Abstract
Purpose
We have previously identified solute-linked carrier family A1 member 5 (SLC1A5) as an overexpressed proteins in a shotgun proteomic analysis of stage I non-small cell lung cancer (NSCLC) when compared to matched controls. We hypothesized that overexpression of SLC1A5 occurs to meet the metabolic demand for lung cancer cell growth and survival.
Experimental Design
To test our hypothesis we first analyzed the protein expression of SLC1A5 used in archival lung cancer tissues by immunohistochemictry (IHC) and immunoblotting (N=98), and cell lines (N=36). To examine SLC1A5 involvement in a.a transportation, we performed kinetic analysis of Gln uptake in lung cancer cell lines in the presence and absence of a pharmacological inhibitor of SLC1A5, gamma-L-Glutamyl-p-Nitroanilide (GPNA). Finally we examined the effect of Gln deprivation and uptake inhibition on cell growth, cell cycle progression and growth signaling pathways of 5 lung cancer cell lines.
Results
Our results demonstrate that 1) SLC1A5 protein is expressed in 95% of squamous cell carcinomas (SCC), 74% of adenocarcinomas (ADC) and 50% of neuroendocrine tumors, 2) SLC1A5 is located at the cytoplasmic membrane, is significantly associated with SCC histology and male gender, and 3) 68% of Gln is transported in a Na+ -dependent manner, 50% of which is attributed to SLC1A5 activity, 4) pharmacological and genetic targeting of SLC1A5 decreased cell growth and viability in lung cancer cells, an effect mediated in part by mTOR- signaling.
Conclusions
These results suggest that SLC1A5 plays a key role in Gln transport controlling lung cancer cells metabolism, growth and survival.
Keywords: Biomarkers, glutamine, transporters, lung cancer
A. Introduction
Lung cancer is the leading cause of cancer-related deaths in the United States (1). NSCLC accounts for about 80% of all lung cancers. Although advances have been made in diagnosis and treatment strategies in the last decade, the prognosis of NSCLC patients remains poor, with a 5-year overall survival of 15 to 20% (2). A small subset of tumors have been found to be driven by mutated oncogenes for which active, but still non-curative therapies are available. Yet the vast majority of tumors have a complex pathogenesis that are still poorly understood (3). Thus, new molecular diagnostic and therapeutic targets are urgently needed to improve the quality of care and survival of lung cancer patients. Although genomic profiling has given important new insights into the mechanisms of carcinogenesis, therapeutic targets and most biomarkers with clinical utility are protein products. Advances in proteomic techniques in recent years have allowed for an in-depth analysis of the changes in protein expression and post translational modifications associated with lung cancer (4, 5). These studies have yielded large inventories of proteins that can potentially be translated to molecular targets or biomarkers of lung cancer, but few of these candidates have been validated or associated with functional relevance to the disease process.
Given the urgent need for a reliable and non-invasive diagnostic test for lung cancer, we have previously compared shotgun proteomic profiles of fresh frozen stage I non-small cell lung cancer to matched control lung specimens and identified several candidates that were significantly overexpressed in NSCLC (6). Solute linked carrier family 1 member A5 (SLC1A5) emerged as a top candidate. SLC1A5 acts as a high affinity transporter of L-glutamine (Gln) in rapidly growing epithelial and tumor cells in culture (7). Neutral amino acids, including Gln, can be transported by four main families of amino acid transporter systems including sodium-dependent systems A, ASC, N and sodium-independent system L (8–11). These transporters are classified based on tissue distribution and affinities for different amino acids. System ASC is the most commonly expressed amino acid transporter in human tumor-derived cells, suggesting it might play a major role in cellular transformation and mediation of Gln-dependent growth (10, 12, 13). Because SLC1A5 belongs to the Na+-dependent ASC family of amino acid transporters we examined whether glutamine is transported in lung cancer cells in a Na+ -dependent manner. In lung cancer, the dependency on Gln for growth, survival and cell cycle progression is well documented (14–16). More recently, Gln conversion into the tricarboxylic acid cycle intermediate alpha-ketoglutarate through glutaminase was shown to be essential for Kras-induced anchorage-independent-growth in A549 cells (17). Other studies demonstrated that reductive carboxylation of glutamine is key for the metabolic reprogramming that enables cancer cells to survive and proliferate under hypoxia in several cancer cellular models, including lung cancer (18–20). Collectively, these studies provide strong evidence for a major role of Gln metabolism in supporting lung cancer cell growth and survival. However, the identity of the transporter(s) that capture Gln in lung cancer cells and how Gln transport is linked to cell growth and survival is still largely unknown. Therefore, the three main objectives of this study were first to evaluate the expression pattern of SLC1A5 protein in different lung cancer pathologic subtypes, second to assess its relevance to the clinical outcomes in lung cancer, and finally, to investigate the functional contribution of SLC1A5 to glutamine uptake and its role in supporting the cell growth and viability of lung cancer cells.
B. Materials and Methods
Patient selection and tissue microarray
Tissue microarrays (TMA) of 98 lung cancer tumor tissues were prepared from paraffin-embedded formalin-fixed (FFPE) blocks. The TMA consisted of 46 adenocarcinomas, 37 squamous cell carcinomas, 4 bronchioalveolar carcinomas, 4 large cell carcinomas, 2 non-small cell lung cancers (NSCLC), 5 carcinoids, and 1 each of adenosquamous, sarcoma, and small cell lung cancer (SCLC). These arrays were constructed according to protocols previously described (21). Archived tissue blocks from consecutive anatomic resections between 1989 to 2002 were retrieved from the files of the Vanderbilt University Medical Center and the Nashville Veterans Administration Medical Center pathology departments. Demographic and clinical characteristics of the 98 patients represented in both TMAs are summarized in Table 1, for details in IHC analysis refer to Supplementary materials.
Table1.
Characteristics of the patients in the TMAs cohort according to cancer status and SLC1A5 expression
| Patients demographics | Total N=98 No (%) |
SLC1A5 (−) No (%) |
SLC1A5 (+) No (%) |
|---|---|---|---|
| Age, Mean (SD) | 56.4±11.5 | ||
| Race | |||
| African American | 7(7) | 0 | 7(100) |
| Caucasian | 91(93) | 17 (17) | 81 (83) |
| Gender | |||
| Men | 56(57) | 6 (11) | 50 (98) |
| Women | 42(43) | 13(31) | 29 (69) |
| Smoking status | |||
| Ex. never-smoker | 71(72) | 13(18) | 56(82) |
| Current-smoker | 27(28) | 3 (11) | 24(88) |
| Pack-years, mean (SD) | 55.78 ±33 | ||
| Histology | |||
| ADC | 46(47) | 12(26) | 34 (74%) |
| SCC | 37(38) | 2 (5) | 35 (95%) |
| Other NSCLC | 9 (9) | 0 | 9 (100%) |
| Neuroendocrine tumors | 6 (6) | 3 (50) | 3 (50%) |
Abbreviations. ADC= adenocarcinoma, NSCLC= non-small cell lung cancer, PKY= pack year (pack per day × number of years smoked), Other NSCLC= Large cell carcinoma+Adenosquamous carcinoma+NSCLC, Neuroendocrine tumors =Small cell lung cancer+large cell Neuroendocrine+Atypical carcinoid.
Cell culture
Human lung cancer cell lines A549 (ADC), H1819 (ADC), HCC15 (SCC), H520 (SCC), and H727 (Carcinoid) (ATCC, Manassas, VA, USA) were maintained in RPMI-1640 medium (Gibco® by Life Technologies, Grand Island, NY, USA) containing 10% heat-inactivated fetal bovine serum (FBS) (Gibco® by Life Technologies, Grand Island, NY, USA), at 37°C, 100% humidity, and 5% CO2. Cells were passaged every 2–3 days to maintain exponential growth.
