it's hard to see any other fate that would not allow access into the bloodstream.
Recent publications have reported that in humans, behavior can be affected if vasopressin or oxytocin is delivered intranasally (3, 11, 52). The only publication that has reported measurements of either peptide in human CSF after intranasal application reported that after intranasal administration of 80 IU vasopressin (160 μg) achieved a peak concentration of ~ 40 pg/ml in CSF (and ~80 pg/ml in plasma) (12). Although this paper is widely cited as evidence that intranasally administered peptide reaches the CNS, it must be noted that the doses administered were large even by the very generous standards of the field, yet the achieved CSF concentrations were relatively modest. This paper reports changes in CSF concentrations that cannot readily be accounted for by passage from the blood – but does not resolve whether the changes arise from entry into the CNS rather than from endogenous release provoked by peripheral actions of the peptide (53).
The blood-brain barrier (BBB) protects the brain and spinal cord from a variety of pathogens and toxic substances, and presents a significant barrier for most charged and/or large molecules (54, 55). This applies to most proteins and peptides, including vasopressin and oxytocin (5). In the 1970s, a large number of studies reported behavioral effects of peripherally administered vasopressin – mainly on memory (56). However, there is convincing evidence that these can be attributed to peripheral effects of vasopressin, including vasopressor actions that, by reinforcing aversive stimulation, promoting enhanced ‘memory’ performance (53). A turning point was a 1983 Nature paper (57) showing that behavioural deficits in the (vasopressin-deficient) Brattleboro rat that could be remediated with systemic vasopressin treatment arose from disrupted sleep patterns in the Brattleboro rat which were normalised when normal antidiuresis was restored.
The passage of oxytocin and vasopressin from blood into brain has been carefully evaluated by Mens and colleagues (58); these workers estimated that after intravenous injection in rats, just 0.002% of the injected dose reached the CNS. Some peptides that do not penetrate the blood-brain barrier have direct actions on the CNS by acting at specialized sites on the blood side of the blood-brain barrier. These sites, which comprise the so-called circumventricular organs, can be identified at (i) the ultrastructural level by the presence of fenestrated capillaries, the (ii) light microscope level by the presence of specific biochemical markers, and (iii) functionally by the sites of penetration into the brain of dyes administered systemically. At least two of these sites, the organum vasculosum of the lamina terminalis and the subfornical organ, contain nerve fibers that are immunoreactive for vasopressin V1b receptors (59). However, these sites are not gateways into the brain for blood-borne substances; molecules that penetrate into these organs do not penetrate further into the brain.
Some drugs enter the brain readily following intranasal application, but this does not in itself imply that there is any privileged access to the brain for all molecules via this pathway. Not only do inhaled substances access the lungs swiftly, but the nasal cavity itself has a rich vascular plexus that may permit rapid entry of topically administered drugs. Thus, for many medications, the rates of absorption and plasma concentrations after intranasal application are similar to those achieved by intravenous administration, and this is particularly true for small, lipophilic molecules that can be delivered in high concentrations as aerosols. For larger molecules and peptides, it has been suggested that pathways involving nerves connecting the nasal passages to the brain are important, together with pathways involving the vasculature, CSF, and lymphatic system. The CSF and lymphatic systems are interconnected; CSF is produced by the choroid plexuses, is maintained at high pressure, and drains into blood via the arachnoid and into lymph via some of the cranial nerve bundles, including the olfactory and trigeminal nerves (60). Thus entry into the brain via the lymphatic system or nerve bundles route must proceed against the direction of bulk flow of the CSF. For some large peptides (e.g. leptin) it seems that specialized transport mechanisms may facilitate penetration (54, 55). However, on the other hand, many mechanisms impair the bioavailability of intranasally delivered drugs, including nasal mucociliary clearance mechanisms (61-63) and drug metabolizing enzymes (64, 65). Oxytocin and vasopressin for example are rapidly broken down by the enzyme oxytocinase which is abundant in several brain regions including the olfactory bulb (66).
