Abstract
Background
Investigations examining psychosocial adjustment among childhood cancer survivors have focused primarily on negative effects and psychopathology. Emergent literature suggests the existence of positive impact or adjustment experienced after cancer, as well. The purpose of this study is to examine the distribution of Perceived Positive Impact (PPI) and its correlates in young adult survivors of childhood cancer.
Methods
6,425 survivors and 360 siblings completed a comprehensive health survey, inclusive of a modified version of the Posttraumatic Growth Inventory (PTGI) as a measure of PPI. Linear regression models were used to examine demographic, disease and treatment characteristics associated with PPI.
Results
Survivors were significantly more likely than siblings to report PPI. Endorsement of PPI was significantly greater among female and non-white survivors, and among survivors exposed to at least one intense therapy, a second malignancy or cancer recurrence. Survivors diagnosed at older ages and fewer years since diagnosis were more likely to report PPI. Income, education and marital/relationship status appeared to have varied relationships to PPI depending upon the subscale being evaluated.
Conclusions
The existence and variability of PPI in survivors in this study suggest that individual characteristics, inclusive of race, gender, cancer type, intensity of treatment, age at diagnosis and time since diagnosis, have unique and specific associations with different aspects of perceived positive outcomes of childhood cancer.
Keywords: Psychosocial, childhood cancer, trauma, event centrality, survivors
BACKGROUND
Research has tended to focus on the ways in which serious life-threatening conditions negatively influences health and functioning. Yet, empirical evidence also suggests that some individuals perceive personal growth or benefits as a result of exposure to traumatic experiences (Calhoun & Tedeschi, 1998; Carver, 1998; Park, 1998; Tedeschi, Calhoun, & Cann, 2007). In the case of pediatric oncology, published investigations examining psychosocial adjustment among childhood cancer survivors have focused primarily on negative effects and psychopathology (Hobbie, et al., 2000; Meeske, Ruccione, Globe, & Stuber, 2001; Stuber, Meeske, Gonzalez, Houskamp, & Pynoos, 1994). However, while limited, emergent research also suggests the possibility that survivors are resilient and report positive life circumstances which they attribute to having had cancer.
Positive outcomes assumedly attributable to cancer are conceptualized in the psycho-oncology literature as benefit finding or perceived benefits (Kallay, 2006; Phipps, Long, & Ogden, 2007; Thornton, 2002), resilience and thriving (Chesler & Parry, 2005; Orbuch, Parry, Chesler, Fritz, & Repetto, 2005; Woodgate, 1999), and posttraumatic growth (Barakat, Alderfer, & Kazak, 2006; Cordova, Cunningham, Carlson, & Andrykowski, 2001; Kamibeppu, et al., 2010; Stanton, Bower, & Low, 2006; Sumalla, Ochoa, & Blanco, 2009; Thornton & Perez, 2006). They are defined as cognitive processes by which those who have experienced life changing or traumatic events apply positive interpretations to and find meaning in the event (Barakat, et al., 2006), and occur when individuals formulate adaptive interpretations or worldviews as a result of experiencing these events (Boals & Schuettler, 2010). Findings from these investigations are consistent with the theoretical notion of “post-traumatic growth” (Tedeschi & Calhoun, 1996), in that they offer evidence that cancer changes or influences survivors’ lives in some or all of the following domains: (1) life perspective (e.g., altered priorities, greater joy or appreciation for one’s life, greater sense of meaning, enhanced religious or spiritual beliefs); (2) relationships (e.g., greater appreciation for one’s relationships, greater sense of intimacy, enhanced emotional expressiveness, increased sensitivity to others); and (3) self-perception (e.g., sense of emotional growth, strength, self-reliance).