Glutamine uptake assays
Measurement of L-glutamine uptake in monolayer cultured NSCLC cells was carried out via the cluster-tray method originally described by Gazzola et al (22). Briefly, cells were plated at 105cells/well in 24-well culture plates (Costar, Cambridge, MA, USA) and allowed to adhere overnight. Before transport assays, the cells were rinsed twice with warm Na+-free Krebs-Ringer Phosphate Buffer (cholKFtP) in which choline chloride and choline phosphate iso-osmotically replaced the corresponding Na' salts, to remove extracellular Na' and amino acids. The radiotracer used was L-[3,4Gln-3H] glutamine (Amersham, Arlington Heights, IL) at 500,000 dpm/µmol of the specific activity (5µCi/ml). For kinetic studies, the amount of unlabeled glutamine in the transport buffer varied from 400 µM to 6.4 mM. Transport values were obtained either in the absence of extracellular Na+ (diffusion and Na-independent uptake) using cholKRP or in the presence of Na+ (total uptake), using NaKRP buffer to determine the Na-dependent rates, reported in units of picomoles per milligram protein per min. All transport measurements were carried out at 37°C and were terminated after 3 minutes (min) by adding ice-cold phosphate-buffered saline solution (PBS) followed by three rapid washes with an ice-cold PBS. Intracellular glutamine was extracted with 0.2 ml/well of 0.2% SDS in 0.2 N NaOH; after 1 hour at RT, 0.1 mL of the lysate was neutralized with 2N HCl and subjected to liquid scintillation t spectrophotometry. The remaining lysate was used for the determination of cellular protein by the Pierce® BCA Protein assay. Rates of glutamine transport were calculated from the counts per minute (cpm) per sample, and the specific activity of the uptake mix (in cpm/nmol). These results were then normalized to cellular protein content using Microsoft Excel. For pharmacological targeting by GPNA, Gln uptake assays were performed in the presence of 0, 100, and 300 µM of GPNA in NaKRP buffer. The transport velocities were calculated from radioactive counts and protein concentration and subsequently expressed as pmol of Gln transported per milligram of protein per minute. Each data point represents the average ± SEM of at least three separate determinations.
Glutamine- dependent proliferation assays
To test the growth dependency on Gln, cells were plated in 12-well tissue culture plates at a density of 2 × 104 cells/well. The following day, the cells were rinsed once with serum free L-glutamine free medium and replaced with RPMI-1640 (Gibco® by Life Technologies, Grand Island, NY, USA) with the following variations: supplemention with EGF (25 ng/ml) and 1× growth factors cocktail (Invitrogen, Carlsbad, USA) that includes insulin, selenium and transferein (Sup), Sup+ 2 mM L-glutamine (+Gln+Sup), RPMI-1640 + L-glutamine but no supplements (+Gln-Sup), no L-glutamine but with supplements (−Gln+Sup) or with neither L-glutamine nor supplements (−Gln-Sup). Cultures were left to grow for 3 days with media changes every 48 hours (h). To test whether SLC1A5 activity mediates the Gln depletion effect, A549 and H520 were cultured in media containing (+Gln+Sup) + 1 mM GPNA, and left to grow for 2 days. To further validate the anti-growth effects of SLC1A5 blockade by GPNA, A549 and H1819 cells were cultured in the optimum growth media (+Gln+Sup) in the presence of increasing doses of GPNA (0, 1, 10 100,1000 µM) for 2 days. Cell growth was monitored by measuring the OD490 by the Cell-Titer 96-Aqueous colorimetric assay (Promega, Madison, WI) at day 0 and after 48h of culture. Relative growth rates were expressed as % growth from day 0 and were calculated using the following equation; (Ti-Tz)/(C-Tz) ×100, where Ti is the cell number after 48 hours (i=inhibition), Tz is the cell number at time 0 and C is the cell number of the control cells that were cultured in optimum growth conditions (+Gln +Sup)(23).
Transfection of short interfering RNA (siRNA)
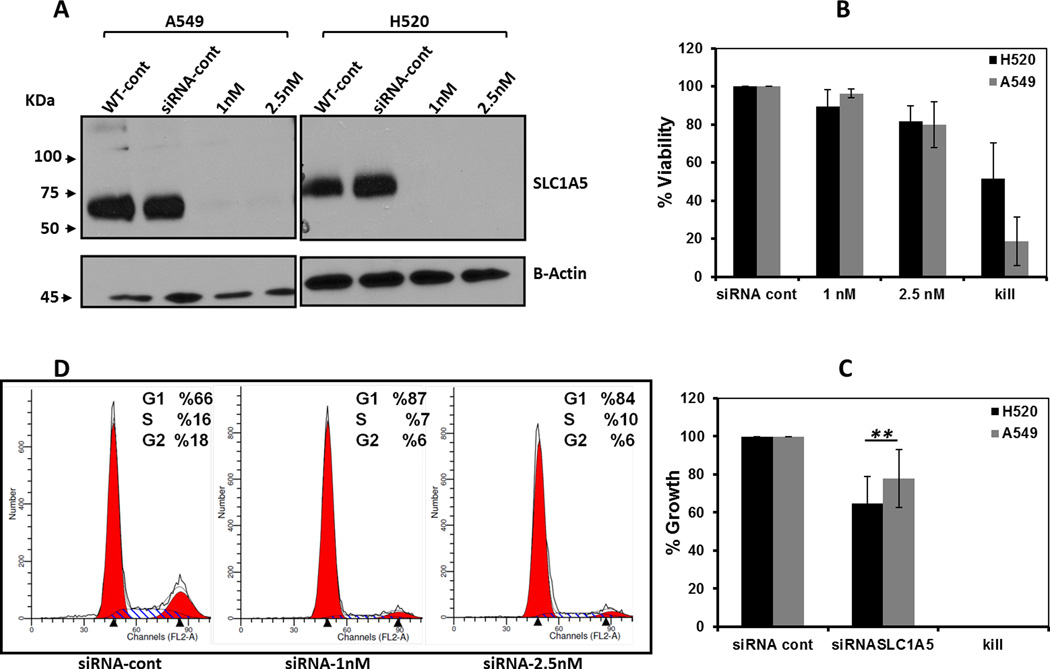
A mixture of 4 21-nucleotide short interfering RNAs (siRNAs) that target human SLC1A5 was synthesized at (Thermo Scientific, Dharmacon, Waltham, MA) and was provided as single reagent. The 4 siRNA sequences targeting human SLC1A5 in the siRNA pool were; 1) UGAUACAAGUGAAGAGUGA, 2) GCAAGGAGGUGCUCGAUUC, 3) GGUCGACCAUAUCUCCUUG, 4) GCCUUUCGCUCAUACUCUA. A non-targeting control siRNA of scrambled nucleotide sequence used as a negative control for nonspecific binding (siRNACont) was purchased from the same company. AllStars Hs Cell Death Control siRNA was used as a positive control to verify both the transfection efficiency and as a positive control for cell viability (Qiagen, Cambridge, MA). A549 and H520 cells were transfected using DhrmaFECT1- reagent (0.2 µL/well) at 1.0 and 2.5 nM siRNAs for 72h before assessing cell growth, viability and cell cycle progression as described in supplementary materials.
Statistical analysis
The association between SLC1A5 expression and clinical variables was analyzed using the Kruskal–Wallis or Wilcoxon rank-sum tests. Kinetic data was fitted to Michaelis–Menten kinetics with data points equal to means ± SEM of n experiments minus nonspecific binding. Statistical analysis for the glutamine uptake kinetics and cell growth assays was performed with GraphPad Prism (GraphPad Software, San Diego, CA, USA). Data comparing two experimental conditions were statistically analyzed by two tailed Students t-test and only results with P < 0.05 were considered to be statistically significant. All experimental data are presented as the mean ± standard error of the mean (SEM) of n independent measurements (as indicated in each figure legends). All treatments within each experiment were performed in triplicate wells and repeated on 3 independent days. To assess the extent of the dose response treatment of GPNA, ordinary regression analysis was employed to evaluate the linear trend between log and log (percent of growth inhibition).