A few studies have used autoradiography to measure the brain distribution of radiolabel after intranasal administration of radiolabelled peptides. These studies failed to find evidence of peptide entry into the CSF, but did report penetration of the radiolabel into brain sites approximately according to their distance from the nose – with highest concentrations of radiolabel found in the olfactory bulb (67, 68). As these studies did not use HPLC to confirm the identity of the radiolabel carrier, it is not established whether the presence of the radiolabel in brain areas reflects penetration of intact peptide, metabolites, or free label. We used fluorescently-tagged vasopressin and found labeling at diverse sites throughout the brain within 20 min after intranasal application. However, we found exactly the same distribution of labeling when we administered the fluorophore alone (data not shown). This suggests that, when fluorescently tagged vasopressin is administered intranasally, cleavage by endogenous enzymes in the nasal mucous (which is exceptionally rich in antiseptic enzyme activity (69), yields free label that is subsequently found in the brain. Again, via which route the label reaches the brain (directly or via the blood stream) remains unclear. However, the ubiquitary distribution of the label in brain tissue at sites relatively remote from the ventricular system does not favor a primary distribution via the CSF.
This raises the question if the effects of intranasal peptides in humans are really mediated by activation of central vasopressin and oxytocin receptors, or by activation of their receptors in peripheral tissue, which then impacts on brain activity as shown in animal studies (53). In human studies, when the subjects receive social stimuli, it is likely that this causes reactions in the viscera (especially heart and gut), and the heart and the gut may themselves contribute to peptide plasma levels (70). The afferent branches of the vagus nerve and other visceral afferents can transfer visceral signals, and the brain may register these as emotional signals. Evidence indicates that the autonomic nervous system is influenced by both endogenous and exogenous oxytocin (71).
Finally, we should not neglect the possibility that neuropeptides applied to the nose act on the nose, especially when applied at concentrations so high that they are likely to have promiscuous actions at many other receptor types, as well as on the vasopressin receptors that are present in olfactory epithelial cells (72). The nasal mucosa is the first line of defense against inhaled toxicants: many inhaled pollutants initiate immediate nasal responses via interaction with nasal trigeminal C-fibers involving the release of a variety of potent mediators including peptides, which exert a variety of effects in nasal mucosa including vasodilation and increased blood flow, increased vascular permeability leading to edemagenesis, and mucous hypersecretion (73).
Taken together, we report a consistent failure to find any significant effects of intranasal administration of vasopressin or oxytocin in rats on objective parameters that are strongly influenced by centrally administered peptide. In studies of Fos expression, we tested the effects of intranasal application of 1μg peptide. This is approximately equivalent to the total content of the posterior pituitary gland, and equates approximately to the total amount of vasopressin secreted in one month by normally hydrated rats: vasopressin and oxytocin act at high affinity G-protein coupled receptors, and are active in the periphery at circulating concentrations in the range 1-10 pg/ml. As injections of 2 ng icv had profound effects on Fos expression at multiple brain sites known to express oxytocin and/or vasopressin receptors, we might expect that passage into the brain of as little as 0.2% of the dose administered intranasally would be reflected in positive outcomes. We also found no effects of intranasal vasopressin on anxiety-related behavior on the elevated plus maze, a test which has been very extensively used to study the role of vasopressin in anxiety-related behaviors. In contrast to a recent report showing behavior changes in an open field test after intranasal administration of oxytocin in voles (28), we failed to detect any effect of intranasal vasopressin in this paradigm. We also found no effects on social behavior in the social recognition test, although this behavior is known to depend on the actions of vasopressin in the olfactory bulb, supposedly the brain site most accessible to intranasal vasopressin. We also found no effects of intranasal vasopressin on mitral cell activity when administered intranasally even at a hundred times higher dose. We therefore have to conclude that vasopressin and oxytocin administered at very large doses intranasally in rats do not enter the brain in amounts sufficient to exert clear effects.