Studies suggest that positive life changes attributable to cancer are reported by a majority of adult cancer patients and survivors (Stanton, et al., 2006; Thornton, 2002). Sumalla et al (2009) estimated that in most cases 80% or more of adult cancer survivors regarded themselves as having benefited in some way from the experience. Indeed, young survivors also are capable of describing subjective perceptions of how they believe having had cancer has affected their lives. For example, 304 childhood cancer survivors (aged 14-25 years) were asked about their experience of positive and negative life changes after their illness (Chesler, 2000), and over half of the sample reported themselves as having enhanced concern for others (60%), ability to cope with tragedy (54%), sense of identity (52%), and spiritual well-being (52%). In companion, qualitative interviews with 50 young adult survivors of childhood cancer (aged 17-29 years at interview, 1 month -17 years of age at diagnosis, at least 3 years post-treatment), a majority of study participants reported positive effects attributable to having had cancer, including increased psychological maturity (65%), greater compassion and empathy (61%), new values and priorities (57%), new strengths (48%), and increased recognition of vulnerability and struggle, with a deeper appreciation for life (44%) (Parry & Chesler, 2005). A recent investigation of 150 adolescent survivors of childhood cancer (aged 11-19 years, at least 1 year post-treatment) indicated that 84.7% of survivors identified at least one positive consequence of having had cancer, and 32% identified four or more positive consequences (Barakat, et al., 2006). Positive influence was associated with older age at diagnosis, greater treatment severity and life threat, and less time since diagnosis. In another study of 199 child and adolescent cancer patients (aged 7-18 years), “benefit-finding” was associated with older age at diagnosis, less time since diagnosis, and being African American (Phipps, et al., 2007).
For many childhood cancer survivors, particularly those who were very young at the time of treatment, their memories of cancer are often created through stories passed on to them by family members. Thus, for a young adult cancer survivor population, perceived positive impact of cancer may be less a reflection of change attributable to cancer and more a function of “event centrality,” the extent to which a stressful or life-threatening event (such as cancer) becomes a salient organizing principle for the individual’s growing sense of self and view of the world (Berntsen & Rubin, 2006; Boals & Schuettler, 2010).
Assessing the perceived positive impact of cancer in childhood survivors is instructive for several reasons. Recent research suggests that subjective perceptions of conditions or phenomenon are better predictors of distress and well-being than are objective health status measures (Cordova & Andrykowski, 2003; Fleer, et al., 2006; Pemberger, et al., 2005; Taieb, Moro, Baubet, Revah-Levy, & Flament, 2003; Zebrack, Yi, Petersen, & Ganz, 2008). Thus, studies of perceived positive impacts and their correlates or predictors will achieve a more comprehensive and balanced understanding of psychosocial adjustment in long-term survivors of childhood cancer. Such understanding can guide the development of interventions that not only prevent or minimize cancer’s negative effects but also promote self-esteem and adjustment, and subsequently facilitate successful achievement of developmental tasks typical of adolescence and young adulthood, such as establishing employment or career paths, forming a family, and achieving autonomy. Furthermore, theoretical models of benefit-finding, perceived positive impact and post-traumatic growth in this field are evolving. Findings from investigations such as this may help to advance theoretical suppositions as they apply to the impact of chronic or serious life-threatening illnesses in young people whose psychosocial and cognitive development are incomplete at the time of exposure to these medical conditions.
Given recent interest in examining potential positive impacts of cancer, this study aims to (1) examine the distribution of perceived positive impact (PPI) of cancer in young adults who are childhood cancer survivors, and compare it to that of siblings; and (2) examine the extent to which cancer-related factors (e.g. age at diagnosis, time since diagnosis, cancer type) and key sociodemographic variables predict the likelihood of young adult survivors attributing positive outcomes to having had cancer. This study overcomes many of the limitations of current research in the field of psychosocial oncology in that it involves a large multi-institutional sample, includes a comparison group, and is powered to examine the simultaneous influences of sociodemographic and cancer-related risk factors on outcomes.
METHODS
Sample and Data Collection
The Childhood Cancer Survivor Study (CCSS) is a large, multi-institutional cohort study that tracks the health status of survivors of childhood cancer diagnosed between 1970 and 1986 who survived at least 5 years post diagnosis. The institutional review board at each participating center reviewed and approved the CCSS protocol and documents sent to participants. Study participants provided informed consent for participation in the study and for release of medical-record information. Detailed descriptions of the study design and characteristics of the cohort are reported elsewhere (Mertens, Walls, Taylor, et al., 2004; Robison, et al., 2009).