C. Results
SLC1A5 is overexpressed at the cytoplasmic membrane and is associated with squamous lung cancer histology and male gender
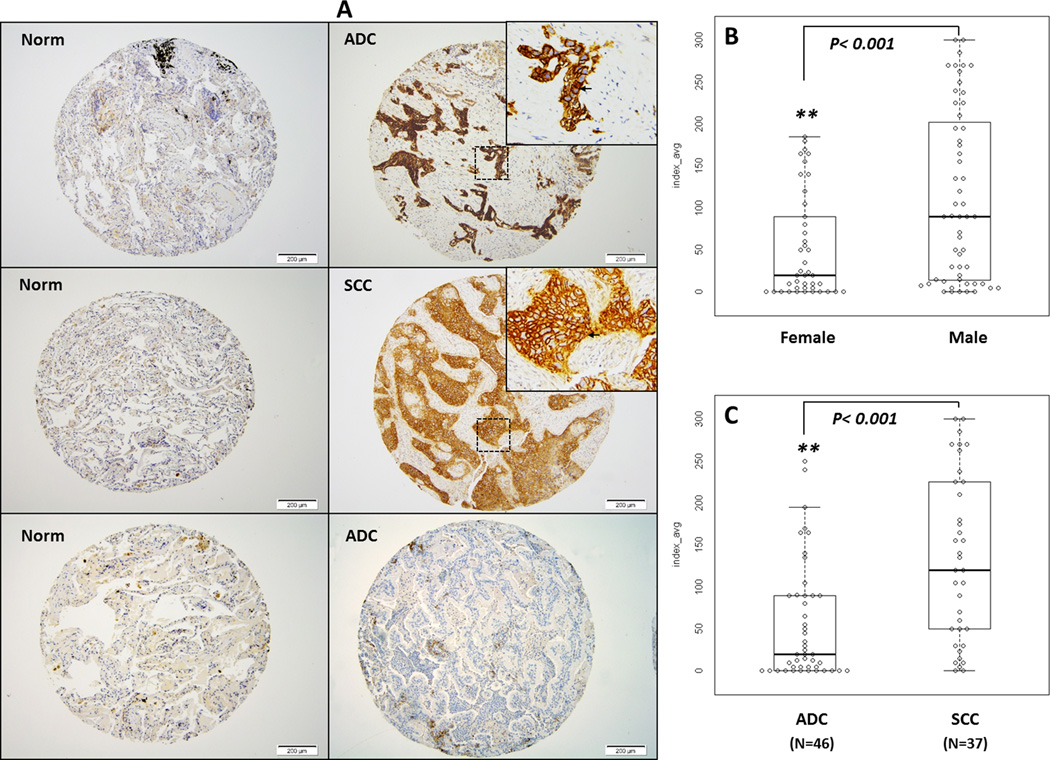
Our shotgun proteomic analysis showed that SLC1A5 protein is overexpressed in tissue extracts of both stage I ADC (N=20) and SCC (N=20) of the lung compared to control lung samples (N=20) from fresh frozen tissue and from formalin-fixed paraffin-embedded FFPE tissues (N=5 of each tissue type) (Supplemental Fig1. A, B). We confirmed these results in a separate set of tissue lysates collected from 3 normal lung tissues and 3 NSCLC tumors (Supplemental Fig. 1C). To evaluate the pattern of expression for SLC1A5 protein in lung tumors, we performed immunohistochemical (IHC) analysis using two tissue microarrays (TMA) of archival primary lung cancer tissues collected from patients diagnosed with different histologic subtypes of lung cancer using a validated human anti-SLC1A5 IgG (http://www.proteinatlas.org/). SLC1A5 signals were seen in 35/38 (95%) of SCC and of 34/46 (74%) ADC subtypes. Other NSCLCs, including large cell carcinoma (LCC), adenosquamous and NSCLC, not otherwise specified were all positive (9/9) (100%). Neuroendocrine tumors, which included atypical and typical carcinoid and small cell lung cancer (SCLC), were also represented in our TMA but only 50% (3/6) stained positive for SLC1A5 (Table 1). The pattern of SLC1A5 staining in both adenocarcinomas and squamous cell carcinomas was along the cytoplasmic membrane, with less intense cytoplasmic staining. Of the normal cellular components, we found zero to 1+ cytoplasmic membrane staining of ciliated respiratory epithelial cells, 2+ to 3+ cytoplasmic membrane staining of basal bronchial/bronchiolar cells, 2+ cytoplasmic membrane staining of bronchial submucosal glandular cells, 1+ to 2+ cytoplasmic membrane staining of reactive type 2 pneumocytes (but no staining in type 1 pneumocytes), 2+ cytoplasmic staining of alveolar macrophages and 1+ to 2+ cytoplasmic and cytoplasmic membrane staining of plasma cells. There was no staining of stromal fibroblasts, smooth muscle, cartilage, endothelial cells or lymphocytes (Fig. 1A). The occurrence of SLC1A5 expression was significantly higher in SCC than in ADC (p<0.001), (Fig. 1B). The most significant correlation between SLC1A5 and clinical variables was observed with gender, being higher in males than females (p<0.001), (Fig. 1C) and with histologic subtype, being higher in SCC than ADC (p<0.001), (Fig. 1C and Table 1). No significant correlation was observed with SLC1A5 expression and the overall survival, pack year history of smoking or age.
Figure 1. SLC1A5 is differentially expressed in NSCLC tumors and located at the level of the cytoplasmic membrane.
(A) Representative images of IHC staining for SLC1A5 protein in a tissue microarray (TMA) constructed from archival lung tissue sections collected from 98 lung cancer patients representing the main lung cancer histologic subtypes. The left panel consists of 3 representative photomicrographs at 100× magnification of sections of normal lung tissue from patients with lung cancer which stained negative for SLC1A5. The right panel consists of representative photomicrographs at 100× magnification of lung cancer sections from patients with stage IIA ADC and IIB SCC. A zoomed in 200× magnification of a small area of the same sections in the upper right corner shows membranous staining pattern of cancer cells. A section of ADC that did not stain for SLC1A5 is also represented here. (B) A box plot of SLCA15 protein IHC index (intensity × % tumor cells) shows significantly higher index of staining (p<0.001) of tumors in males (N=56) than in females (N=42). (C) A box plot of SLCA15 protein IHC index shows significantly increased IHC staining index in SCC compared to ADC (p<0.001). A detailed summary of our IHC analysis is provided in table 1.
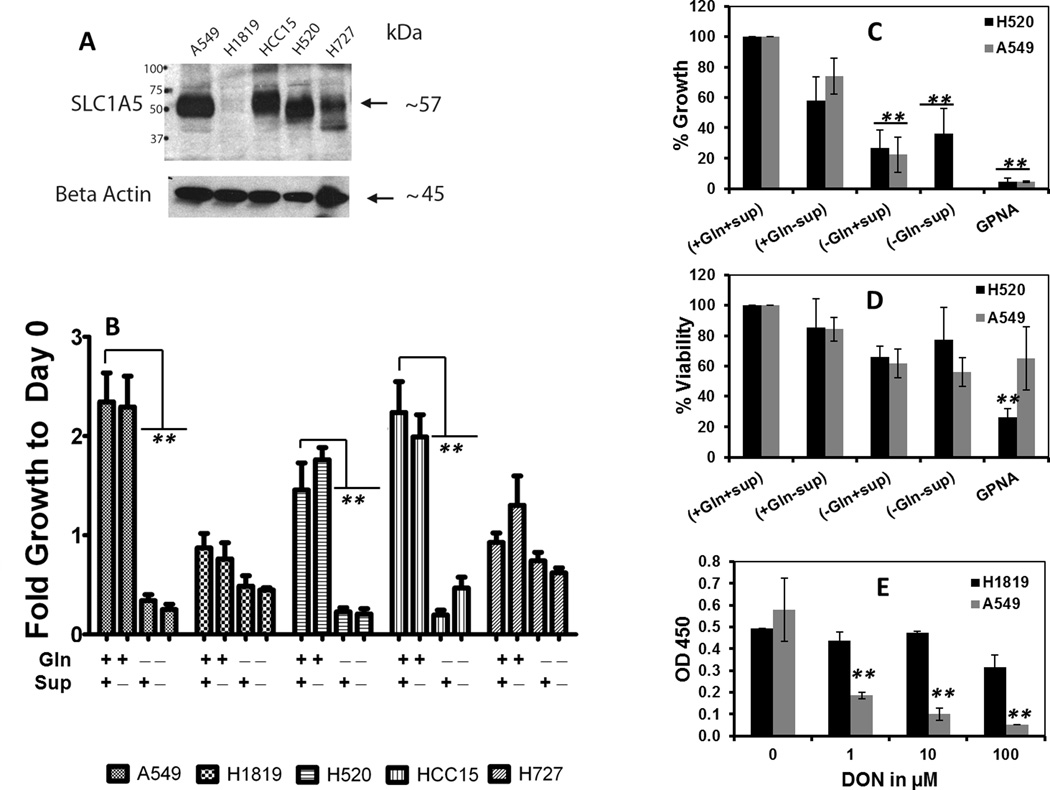
The IHC results from lung cancer cell lines showed that all but one adenocarcinoma cell line (H2009) (supplemental Table 1, not shown in IHC) and one SCLC cell line (H345) stained positive for SLC1A5 (supplemental Table 1 and Supplemental Fig.2A). Similar to lung primary tumors, we found that the subcellular location of SLC1A5 was predominantly membranous (Supplementary Fig.2A) in all lung cancer cell lines. Western blot analysis using a rabbit polyclonal anti SLC1A5 antibody (24, 25) confirmed that the expression of this protein is higher in malignant lung cancer cell lines, A549 (ADC), HCC15 (SCC), H520 (SCC) and H460 (LCC) (Supplemental Fig. 2B, C). The specificity of this antibody and the confirmation of the SLC1A5 expression in A549 and H1819 cell lines were verified using 3 different antibodies (Supplemental Fig. 3A). One ADC cell line, (H1819), was negative for SLC1A5. H727, a carcinoid cell line, had low levels of the protein. The Western blot results from lung cancer cell lines are consistent with the pattern of expression of SLC1A5 from shotgun proteomics (Supplemental Fig.1A–C) and from IHC of tumor TMA (Fig1. A).These results demonstrate strong cell membrane immunostaining for SLC1A5 in most lung cancers, more so in SCC and in men.