Acknowledgements
We would like to thanks previous honors and master students from the University of Edinburgh, Christopher Oldroyd, Jonathon Shaw, Charlotte Fleming and Angela Chen, for their help in analyzing the immediate early gene studies, Professor Rainer Landgraf (Munich, Germany) for analyzing the plasma samples and Rita Murau (Magdeburg, Germany) for the help with the behavior testing. Work was supported by grants from the BBSRC and the Edinburgh Patrick Wild Centre (ML), DFG (EN366/6-1) (ME), NIH R43CA150703 02A1 (MFC) and European Community's Seventh Framework Programme (FP7_KBBE_2009_245009; Neurofast) (GL). The funding source had no role in study design; in the collection, analysis, and interpretation of data; in the writing of the report; or in the decision to submit the paper for publication.
References
- 1.Insel TR. The challenge of translation in social neuroscience: a review of oxytocin, vasopressin, and affiliative behavior. Neuron. 2010;65:768–779. doi: 10.1016/j.neuron.2010.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Heinrichs M, von Dawans B, Domes G. Oxytocin, vasopressin, and human social behavior. Front Neuroendocrinol. 2009;30:548–557. doi: 10.1016/j.yfrne.2009.05.005. [DOI] [PubMed] [Google Scholar]
- 3.Meyer-Lindenberg A, Domes G, Kirsch P, Heinrichs M. Oxytocin and vasopressin in the human brain: social neuropeptides for translational medicine. Nat Rev Neurosci. 2011;12:524–538. doi: 10.1038/nrn3044. [DOI] [PubMed] [Google Scholar]
- 4.Neumann ID, Landgraf R. Balance of brain oxytocin and vasopressin: implications for anxiety, depression, and social behaviors. Trends Neurosci. 2012;35:649–659. doi: 10.1016/j.tins.2012.08.004. [DOI] [PubMed] [Google Scholar]
- 5.Ermisch A, Ruhle HJ, Landgraf R, Hess J. Blood-brain barrier and peptides. J Cereb Blood Flow Metab. 1985;5:350–357. doi: 10.1038/jcbfm.1985.49. [DOI] [PubMed] [Google Scholar]
- 6.Landgraf R. Plasma oxytocin concentrations in man after different routes of administration of synthetic oxytocin. Exp Clin Endocrinol. 1985;85:245–248. doi: 10.1055/s-0029-1210444. [DOI] [PubMed] [Google Scholar]
- 7.Newton M, Egli GE. The effect of intranasal administration of oxytocin on the let-down of milk in lactating women. Am J Obstet Gynecol. 1958;76:103–107. doi: 10.1016/s0002-9378(16)36872-7. [DOI] [PubMed] [Google Scholar]
- 8.Hendricks CH, Gabel RA. Use of intranasal oxytocin in obstetrics. 1. A laboratory evaluation. Am J Obstet Gynecol. 1960;79:780–788. doi: 10.1016/0002-9378(60)90636-0. [DOI] [PubMed] [Google Scholar]
- 9.Seckl JR, Dunger DB. Diabetes insipidus. Current treatment recommendations. Drugs. 1992;44:216–224. doi: 10.2165/00003495-199244020-00006. [DOI] [PubMed] [Google Scholar]
- 10.Fjellestad-Paulsen A, Tubiana-Rufi N, Harris A, Czernichow P. Central diabetes insipidus in children. Antidiuretic effect and pharmacokinetics of intranasal and peroral 1-deamino-8-D-arginine vasopressin. Acta Endocrinol. 1987;115:307–312. [PubMed] [Google Scholar]
- 11.Guastella AJ, MacLeod C. A critical review of the influence of oxytocin nasal spray on social cognition in humans: evidence and future directions. Horm Behav. 2012;61:410–418. doi: 10.1016/j.yhbeh.2012.01.002. [DOI] [PubMed] [Google Scholar]