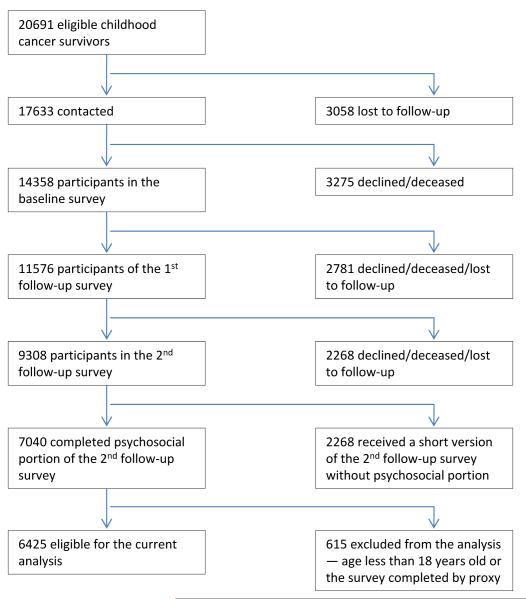
In 1995-1996, eligible study participants were mailed a 24-page baseline questionnaire. All subjects who completed that baseline survey received a subsequent follow-up questionnaire sometime in 2002-2004, in which were included standardized measures of health-related quality of life, psychological distress, and the outcomes and correlates reported here. Of 20,691 survivors of childhood cancer identified for the original cohort, 3058 (14.8%) were lost to follow-up despite extensive efforts to locate them. Among the remaining 17,633 survivors, 14,358 (81.4%) completed the baseline questionnaire. In addition, a sample of the participating survivor population were randomly selected and asked to nominate their nearest age sibling to be a part of the comparison group. Of the 4,782 siblings nominated, 3,899 siblings (81.5%) participated in the baseline survey. In 2003, 11,576 (80.6%) of the original cohort were located and requested to participate in a follow-up survey. Of these, 9,308 (80.4%) completed and returned a survey, with 6,425 being over 18 years of age and having completed all survey items required for this study by themselves and thus included in the analysis reported here. Also, a sub-sample of 500 siblings over age 18 was randomly selected to survey. Of these, 360 (72.0%) completed all of the items of interest to this study. Figure 1 offers a graphic representation of subject recruitment and retention of the CCSS survivor group.
Figure 1.
Although siblings of childhood cancer survivors also are influenced by the life disruption associated with cancer in the family, they can still serve as an adequate same-aged peer comparison group (Zebrack, Zeltzer, Whitton, Berkow, & Chesler, 2003). Recent research suggests that siblings approximate a psychologically healthy comparison group, in that rates of posttraumatic stress and emotional distress in adult siblings of adult survivors of childhood cancer are similar to those found in population norms (Buchbinder, et al., In Press; Stuber, et al., 2010).
Measures
Perceived positive impact (PPI), the primary outcome variable, was assessed using a modified version of the Posttraumatic Growth Inventory (PTGI), a 21-item scale comprising subscales suggestive of positive growth in five domains: Relating to Others, New Possibilities, Personal Strength, Spiritual Change, and Appreciation for Life (Tedeschi & Calhoun, 1996). Modified wording of the question stem instructed survivor respondents to indicate how much their cancer experience has influenced their life. Siblings were instructed to indicate how much their life was influenced by their sibling’s cancer experience. Each item was rated on a six-point scale, with values ranging from 0 (“I am NOT influenced by my/my sibling’s experience”) to 5 (“I am influenced to a VERY GREAT degree as a result of my/my sibling’s experience”). Items are summed to derive five subscale scores. Higher scores suggest increasing levels of PPI.
Sociodemographic and cancer-specific variables were analyzed as potential correlates of PPI in the survivors. Self-reported sociodemographic variables included age at study, gender, employment status, marital/relationship status, education, race, and household income. Cancer-related variables were derived via medical records abstraction (Mertens, et al., 2004; Robison, et al., 2009), and included type of cancer, age at diagnosis, relapse or second malignancy (Yes/No), and years since diagnosis. A dichotomous “intensity of treatment” variable (no intense therapy; at least 1 intense therapy) was created based on high risk chemotherapy exposures (anthracyclines/ alkalating agents, other) and/or exposures to high risk types and locations of radiotherapy (RT to brain or spine,), as compared to no chemotherapy or radiation (Stuber, et al., 2010).