Glutamine uptake in lung cancer cells is mediated in part by SLC1A5
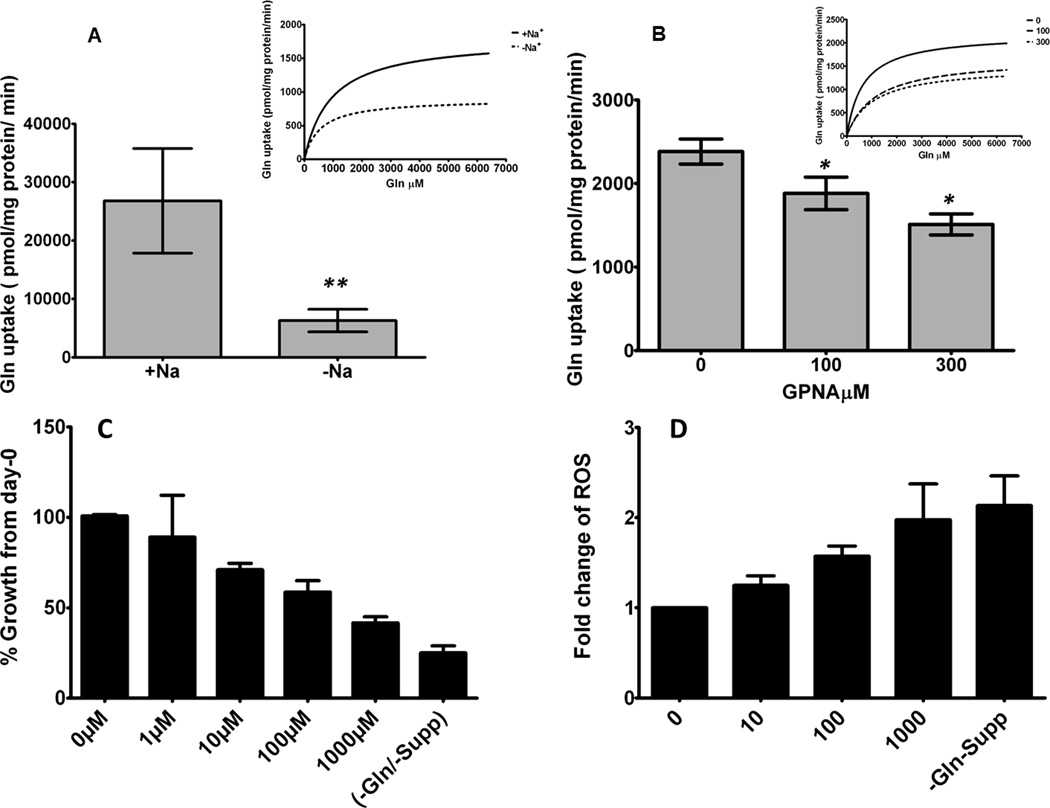
We tested the Na+ dependency of the cellular uptake of L-glutamine in A549 cells using L-[G-3H] in Krebs-Ringer solution (Fig. 2A–B)(26). Our results depicted in Fig. 2A and Supplementary table 2 showed that 68 % of cellular Gln uptake occurs in a Na+-dependent manner. To test the contribution of SLC1A5 in Gln uptake by lung cancer cells, we performed Gln uptake assays in A549 cells in the absence or in the presence of the glutamine analogue, gamma-L-Glutamyl-p-Nitroanilide, hydrochloride (GPNA), a competitive inhibitor of Gln uptake that binds specially to SLC1A5 (27, 28). Gln uptake kinetics in A549 cells demonstrated a dose-dependent inhibition of Gln uptake of 15% (p<0.05) and 32% (p<0.005) by incubating at 100 and 300 µM of GPNA for 3 min, respectively (Fig. 2B). No further inhibition of Gln uptake was observed when concentrations of GPNA were increased up to 900 µM under the same conditions (data not shown). These results suggest that the majority of the cellular Gln uptake in A549 occurs in a Na+-dependent manner and that approximately 50% of it is mediated by SLC1A5. To test the effect of pharmacological blockade of SLC1A5-mediated Gln uptake in lung cancer cell growth, we incubated A549 cells in the presence of increasing concentrations of GPNA for 48 hours. A dose-response growth inhibition was observed (Fig. 2C) compared to growth of cells without L-glutamine and supplement for the same duration. These results present the first evidence that targeting SLC1A5 activity in lung cancer cells can directly affect cell growth.
Figure 2. Gln uptake is Na+-dependent and mediated partially by SLC1A5.
To examine if intracellular transportation of Gln, human ADC A549 cells were seeded at the indicated cell densities into 24-well culture plates (0.5 mL/well). (A) The Na+-dependent uptake of 1.6mM of L-glutamine was monitored for 3 minute at 37 C. Each point represents the average ± SEM for quadruplicate determinations. This figure also illustrates the Michaelis–Menten kinetics of glutamine rates in cholKRP (-Na+) or NaKRP (+Na+) (A, upper right), (+Na+) > (−Na+). (**p < 0.005 [n = 3]). (B) Vmax values of Gln uptake kinetics of A549 cells in the presence of 0, 100 and 300 µM GPNA and the upper right panel shows the Michaelis–Menten kinetics of glutamine rates. (C) dose-response inhibition of % growth of A549 after incubation in full growth media (+Gln+supp) containing increasing doses of GPNA. (D) The intracellular ROS levels in A549 cells were measured by measuring the florescence signal of H2 DCFDA (Ex488nm/ Emiss525nm) using microtiter plate reader after 24 h incubation in increasing doses of GPNA. These data are representative of at least three independent observations and results are average ± SEM.
SLC1A5 expression regulates growth dependency on glutamine in NSCLC cells
The role of SLC1A5 expression in regulating glutamine metabolism-dependent lung cancer cell growth has not been investigated. We therefore cultured five lung cancer cell lines that varied in their level of protein expression of SLC1A5 (Fig. 3A and supplemental Fig. 3), and cultured them in media that varied in Gln and growth factors concentrations for 72h as described in the Methods section. Under Gln deprived condition, cell growth was significantly decreased in cell lines that had high levels of SLC1A5 expression (A549, HCC15 and H520) but no significant effect was observed in cell lines that had low (H727) or undetectable levels of SLC1A5 (H1819) (Fig. 3B). Interestingly, H1819 (SLC1A5 null) grew much slower even under optimum growth conditions (+Gln+Sup) compared to A549, H520 and HCC15, all of which overexpress SLC1A5. Pharmacological treatment of A549 cells (over expresses SLC1A5) with incerasing doses of 6-diazo- -oxo-L- norleucine (DON), a glutamine antagonist (29) for 4 days resulted in significant growth inhibition in these cells while H1819 (SLC1A5 null) was unaffected (Fig 3, E). These results suggest that SLC1A5 expression regulates at least in part lung cancer cell growth dependency on glutamine. To examine the specific contribution of SLC1A5 in lung cancer cell dependency on glutamine we treated A549 and H520 cells (overexpress SLC1A5) with 1 mM GPNA for 48h. Our results depicted in Fig3. C, D show a significant decrease in cell growth in both cell A549 and H520 cell lines while viability was less affected.
Figure 3. Glutamine is required for growth of lung cancer cells in vitro.