- 12.Born J, Lange T, Kern W, McGregor GP, Bickel U, Fehm HL. Sniffing neuropeptides: a transnasal approach to the human brain. Nat Neurosci. 2002;5:514–516. doi: 10.1038/nn849. [DOI] [PubMed] [Google Scholar]
- 13.Churchland PS, Winkielman P. Modulating social behavior with oxytocin: how does it work? What does it mean? Horm Behav. 2012;61:392–399. doi: 10.1016/j.yhbeh.2011.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Engelmann M, Wotjak CT, Neumann I, Ludwig M, Landgraf R. Behavioral consequences of intracerebral vasopressin and oxytocin: focus on learning and memory. Neurosci Biobehav Rev. 1996;20:341–358. doi: 10.1016/0149-7634(95)00059-3. [DOI] [PubMed] [Google Scholar]
- 15.Giri PR, Dave JR, Tabakoff B, Hoffman PL. Arginine vasopressin induces the expression of c-fos in the mouse septum and hippocampus. Mol Brain Res. 1990;7:131–137. doi: 10.1016/0169-328x(90)90090-z. [DOI] [PubMed] [Google Scholar]
- 16.Andreae LC, Herbert J. Expression of c-fos in restricted areas of the basal forebrain and brainstem following single or combined intraventricular infusions of vasopressin and corticotropin-releasing factor. Neuroscience. 1993;53:735–748. doi: 10.1016/0306-4522(93)90620-u. [DOI] [PubMed] [Google Scholar]
- 17.Olson BR, Freilino M, Hoffman GE, Stricker EM, Sved AF, Verbalis JG. c-Fos expression in rat brain and brainstem nuclei in response to treatments that alter food intake and gastric motility. Mol Cell Neurosci. 1993;4:93–106. doi: 10.1006/mcne.1993.1011. [DOI] [PubMed] [Google Scholar]
- 18.Windle RJ, Kershaw YM, Shanks N, Wood SA, Lightman SL, Ingram CD. Oxytocin attenuates stress-induced c-fos mRNA expression in specific forebrain regions associated with modulation of hypothalamo-pituitary-adrenal activity. J Neurosci. 2004;24:2974–82. doi: 10.1523/JNEUROSCI.3432-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Paban V, Alescio-Lautier B, Devigne C, Soumireu-Mourat B. Fos protein expression induced by intracerebroventricular injection of vasopressin in unconditioned and conditioned mice. Brain Res. 1999;825:115–131. doi: 10.1016/s0006-8993(99)01232-9. [DOI] [PubMed] [Google Scholar]
- 20.Kita I, Yoshida Y, Nishino S. An activation of parvocellular oxytocinergic neurons in the paraventricular nucleus in oxytocin-induced yawning and penile erection. Neurosci Res. 2006;54:269–275. doi: 10.1016/j.neures.2005.12.005. [DOI] [PubMed] [Google Scholar]
- 21.Wakerley JB, Terenzi MG, Housham SJ, Jiang QB, Ingram CD. Electrophysiological effects of oxytocin within the bed nuclei of the stria terminalis: influence of reproductive stage and ovarian steroids. Prog Brain Res. 1998;119:321–334. doi: 10.1016/s0079-6123(08)61578-2. [DOI] [PubMed] [Google Scholar]
- 22.Ludwig M, Leng G. Autoinhibition of supraoptic nucleus vasopressin neurons in vivo: a combined retrodialysis/electrophysiological study in rats. Eur J Neurosci. 1997;9:2532–2540. doi: 10.1111/j.1460-9568.1997.tb01682.x. [DOI] [PubMed] [Google Scholar]