Statistical Analyses
Exploratory data analysis was performed to examine the distributions of variables of interest. To test the psychometric properties of the PTGI (as a measure of PPI) in a childhood cancer survivor population, we conducted a confirmatory factor analysis (CFA) in the eligible survivor population (i.e., aged 18 years or older at the survey and completed all 21 PTGI items, n=6425) using a five-factor solution suggested by prior administrations of the PTGI instrument (Calhoun & Tedeschi, 1998; Taku, Cann, Calhoun, & Tedeschi, 2008). The weighted least squares method was used, as the multivariate normality assumption was in doubt. To statistically test the fit of the five-factor model with that of a single-factor model, bootstrap was used, assessing the significance of the differences in three measures of goodness of fit – (1) the goodness of fit index (GFI), (2) the adjusted goodness of fit index (AGFI), and (3) the root mean square error of approximation (RMSEA), between the two models. In addition, we assessed internal consistency of each of the five factors by Cronbach’s alpha.
Demographic factors were compared between the survivors and siblings with statistical significance evaluated using bootstrap, which takes into account potential within family correlation between a survivor and his/her sibling. The continuous scores of the five PTGI subscales among survivors were compared with those among siblings, using the modification of linear regression by Generalized Estimating Equations to account for the potential within-family correlation (Liang & Zeger, 1986), adjusting for gender and two variables that differ significantly between the survivors and siblings in this sample: age at study and race. Effect size and confidence intervals are reported. Effect size is defined as the difference of a score between the survivor group and the sibling group divided by standard deviation of the latter. This approach is justified based on viewing the sibling group as an appropriate reference population. Adjusted differences in scores were similarly expressed as adjusted effect sizes. Similarly, relationships between the five PTGI subscales and selected correlates were assessed using linear regression models. A backward variable-selection method was employed to build a summary model that describes the independent and simultaneous associations of each subscale with demographic and clinical factors. In these models, age at diagnosis and years since cancer diagnosis were assumed to have linear effects. This linear assumption was examined using natural cubic splines (Harrell, 2001), given the possibility that the relationships of these variables with the PTGI might be curvilinear (Kleim & Ehlers, 2009); however, results indicated that the linear assumption was reasonable and, therefore, used in the analysis. We considered treatment exposures within the first five years from the original diagnosis of cancer in defining treatment variables. All statistical analyses were performed using SAS Version 9.2 (SAS Institute, 1996) and 2-sided statistical inferences were employed throughout the analyses.
RESULTS
Demographic characteristics of the survivors and siblings participating in this study are summarized in Table 1, along with cancer-related descriptive data of participating survivors. Cancer survivors were similar to siblings in gender and education level, but were more likely to be younger at interview (mean 32.3 years versus 33.9 years among siblings, p< .001), non-white (p<.001), not employed (p=.04), not married (p<.001) and to have a lower income (p=.009). On average, survivors were 8.7 years of age at time of diagnosis (SD=5.9), and 23.6 years beyond their diagnosis (SD=4.5). As reported elsewhere (Stuber, et al., 2010), descriptive demographic and cancer related characteristics of survivors and siblings who completed the current survey of interest to this study were compared with those who did not complete it (but did complete the original CCSS baseline survey) using Chi-square tests. Similarly, siblings included in this study were compared to siblings who completed the baseline questionnaire but not the survey of interest to this study. Compared to survivors who completed the baseline survey, follow-up survey respondents for this study were significantly more likely to be older, female, white, employed, married/partnered, and older age at diagnosis and fewer years since diagnosis. Survivor participants did not differ from non-participants by cancer diagnosis, survival time, or on a standardized measure of psychological distress assessed at baseline. As expected, based on their random selection for participation in the psychosocial portion of the questionnaire, sibling participants did not differ from non-participants by gender, age, race, educational attainment, employment, marital status, or baseline psychological distress.
Table 1.