To test whether SLC1A5 expression is correlated with Gln-dependent growth of lung cancer cells in vitro, 5 lung cancer cell lines that vary in their expression level of SLC1A5 protein (A) were grown in culture media that are supplemented with EGF, Insulin, selenium and transferein and 2mM of L-glutamine (+Gln+Sup), or L-glutamine but no supplements (+Gln-Sup), or no L-glutamine but with supplements (−Gln+Supp) or not supplemented with neither L-glutamine not supplements. (B) The fold change in cell growth was analyzed at day 3 by Cell-Titer 96-Aqueous Colorimetric Assay as a change of OD at 490nm signal from day 0. Sensitivity of growth inhibition to Gln deprivation was significant in cell lines that overexpress SLC1A5, A549, H520 and HCC15 but not in cell lines that have low or undetectable level of the protein H1819 and H727. (C) The effect of Gln deprivation on cell viability in SLC1A5 expressing cell lines A549 and H520 were measured by Trypan blue Exclusion Dye method after 48 h of culturing in media that either supplemented or deprived of Gln. (D) The anti-growth effect of Gln deprivation was measure by calculating the net increase or decrease of cellular growth after 48 hour of culturing under media that either supplemented or deprived of Gln or fully supplemented media + 1mM of GPNA using the following equation (Ti−Tz)/(C−Tz)×100. Where Ti is the cell number after 48 hours of treatment of growth stimuli or inhibitors (i=inhibition), Tz is the cell number at time zero and C is the cell number of the control cells that were cultured in optimum growth conditions (+Gln +Sup). (E) Dose response decline in cell growth after treatment of A549 and H1819 with increasing concentrations of DON for 48 hours. Statistical significance was assessed by Students t-test and was denoted as *= p≤ 0.05, **= p≤ 0.005 from 3 independent assays.
Blockade of SLC1A5-related Gln uptake in lung cancer cells increases release of intracellular ROS
Our shotgun proteomic data from fresh frozen tissue of stage I lung cancer showed an increased expression of both alanyl amino peptidase (ANPEP) and glutathione synthetase (GSS), two key enzymes in the glutathione synthesis pathway (data not shown). Because glutamine acts as a precursor in the biosynthesis of glutathione (30) and because GSH functions as a scavenger for intracellular excess of reactive oxygen species (ROS) (31, 32), we tested whether inhibiting SLC1A5-mediated Gln uptake in A549 cells would increase the intracellular level of ROS. When treated with increasing doses of GPNA for 24h, A549 cells exhibited rising levels of intracellular ROS as measured by the relative fluorescence signal of the oxidized form of H2DCFDA (Fig. 2D). To test whether the anti-growth effect and the increase of intracellular ROS release were mediated by SLC1A5-Gln activity, we measured Gln uptake, cell growth and intracellular ROS release in A549 cells which express high levels of SLC1A5 compared to H1819 (Supplemental Fig. 2B). The Gln uptake of H1819 was significantly lower (by 40%) (p<0.005) than that of A549 and was comparable to the same uptake level of A549 cells when treated with SLC1A5 inhibitor GPNA at 300 µM (p<0.05). GPNA did not affect of Gln uptake in H1819 compared to A549 cells, which are inhibited by 33% at 300 µM. Pharmacological blockade of SLC1A5 with increasing doses of GPNA for 48 hours caused a dose-dependent increase in intracellular ROS release in A549 but not in H1819 (Supplemental Fig. 3C). Similarly, a dose-dependent decrease of cell growth was observed in A549 (over expressing SLC1A5) but not in H1819 (SLC1A5 null) when cells were cultured in media containing increasing concentrations of SLC1A5 inhibitor GPNA (Supplemental Fig. 3D). Collectively, these results suggest that Gln uptake is mediated in part by SLC1A5 transport activity in lung cancer cells and that blockade of this activity decreases cell growth and increases release of intracellular ROS.
Targeting SLC1A5 causes G1 arrest by inhibiting mTOR signaling
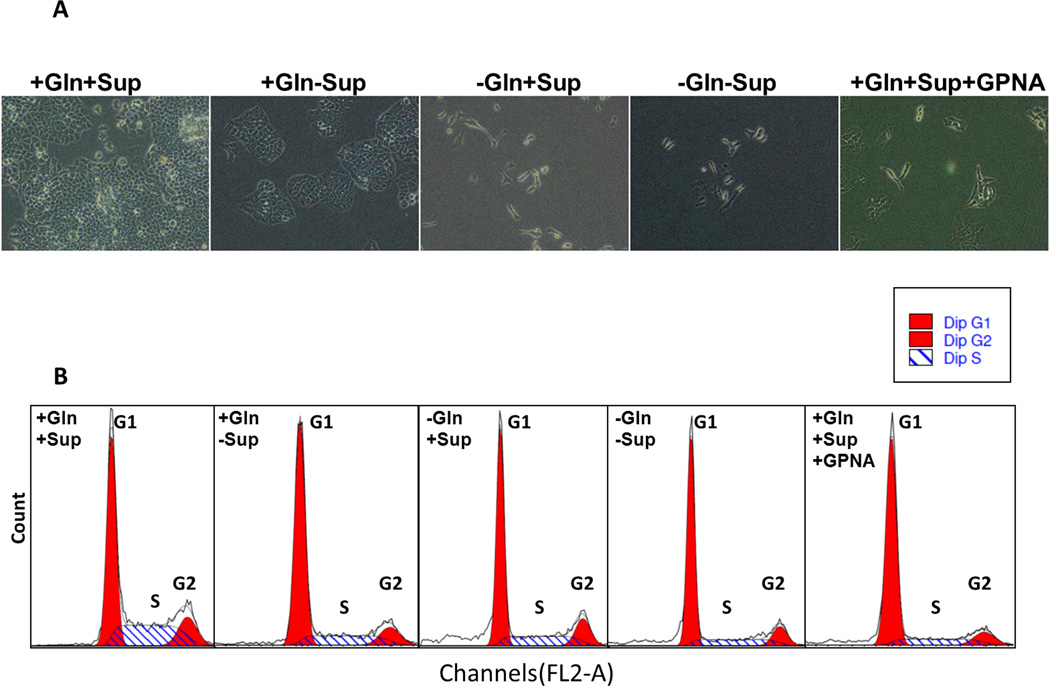
To evaluate the effects of glutamine depletion and glutamine-dependent uptake by SLC1A5 on cell cycle progression, A549 cells were cultured for 48h with either supplemented growth media (+Gln+Sup), growth supplement -depleted media (+Gln-Sup), glutamine-depleted medium (−Gln+Sup), media that lacked both glutamine and growth supplements (−Gln-Sup) or in (+Gln+Sup) media + 5 mM GPNA. Glutamine depletion only (−Gln +Sup) resulted in complete inhibition of growth as illustrated by the increased percentage of cells arrested at G1 phase and decreased percentage of cells at S and G2/M phases but cell viability was unaffected (Fig. 4A–B). A similar effect was observed in cells treated with GPNA. To test whether the anti-proliferative effect of Gln depletion can be attributed to SLC1A5 activity, we targeted SLC1A5 genetically for down regulation by using a specific short interfering RNA oligonucleotide (siRNA). A significant knock down of SLC1A5 protein was confirmed by Western blotting after 72h incubation with two different concentrations of anti SLC1A5 siRNA (Fig. 5A). Similar to Gln depletion and GPNA treatment, a significant reduction in viability and growth were observed when SLC1A5 was down regulated by siRNA (Fig. 5B–C). The down regulation of SLC1A5 by siRNA also resulted in cell cycle arrest at G1.
Figure 4. Effect of Gln depletion and SLC1A5 inhibition on cell cycle progression.
(A) Representative bright field (20× magnification) images of A549 cells after 48 hours culturing in various growth conditions as described below. (B) DNA histograms of cell cycle phase distribution of 104 cells after 48 hours of culturing A549 cells in growth media supplemented with EGF, insulin, selenium and transferein and 2mM of L-glutamine (+Gln +Sup), or L-glutamine but no supplements (+Gln-Sup), or no L-glutamine but with supplements (−Gln+Supp) or not supplemented with neither L-glutamine not supplements or (+Gln +Sup) in that contain 5mM of GPNA.
Figure 5. Genetic targeting of SLC1A5 affects cell growth and viability.
(A) Western blot analysis verification of the down regulation of SLC1A5 protein in A549 and H520 cells transfected with scramble siRNA at 25nM or anti-SLC1A5 with indicated concentrations. After siRNA 72h of transfection, cell viability (B) and cell growth compared to day 0 (C) were analyzed by direct cell count using Trypan Blue dye exclusion method.(D) Cell cycle analysis was performed in A549 cells after 72h of transfection with the indicated siRNA concentrations. The data shown as mean ± SD represent three independent experiments.