- 23.Gouzenes L, Desarmenien MG, Hussy N, Richard P, Moos FC. Vasopressin regularizes the phasic firing pattern of rat hypothalamic magnocellular vasopressin neurons. J Neurosci. 1998;18:1879–1885. doi: 10.1523/JNEUROSCI.18-05-01879.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tobin VA, Hashimoto H, Wacker DW, Takayanagi Y, Langnaese K, Caquineau C, Noack J, Landgraf R, Onaka T, Leng G, Meddle SL, Engelmann M, Ludwig M. An intrinsic vasopressin system in the olfactory bulb is involved in social recognition. Nature. 2010;464:413–417. doi: 10.1038/nature08826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Donaldson ZR, Young LJ. Oxytocin, vasopressin, and the neurogenetics of sociality. Science. 2008;322:900–904. doi: 10.1126/science.1158668. [DOI] [PubMed] [Google Scholar]
- 26.Goodson JL. Nonapeptides and the evolutionary patterning of sociality. Prog Brain Res. 2008;170:3–15. doi: 10.1016/S0079-6123(08)00401-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Young LJ, Wang Z. The neurobiology of pair bonding. Nat Neurosci. 2004;7:1048–1054. doi: 10.1038/nn1327. [DOI] [PubMed] [Google Scholar]
- 28.Bales KL, Perkeybile AM, Conley OG, Lee MH, Guoynes CD, Downing GM, Yun CR, Solomon M, Jacob S, Mendoza SP. Chronic intranasal oxytocin causes long-term impairments in partner preference formation in male prairie voles. Biol Psychiatry. 2012 doi: 10.1016/j.biopsych.2012.08.025. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lukas M, Neumann ID. Nasal application of neuropeptide S reduces anxiety and prolongs memory in rats: social versus non-social effects. Neuropharmacology. 2012;62:398–405. doi: 10.1016/j.neuropharm.2011.08.016. [DOI] [PubMed] [Google Scholar]
- 30.Ionescu IA, Dine J, Yen YC, Buell DR, Herrmann L, Holsboer F, Eder M, Landgraf R, Schmidt U. Intranasally administered neuropeptide S (NPS) exerts anxiolytic effects following internalization into NPS receptor-expressing neurons. Neuropsychopharmacology. 2012;37:1323–1337. doi: 10.1038/npp.2011.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jiang M, Griff ER, Ennis M, Zimmer LA, Shipley MT. Activation of locus coeruleus enhances the responses of olfactory bulb mitral cells to weak olfactory nerve input. J Neurosci. 1996;16:6319–6329. doi: 10.1523/JNEUROSCI.16-19-06319.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Engelmann M, Hadicke J, Noack J. Testing declarative memory in laboratory rats and mice using the nonconditioned social discrimination procedure. Nat Protoc. 2011;6:1152–1162. doi: 10.1038/nprot.2011.353. [DOI] [PubMed] [Google Scholar]
- 33.Wall PM, Messier C. Methodological and conceptual issues in the use of the elevated plus-maze as a psychological measurement instrument of animal anxiety-like behavior. Neurosci Biobehav Rev. 2001;25:275–286. doi: 10.1016/s0149-7634(01)00013-6. [DOI] [PubMed] [Google Scholar]
- 34.Denenberg VH. Open-field behavior in the rat: what does it mean? Ann N Y Acad Sci. 1969;159:852–859. doi: 10.1111/j.1749-6632.1969.tb12983.x. [DOI] [PubMed] [Google Scholar]
- 35.Landgraf R, Neumann I, Holsboer F, Pittman QJ. Interleukin-1 beta stimulates both central and peripheral release of vasopressin and oxytocin in the rat. Eur J Neurosci. 1995;7:592–798. doi: 10.1111/j.1460-9568.1995.tb00663.x. [DOI] [PubMed] [Google Scholar]
- 36.Le Moal M, Dantzer R, Michaud B, Koob GF. Centrally injected arginine vasopressin (AVP) facilitates social memory in rats. Neurosci Lett. 1987;77:353–359. doi: 10.1016/0304-3940(87)90527-1. [DOI] [PubMed] [Google Scholar]
- 37.Dantzer R, Bluthe RM, Koob GF, Le Moal M. Modulation of social memory in male rats by neurohypophyseal peptides. Psychopharmacology. 1987;91:363–368. doi: 10.1007/BF00518192. [DOI] [PubMed] [Google Scholar]
- 38.Engelmann M, Landgraf R. Microdialysis administration of vasopressin into the septum improves social recognition in Brattleboro rats. Physiol Behav. 1994;55:145–149. doi: 10.1016/0031-9384(94)90022-1. [DOI] [PubMed] [Google Scholar]
- 39.Dluzen DE, Muraoka S, Engelmann M, Landgraf R. The effects of infusion of arginine vasopressin, oxytocin, or their antagonists into the olfactory bulb upon social recognition responses in male rats. Peptides. 1998;19:999–1005. doi: 10.1016/s0196-9781(98)00047-3. [DOI] [PubMed] [Google Scholar]
- 40.Appenrodt E, Schnabel R, Schwarzberg H. Vasopressin administration modulates anxiety-related behavior in rats. Physiol Behav. 1998;64:543–547. doi: 10.1016/s0031-9384(98)00119-x. [DOI] [PubMed] [Google Scholar]
- 41.Klimkiewicz T. Memory effects of arginine vasopressin (AVP) and [7-9] fragment of its peptide chain in rats. Acta Neurobiol Exp. 2001;61:267–276. doi: 10.55782/ane-2001-1402. [DOI] [PubMed] [Google Scholar]
- 42.Ganten D, Unger T, Lang RE. The dual role of angiotensin and vasopressin as plasma hormones and neuropeptides in cardiovascular regulation. J Pharmacol. 1985;16:51–68. [PubMed] [Google Scholar]
- 43.Petersson M, Uvnas-Moberg K. Effects of an acute stressor on blood pressure and heart rate in rats pretreated with intracerebroventricular oxytocin injections. Psychoneuroendocrinology. 2007;32:959–965. doi: 10.1016/j.psyneuen.2007.06.015. [DOI] [PubMed] [Google Scholar]
- 44.Poulin P, Komulainen A, Takahashi Y, Pittman QJ. Enhanced pressor responses to ICV vasopressin after pretreatment with oxytocin. Am J Physiol. 1994;266:R592–R598. doi: 10.1152/ajpregu.1994.266.2.R592. [DOI] [PubMed] [Google Scholar]
- 45.Gutkowska J, Jankowski M. Oxytocin revisited: its role in cardiovascular regulation. J Neuroendocrinol. 2012;24:599–608. doi: 10.1111/j.1365-2826.2011.02235.x. [DOI] [PubMed] [Google Scholar]
- 46.Lloyd S. Changes in the vascular responses of the rat during pregnancy. J Physiol. 1959;149:586–592. doi: 10.1113/jphysiol.1959.sp006362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chen YL, Shepherd C, Spinelli W, Lai FM. Oxytocin and vasopressin constrict rat isolated uterine resistance arteries by activating vasopressin V1A receptors. Eur J Pharmacol. 1999;376:45–51. doi: 10.1016/s0014-2999(99)00351-9. [DOI] [PubMed] [Google Scholar]
- 48.Costa ESRH, Pereira-Junior PP, Oliveira PF, Olivares EL, Werneck-de-Castro JP, Mello DB, Nascimento JH, Campos-de-Carvalho AC. Cardiac effects of oxytocin: is there a role for this peptide in cardiovascular homeostasis? Regul Pept. 2005;132:107–112. doi: 10.1016/j.regpep.2005.09.011. [DOI] [PubMed] [Google Scholar]