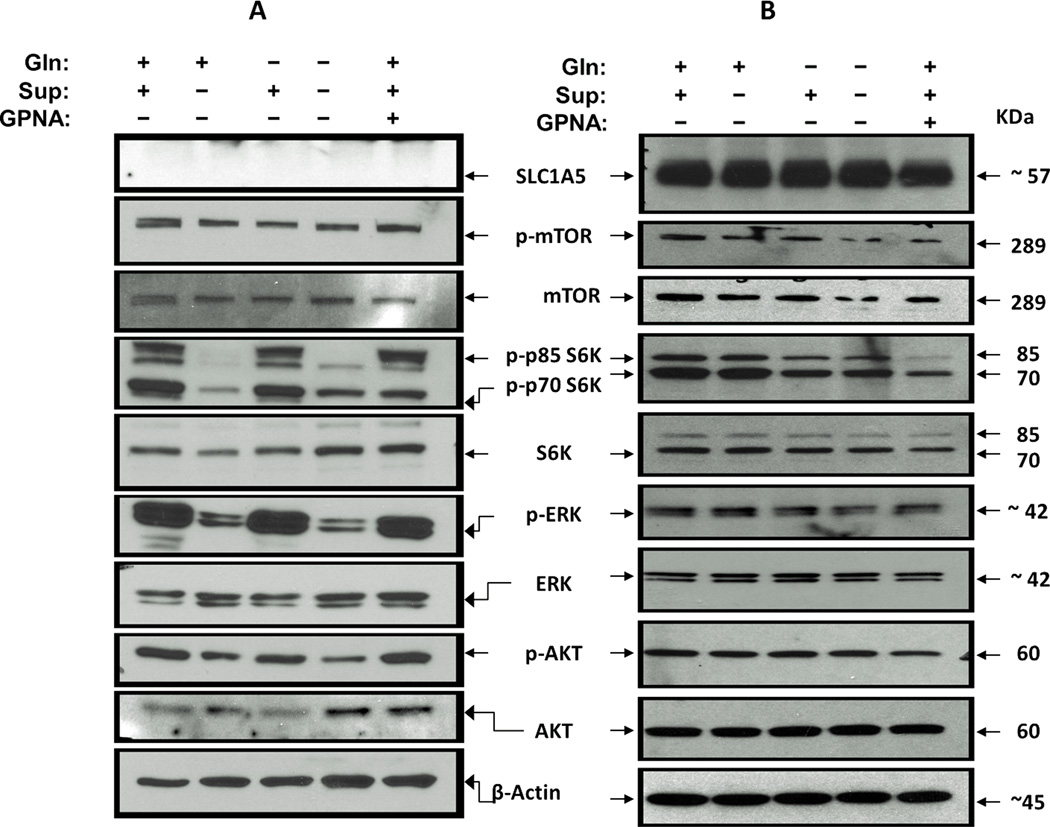
Although the literature suggests that increased cell surface expression of SLC1A5 can be explained by cancer-dependency on glutamine metabolism to support its high demand for energy and macromolecular biosynthesis (33–35), more recent studies revealed that SLC1A5 mediation of glutamine transport is independent from glutamine metabolism and necessary for activating critical survival and cellular growth signaling cascades including mTOR, and ERK pathways in some cancer types (24, 28, 36). To test whether Gln uptake into lung cancer cells induces mTOR signaling, we first starved H520 cells of L-glutamine and growth factors for 24h. The next day, cells were supplemented the medium with Glutamine (+Gln-Sup) or glutamine-free (−Gln+Sup) but with growth factors (EGF and Insulin) or full growth media + 5mM GPNA for 1 hour. Cells were then harvested, lysed and the status of mTOR pathway activation was analyzed by Western blotting. As shown in Fig. 6B, the level of phosphorylated mTOR (p-mTOR) was reduced compared to total mTOR protein by Gln depletion or GPNA treatment. The reduction of the level of p-mTOR resulted in a decrease in the activation of its downstream targets, p85 S6K and p70 S6K compared to their total protein levels. In contrast with mTOR activation we did not detect a significant change in either Akt or ERK signaling cascades as indicated by the level of phsophorylated pAKT and p-ERK levels (Fig. 6B). Contrary to H520, signaling through mTOR were unaffected by either Gln deprivation of GPNA treatment in H1819 cells (SLC1A5 null) while, deprivation of growth supplements attenuate both mTOR and ERK activation in this cell line (Fig. 6A). These results are consistent with previous studies that demonstrated the ability of extracellular glutamine to activate growth signaling cascades such as mTOR signaling and suggest that Gln plays a role as a growth signaling stimulus (24, 28). Whether this mechanism is universal in all SLC1A5 expressing lung cancer cells is unknown.
Figure 6. SLC1A5-mediated Gln uptake activates mTOR signaling.
mTOR, AKT and ERK signaling activation was evaluated using western blot analysis of the phosphorylation of p-mTOR Signaling in H520 (SCC) and H1819 (ADC) cell lines in response to Gln deprivation or SLC1A5 blockade. Cells were deprived of growth factors and L-glutamine (starved) for 24h were treated with RPMI-1640 media that were supplemented EGF, Insulin, selenium and transferein and 2mM of L-glutamine (+Gln+Sup), L-glutamine but no supplements (+Gln-Sup), or no L-glutamine but with supplements (−Gln+Supp) or not supplemented with neither L-glutamine not supplements or the full growth media + 5mM of GPNA (+Gln+Sup) for 60 min. (A) Gln deprivation or GPNA treatment has no effect on mTOR or ERK signaling in H1819 cells (SLC1A5 null). (B) In H520 (SLC1A5 overexpressed) the activation of mTOR signaling cascade was attenuated as evident by the levels of phosphorylated mTOR and the phosphorylation of its p70S6K and p85S6K p. Gln deprivation or GPNA treatment has no effect in either AKT or ERK signaling in H520 cells.
D. Discussion
We report for the first time the clinical relevance as candidate diagnostic biomarker and the biological function of SLC1A5 in lung cancer. Our results show that SLC1A5 mediates glutamine transport required for lung cancer cell growth and survival. Specifically, we report that 1. SLC1A5 is overexpressed at the cytoplasmic membrane and is associated with squamous lung cancer histology and male gender, 2. glutamine uptake in lung cancer cells is mediated in part by SLC1A5, 3. SLC1A5 expression regulates growth dependency on glutamine in NSCLC cells, 4. blockade of SLC1A5-related Gln uptake in lung cancer cells increases release of intracellular ROS, and 5. targeting SLC1A5 causes G1 arrest by inhibiting mTOR signaling.
SLC1A5 expression is significantly associated with but not restricted to SCC histology (Fig 1 and Supplemental Fig 1) and is significantly correlated with higher tumor expression levels in men (Fig 1, B and Table 1). The biological reasons for this gender correlation are not fully understood. One potential explanation is that sex hormones may regulate SLC1A5 and other amino acids transporters by may explain these findings and deserve further investigation. The expression pattern of amino acid transporters in different solid tumors including liver (10, 12), breast (12) and colon cancer (37) is emerging in the literature. In lung cancer, a recent histological study in a cohort of 160 NSCLCs found that Lat1, a sodium-independent amino acid transporter was expressed in 79.6% (43/54) of non-adenocarcinoma and in 15.1% (16/106) of adenocarcinomas (38). The same study also showed that the expression of Lat1 is significantly correlated with markers of glycolysis, angiogenesis, PI3k/Akt, EGFR, and mTOR pathways. Furthermore, the same group found Lat1 to be correlated with chemoresistance and poor prognosis in NSCLC (39). Short of functional data, this report provides preliminary evidence for the potential importance of amino acids transporters in lung cancer progression. Future functional studies should be designed to determine the relative contribution of Lat1 to glutamine uptake in lung cancer cells.