- 49.Petty MA, Lang RE, Unger T, Ganten D. The cardiovascular effects of oxytocin in conscious male rats. Eur J Pharmacol. 1985;112:203–210. doi: 10.1016/0014-2999(85)90497-2. [DOI] [PubMed] [Google Scholar]
- 50.Huntingford PJ. Intranasal use of synthetic oxytocin in management of breast-feeding. Br Med J. 1961;1:709–711. doi: 10.1136/bmj.1.5227.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hoover RT. Intranasal oxytocin in eighteen hundred patients. A study on its safety as used in a community hospital. Am J Obstet Gynecol. 1971;110:788–794. doi: 10.1016/0002-9378(71)90576-x. [DOI] [PubMed] [Google Scholar]
- 52.MacDonald E, Dadds MR, Brennan JL, Williams K, Levy F, Cauchi AJ. A review of safety, side-effects and subjective reactions to intranasal oxytocin in human research. Psychoneuroendocrinology. 2011;36:1114–1126. doi: 10.1016/j.psyneuen.2011.02.015. [DOI] [PubMed] [Google Scholar]
- 53.Le Moal M, Koob GF, Koda LY, Bloom FE, Manning M, Sawyer WH, Rivier J. Vasopressor receptor antagonist prevents behavioural effects of vasopressin. Nature. 1981;291:491–493. doi: 10.1038/291491a0. [DOI] [PubMed] [Google Scholar]
- 54.Thorne RG, Frey WH., 2nd Delivery of neurotrophic factors to the central nervous system: pharmacokinetic considerations. Clin Pharmacokinet. 2001;40:907–946. doi: 10.2165/00003088-200140120-00003. [DOI] [PubMed] [Google Scholar]
- 55.Hanson LR, Frey WH., 2nd Intranasal delivery bypasses the blood-brain barrier to target therapeutic agents to the central nervous system and treat neurodegenerative disease. BMC Neurosci. 2008;9:S5. doi: 10.1186/1471-2202-9-S3-S5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.de Wied D, Gaffori O, van Ree JM, de Jong W. Central target for the behavioural effects of vasopressin neuropeptides. Nature. 1984;308:276–278. doi: 10.1038/308276a0. [DOI] [PubMed] [Google Scholar]
- 57.Danguir J. Sleep deficits in rats with hereditary diabetes insipidus. Nature. 1983;304:163–164. doi: 10.1038/304163a0. [DOI] [PubMed] [Google Scholar]
- 58.Mens WB, Witter A, van Wimersma Greidanus TB. Penetration of neurohypophyseal hormones from plasma into cerebrospinal fluid (CSF): half-times of disappearance of these neuropeptides from CSF. Brain Res. 1983;262:143–149. doi: 10.1016/0006-8993(83)90478-x. [DOI] [PubMed] [Google Scholar]
- 59.Hernando F, Schoots O, Lolait SJ, Burbach JP. Immunohistochemical localization of the vasopressin V1b receptor in the rat brain and pituitary gland: anatomical support for its involvement in the central effects of vasopressin. Endocrinology. 2001;142:1659–1668. doi: 10.1210/endo.142.4.8067. [DOI] [PubMed] [Google Scholar]
- 60.Knopf PM, Cserr HF, Nolan SC, Wu TY, Harling-Berg CJ. Physiology and immunology of lymphatic drainage of interstitial and cerebrospinal fluid from the brain. Neuropathol Appl Neurobiol. 1995;21:175–180. doi: 10.1111/j.1365-2990.1995.tb01047.x. [DOI] [PubMed] [Google Scholar]
- 61.Wioland MA, Fleury-Feith J, Corlieu P, Commo F, Monceaux G, Lacau-St-Guily J, Bernaudin JF. CFTR, MDR1, and MRP1 immunolocalization in normal human nasal respiratory mucosa. J Histochem Cytochem. 2000;48:1215–1222. doi: 10.1177/002215540004800905. [DOI] [PubMed] [Google Scholar]
- 62.Graff CL, Pollack GM. P-Glycoprotein attenuates brain uptake of substrates after nasal instillation. Pharm Res. 2003;20:1225–1230. doi: 10.1023/a:1025053115583. [DOI] [PubMed] [Google Scholar]
- 63.Kandimalla KK, Donovan MD. Localization and differential activity of P-glycoprotein in the bovine olfactory and nasal respiratory mucosae. Pharm Res. 2005;22:1121–1128. doi: 10.1007/s11095-005-5420-3. [DOI] [PubMed] [Google Scholar]
- 64.Sarkar MA. Drug metabolism in the nasal mucosa. Pharm Res. 1992;9:1–9. doi: 10.1023/a:1018911206646. [DOI] [PubMed] [Google Scholar]
- 65.Minn A, Leclerc S, Heydel JM, Minn AL, Denizcot C, Cattarelli M, Netter P, Gradinaru D. Drug transport into the mammalian brain: the nasal pathway and its specific metabolic barrier. J Drug Target. 2002;10:285–296. doi: 10.1080/713714452. [DOI] [PubMed] [Google Scholar]
- 66.Noble F, Banisadr G, Jardinaud F, Popovici T, Lai-Kuen R, Chen H, Bischoff L, Parsadaniantz SM, Fournie-Zaluski MC, Roques BP. First discrete autoradiographic distribution of aminopeptidase N in various structures of rat brain and spinal cord using the selective iodinated inhibitor [125I]RB 129. Neuroscience. 2001;105:479–488. doi: 10.1016/S0306-4522(01)00185-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Thorne RG, Pronk GJ, Padmanabhan V, Frey WH., 2nd Delivery of insulin-like growth factor-I to the rat brain and spinal cord along olfactory and trigeminal pathways following intranasal administration. Neuroscience. 2004;127:481–496. doi: 10.1016/j.neuroscience.2004.05.029. [DOI] [PubMed] [Google Scholar]
- 68.Ross TM, Martinez PM, Renner JC, Thorne RG, Hanson LR, Frey WH., 2nd Intranasal administration of interferon beta bypasses the blood-brain barrier to target the central nervous system and cervical lymph nodes: a non-invasive treatment strategy for multiple sclerosis. J Neuroimmunol. 2004;151:66–77. doi: 10.1016/j.jneuroim.2004.02.011. [DOI] [PubMed] [Google Scholar]
- 69.Watelet JB, Strolin-Benedetti M, Whomsley R. Defence mechanisms of olfactory neuroepithelium: mucosa regeneration, metabolising enzymes and transporters. B-Ent. 2009;5:21–37. [PubMed] [Google Scholar]
- 70.Yu Q, Ji R, Gao X, Fu J, Guo W, Song X, Zhao X, Burnstock G, Shi X, He C, Xiang Z. Oxytocin is expressed by both intrinsic sensory and secretomotor neurons in the enteric nervous system of guinea pig. Cell Tissue Res. 2011;344:227–237. doi: 10.1007/s00441-011-1155-0. [DOI] [PubMed] [Google Scholar]
- 71.Porges SW. The polyvagal theory: neurohypophysiological foundations of emotions, attachement, communication, and self-regulation. W.W. Norton; New York: 2011. [Google Scholar]
- 72.Levasseur G, Baly C, Grebert D, Durieux D, Salesse R, Caillol M. Anatomical and functional evidence for a role of arginine-vasopressin (AVP) in rat olfactory epithelium cells. Eur J Neurosci. 2004;20:658–670. doi: 10.1111/j.1460-9568.2004.03516.x. [DOI] [PubMed] [Google Scholar]
- 73.Lund VJ. Nasal physiology: neurochemical receptors, nasal cycle, and ciliary action. Allergy Asthma Proc. 1996;17:179–184. doi: 10.2500/108854196778996877. [DOI] [PubMed] [Google Scholar]