SLC1A5 has two known functions in normal and cancer cells: as a retroviral receptor during placental development and cancer-endothelial cell fusion in breast cancer (25, 40), and as a neutral amino acid transporter with high affinity for glutamine (11, 41). Earlier studies in lung cancer models in vitro and in vivo demonstrated that glutamine is essential for both growth and viability of the cells (14–16). Therefore, we focused our efforts on investigating the contribution of SLC1A5 to glutamine transport in lung cancer cell lines. We hypothesized that the enhanced expression of SLC1A5 in lung tumors and cell lines could be an adaptation mechanism that enables lung cancer cells to efficiently capture the overly abundant Gln from the extracellular milieu. Our results indicate that most of the Gln uptake by A549 cells occurs in a Na+-dependent fashion and that half of that is mediated by SLC1A5. Pharmacological and genetic targeting of SLC1A5 significantly attenuates cell growth by forcing the cells to arrest at G1 phase. Since SLC1A5 can transport aliphatic neutral amino acids including glutamine (10, 41), we anticipate that a part of the growth-inhibitory effect of its blockade can be attributed to reducing the uptake of other neutral amino acids. Nonetheless, because SLC1A5 has high affinity to Gln which is the most abundant amino acid in circulation, and because SLC1A5 contributes to 50% of the Na+-dependent Gln transport (Fig. 2 and Supplementary table 2), SLC1A5 activity is most likely responsible for the phenotypic effects observed. These data also suggest that other Na+-dependent transporters such as SN1 (SLC38A3) and/or SN2 (SLC38A5) may contribute to Gln uptake in cancer cells. Future studies are needed to evaluate the relative contribution of other amino acids transporters in lung cancer.
The anti-growth effect of siRNA down regulation of SLC1A5 in lung cancer cells demonstrated in our results is consistent with previous studies that used antisense methods to down-regulate SLC1A5 in liver cancer cell lines (24). The direct impact of SLC1A5 down regulation on cell cycle progression in lung cancer provides the first strong evidence that SLC1A5 is a link between Gln availability and cell division. Additionally and consistent with the emerging pro-survival role of L- glutamine in cancer progression (18, 19), we found that the protein level of glutathione synthetase (GSS), a rate limiting enzyme which catalyzes the conversion of gamma-L-glutamyl-L-cysteine to glutathione (42), was higher in A549 compared to H1819. Interestingly, the expression pattern of GSS mirrors that of SLC1A5 in these two cell lines. This is consistent with our shotgun proteomic data that showed GSS to be overexpressed in both SCC and ADC stage I NSCLC tissues compared to control counter parts (data not shown). To test the hypothesis that Gln transported by SLC1A5 contributes to GSH synthesis, we inhibited the transporter activity with GPNA. Blockade of SLC1A5 resulted in an increase of intracellular ROS release in A549 (SLC1A5 positive), but not in H1819 (SLC1A5 negative) with increasing doses of GPNA (Supplemental Fig. 3C). These results suggest that SLC1A5 expression may be one component of a wider metabolic reprogramming scheme that is adapted by lung cancer cells to combat oxidative stress in their microenvironment.
Recent studies revealed a new role of SLC1A5 activity independent from glutamine metabolism and necessary for activating critical survival and cellular growth signaling cascades including mTOR and ERK pathways in some cancer types (24, 28). This role for Gln and its transporter in cancer cells goes beyond traditional metabolic functions of amino acids. Consistent with these reports, our results (Fig. 6B) suggest that glutamine uptake via SLC1A5 can activate mTOR signaling independent from growth factors in H520 SCC cells. Altogether, the results of this study suggest that growth-dependency on glutamine is linked to SLC1A5 expression and activity and that inhibition of its Gln-uptake activity has cytostatic effects in lung cancer cells in vitro.
In conclusion, our results provide for the first time a functional link between SLC1A5 activity and the growth-dependency on glutamine observed in lung cancer cells. The cytostatic consequences of targeting SLC1A5 activity could be explained in part by inactivation of mTOR signaling in addition to the depletion of the intracellular glutamine pool necessary for biosynthesis of macromolecules in lung cancer cells. The differential expression of SLC1A5, its cell surface location and its function as an amino acid transporter make it an attractive target in lung cancer.
Supplementary Material
Statement of translation relevance.
We herein report for the first time the clinical relevance of SLC1A5 as a diagnostic biomarker and its biological function in lung cancer. Our results show that SLC1A5 mediates glutamine transport that is required for lung cancer cell growth and survival. These findings identify SLC1A5 as a primary transporter of glutamine in lung cancer cells, helping to explain their growth dependency on glutamine. The enhanced cell surface expression of SLC1A5, its activity as an amino acid transporter and its vital role in supporting lung cancer growth make it an ideal therapeutic target.
ACKNOWLEDGMENTS
We would like to thank Ms. Snjezana Zaja-Milatovic for her help with handling and processing tissue and cell line arrays, Dr. Adriana Gonzalez for her help in the pathologic classification of the tumors in our TMAs and Dr. Brian Lehmman for his help with the siRNA experiments.
Funding
This work was supported by Vanderbilt In vivo Cellular and Molecular Imaging Center (ICMIC) Career Development Award to MH, a NCI CA102353 to PPM and a project in the Vanderbilt SPORE in lung cancer (P50CA090949) to PPM.
Footnotes
CONFLICT OF INTEREST
No potential conflicts of interest were disclosed.
References
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Hoffman PC, Mauer AM, Vokes EE. Lung cancer. Lancet. 2000;355:479–485. doi: 10.1016/S0140-6736(00)82038-3. [DOI] [PubMed] [Google Scholar]
- 3.Carbone DP. Molecular modalities in the treatment of lung cancer. Oncology (Huntingt) 1999;13:142–147. [PubMed] [Google Scholar]
- 4.Rikova K, Guo A, Zeng Q, Possemato A, Yu J, Haack H, et al. Global survey of phosphotyrosine signaling identifies oncogenic kinases in lung cancer. Cell. 2007;131:1190–1203. doi: 10.1016/j.cell.2007.11.025. [DOI] [PubMed] [Google Scholar]
- 5.Campa MJ, Wang MZ, Howard B, Fitzgerald MC, Patz EF., Jr Protein expression profiling identifies macrophage migration inhibitory factor and cyclophilin a as potential molecular targets in non-small cell lung cancer. Cancer Res. 2003;63:1652–1656. [PubMed] [Google Scholar]
- 6.Kikuchi T, Hassanein M, Amann JM, Liu Q, Slebos RJ, Rahman SM, et al. In-depth proteomic analysis of non-small cell lung cancer to discover molecular targets and candidate biomarkers. Mol Cell Proteomics. 2012 doi: 10.1074/mcp.M111.015370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Witte D, Ali N, Carlson N, Younes M. Overexpression of the neutral amino acid transporter ASCT2 in human colorectal adenocarcinoma. Anticancer Res. 2002;22:2555–2557. [PubMed] [Google Scholar]
- 8.Albers A, Broer A, Wagner CA, Setiawan I, Lang PA, Kranz EU, et al. Na+ transport by the neural glutamine transporter ATA1. Pflugers Arch. 2001;443:92–101. doi: 10.1007/s004240100663. [DOI] [PubMed] [Google Scholar]
- 9.Broer S, Brookes N. Transfer of glutamine between astrocytes and neurons. J Neurochem. 2001;77:705–719. doi: 10.1046/j.1471-4159.2001.00322.x. [DOI] [PubMed] [Google Scholar]
- 10.Bode BP, Kaminski DL, Souba WW, Li AP. Glutamine transport in isolated human hepatocytes and transformed liver cells. Hepatology. 1995;21:511–520. [PubMed] [Google Scholar]
- 11.Bode BP, Souba WW. Modulation of cellular proliferation alters glutamine transport and metabolism in human hepatoma cells. Ann Surg. 1994;220:411–422. doi: 10.1097/00000658-199410000-00001. discussion 22-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Collins CL, Wasa M, Souba WW, Abcouwer SF. Determinants of glutamine dependence and utilization by normal and tumor-derived breast cell lines. J Cell Physiol. 1998;176:166–178. doi: 10.1002/(SICI)1097-4652(199807)176:1<166::AID-JCP18>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 13.Fuchs BC, Bode BP. Amino acid transporters ASCT2 and LAT1 in cancer: partners in crime? Semin Cancer Biol. 2005;15:254–266. doi: 10.1016/j.semcancer.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 14.Brower M, Carney DN, Oie HK, Gazdar AF, Minna JD. Growth of cell lines and clinical specimens of human non-small cell lung cancer in a serum-free defined medium. Cancer Res. 1986;46:798–806. [PubMed] [Google Scholar]
- 15.Huber KR, Mayer EP, Mitchell DF, Roberts J. Cell cycle phase perturbations by 6-diazo-5-oxo-L-norleucine and acivicin in normal and neoplastic human cell lines. Br J Cancer. 1987;55:653–656. doi: 10.1038/bjc.1987.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rivera S, Azcon-Bieto J, Lopez-Soriano FJ, Miralpeix M, Argiles JM. Amino acid metabolism in tumour-bearing mice. The Biochemical journal. 1988;249:443–449. doi: 10.1042/bj2490443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Weinberg F, Hamanaka R, Wheaton WW, Weinberg S, Joseph J, Lopez M, et al. Mitochondrial metabolism and ROS generation are essential for Kras-mediated tumorigenicity. Proc Natl Acad Sci U S A. 2010;107:8788–8793. doi: 10.1073/pnas.1003428107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Metallo CM, Gameiro PA, Bell EL, Mattaini KR, Yang J, Hiller K, et al. Reductive glutamine metabolism by IDH1 mediates lipogenesis under hypoxia. Nature. 2011 doi: 10.1038/nature10602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wise DR, Ward PS, Shay JE, Cross JR, Gruber JJ, Sachdeva UM, et al. Hypoxia promotes isocitrate dehydrogenase-dependent carboxylation of alpha-ketoglutarate to citrate to support cell growth and viability. Proc Natl Acad Sci U S A. 2011;108:19611–19616. doi: 10.1073/pnas.1117773108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gaglio D, Metallo CM, Gameiro PA, Hiller K, Danna LS, Balestrieri C, et al. Oncogenic K-Ras decouples glucose and glutamine metabolism to support cancer cell growth. Mol Syst Biol. 2011;7:523. doi: 10.1038/msb.2011.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Massion PP, Kuo WL, Stokoe D, Olshen AB, Treseler PA, Chin K, et al. Genomic copy number analysis of non-small cell lung cancer using array comparative genomic hybridization: implications of the phosphatidylinositol 3-kinase pathway. Cancer Res. 2002;62:3636–3640. [PubMed] [Google Scholar]
- 22.Gazzola GC, Dall'Asta V, Franchi-Gazzola R, White MF. The cluster-tray method for rapid measurement of solute fluxes in adherent cultured cells. Anal Biochem. 1981;115:368–374. doi: 10.1016/0003-2697(81)90019-1. [DOI] [PubMed] [Google Scholar]
- 23.Shoemaker RH. The NCI60 human tumour cell line anticancer drug screen. Nat Rev Cancer. 2006;6:813–823. doi: 10.1038/nrc1951. [DOI] [PubMed] [Google Scholar]
- 24.Fuchs BC, Finger RE, Onan MC, Bode BP. ASCT2 silencing regulates mammalian target-of-rapamycin growth and survival signaling in human hepatoma cells. Am J Physiol Cell Physiol. 2007;293:C55–C63. doi: 10.1152/ajpcell.00330.2006. [DOI] [PubMed] [Google Scholar]
- 25.Bjerregaard B, Holck S, Christensen IJ, Larsson LI. Syncytin is involved in breast cancer-endothelial cell fusions. Cell Mol Life Sci. 2006;63:1906–1911. doi: 10.1007/s00018-006-6201-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gazzola GC, Dall'Asta V, Bussolati O, Makowske M, Christensen HN. A stereoselective anomaly in dicarboxylic amino acid transport. J Biol Chem. 1981;256:6054–6059. [PubMed] [Google Scholar]
- 27.Esslinger CS, Cybulski KA, Rhoderick JF. Ngamma-aryl glutamine analogues as probes of the ASCT2 neutral amino acid transporter binding site. Bioorg Med Chem. 2005;13:1111–1118. doi: 10.1016/j.bmc.2004.11.028. [DOI] [PubMed] [Google Scholar]
- 28.Nicklin P, Bergman P, Zhang B, Triantafellow E, Wang H, Nyfeler B, et al. Bidirectional transport of amino acids regulates mTOR and autophagy. Cell. 2009;136:521–534. doi: 10.1016/j.cell.2008.11.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ovejera AA, Houchens DP, Catane R, Sheridan MA, Muggia FM. Efficacy of 6-diazo-5-oxo-L-norleucine and N-[N-gamma-glutamyl-6-diazo-5-oxo-norleucinyl]-6-diazo-5-oxo-norleucine against experimental tumors in conventional and nude mice. Cancer Res. 1979;39:3220–3224. [PubMed] [Google Scholar]
- 30.Griffith OW, Mulcahy RT. The enzymes of glutathione synthesis: gamma-glutamylcysteine synthetase. Adv Enzymol Relat Areas Mol Biol. 1999;73:209–267. doi: 10.1002/9780470123195.ch7. xii. [DOI] [PubMed] [Google Scholar]
- 31.Meister A. Glutathione, ascorbate, and cellular protection. Cancer Res. 1994;54:1969s–1975s. [PubMed] [Google Scholar]
- 32.Meister A, Anderson ME. Glutathione. Annu Rev Biochem. 1983;52:711–760. doi: 10.1146/annurev.bi.52.070183.003431. [DOI] [PubMed] [Google Scholar]
- 33.Fuchs BC, Perez JC, Suetterlin JE, Chaudhry SB, Bode BP. Inducible antisense RNA targeting amino acid transporter ATB0/ASCT2 elicits apoptosis in human hepatoma cells. Am J Physiol Gastrointest Liver Physiol. 2004;286:G467–G478. doi: 10.1152/ajpgi.00344.2003. [DOI] [PubMed] [Google Scholar]
- 34.Yang C, Sudderth J, Dang T, Bachoo RG, McDonald JG, DeBerardinis RJ. Glioblastoma cells require glutamate dehydrogenase to survive impairments of glucose metabolism or Akt signaling. Cancer Res. 2009;69:7986–7993. doi: 10.1158/0008-5472.CAN-09-2266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.DeBerardinis RJ. 2010 Keystone Symposium: Metabolism and Cancer Progression. Future Oncol. 6:893–895. doi: 10.2217/fon.10.52. [DOI] [PubMed] [Google Scholar]
- 36.DeBerardinis RJ, Mancuso A, Daikhin E, Nissim I, Yudkoff M, Wehrli S, et al. Beyond aerobic glycolysis: transformed cells can engage in glutamine metabolism that exceeds the requirement for protein and nucleotide synthesis. Proc Natl Acad Sci U S A. 2007;104:19345–19350. doi: 10.1073/pnas.0709747104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kekuda R, Torres-Zamorano V, Fei YJ, Prasad PD, Li HW, Mader LD, et al. Molecular and functional characterization of intestinal Na(+)-dependent neutral amino acid transporter B0. Am J Physiol. 1997;272:G1463–G1472. doi: 10.1152/ajpgi.1997.272.6.G1463. [DOI] [PubMed] [Google Scholar]
- 38.Kaira K, Oriuchi N, Takahashi T, Nakagawa K, Ohde Y, Okumura T, et al. LAT1 expression is closely associated with hypoxic markers and mTOR in resected non-small cell lung cancer. Am J Transl Res. 2011;3:468–478. [PMC free article] [PubMed] [Google Scholar]
- 39.Kaira K, Takahashi T, Murakami H, Shukuya T, Kenmotsu H, Naito T, et al. Relationship between LAT1 expression and response to platinum-based chemotherapy in non-small cell lung cancer patients with postoperative recurrence. Anticancer Res. 2011;31:3775–3782. [PubMed] [Google Scholar]
- 40.Blond JL, Lavillette D, Cheynet V, Bouton O, Oriol G, Chapel-Fernandes S, et al. An envelope glycoprotein of the human endogenous retrovirus HERV-W is expressed in the human placenta and fuses cells expressing the type D mammalian retrovirus receptor. J Virol. 2000;74:3321–3329. doi: 10.1128/jvi.74.7.3321-3329.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Utsunomiya-Tate N, Endou H, Kanai Y. Cloning and functional characterization of a system ASC-like Na+-dependent neutral amino acid transporter. J Biol Chem. 1996;271:14883–14890. doi: 10.1074/jbc.271.25.14883. [DOI] [PubMed] [Google Scholar]
- 42.Finkle D, Quan ZR, Asghari V, Kloss J, Ghaboosi N, Mai E, et al. HER2-targeted therapy reduces incidence and progression of midlife mammary tumors in female murine mammary tumor virus huHER2-transgenic mice. Clin Cancer Res. 2004;10:2499–2511. doi: 10.1158/1078-0432.ccr-03-0448. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.