Descriptive statistics.
| Siblings n=360(%) |
Survivors n=6425(%) |
|
|---|---|---|
| Age at interview ** | ||
| 18-29 | 132(36.7) | 2598 (40.4) |
| 30-39 | 135(37.5) | 2718 (42.3) |
| 40+ | 93(25.8) | 1109 (17.3) |
| Race ** | ||
| White non-Hispanic | 322(93.6) | 5603 (87.5) |
| All others | 22(6.4) | 798 (12.5) |
| Gender | ||
| Male | 172(47.8) | 3064 (47.7) |
| Female | 188(52.2) | 3361 (52.3) |
| Education | ||
| <= High school graduate | 52(14.5) | 953 (14.9) |
| Some college | 127(35.5) | 2311 (36.2) |
| >= College graduate | 179(50.0) | 3118 (48.9) |
| Employed * | ||
| Yes | 298(83.0) | 4999 (78.4) |
| No | 61(17.0) | 1381 (21.6) |
| Personal income ** | ||
| <$20,000 | 109(34.8) | 2658 (42.6) |
| $20,000+ | 204(65.2) | 3588 (57.4) |
| Ever married ** | ||
| Yes | 256(71.5) | 3731 (58.6) |
| No | 102(28.5) | 2635 (41.4) |
| Survivor Diagnosis | ||
| Leukemia | 2123 (33.0) | |
| CNS | 669 (10.4) | |
| HD | 912 (14.2) | |
| NHL | 507 (7.9) | |
| Kidney (Wilms) | 620 (9.6) | |
| Neuroblastoma | 405 (6.3) | |
| Soft tissue sarcoma | 593 (9.2) | |
| Bone cancer | 596 (9.3) | |
| Survivor age at Diagnosis | ||
| 0-4 | 2355 (36.7) | |
| 5-9 | 1446 (22.5) | |
| 10-14 | 1378 (21.4) | |
| 15-20 | 1246 (19.4) | |
| Years since Survivor Diagnosis | ||
| 15-19 | 1753 (27.3) | |
| 20-24 | 2296 (35.7) | |
| 25-29 | 1649 (25.7) | |
| 30-34 | 727 (11.3) | |
| 2nd malignancy or recurrence in survivor | ||
| Yes | 1150 (17.9) | |
| No | 5275 (82.1) | |
| At least one intense therapy | ||
| Yes | 4686 (78.6) | |
| No | 1275 (21.4) |
p < 0.05,
p < 0.001
Confirmatory Factor Analysis
The GFI derived from the five-factor confirmatory factor analysis was 0.88, the AGFI was 0.85, and the RMSEA was 0.05. RMSEA is an index of fit that is less influenced by sample size, with recommended levels being 0.05 or below (Floyd & Widaman, 1995). Cronbach’s alpha was 0.92, 0.89, 0.86, 0.89, 0.84 for subscales indicating “new possibilities,” “relating to others,” “personal strength,” “spiritual change,” and “appreciation for life,” respectively. These indices suggest a reasonable fit of the five-factor structure to this survivor sample. CFA was repeated with a single factor to determine whether the five-factor model explained the variability in responses significantly better than the single-factor model. The single-factor model had significantly reduced fit indices (GFI = 0.84, AGFI = 0.80, RMSEA = 0.06; p<0.001). Thus, the five-factor model accounted for the variance of responses significantly better than the single-factor model.
Survivor-Sibling Comparisons
A comparison of survivor and sibling scores for PPI is summarized in Table 2. Mean scores on all subscales, adjusted for differences in age, gender, and race, were significantly higher among survivors than siblings. In all instances, survivors were more likely than siblings to indicate that they felt that their lives had been influenced by cancer. Effect sizes were generally small to moderate.
Table 2.
Age-, gender-, and race- adjusted scores and score difference between survivors and siblings.
| Subscale Score | Survivor mean score |
Sibling mean score |
Difference | Effect Size* (95% CI) |
p- value |
|---|---|---|---|---|---|
| Relating to Others | 18.59 | 16.41 | 2.18 (1.12 - 3.25) | 0.22 (0.11 - 0.32) | <.001 |
| New Possibilities | 10.75 | 8.35 | 2.40 (1.70 - 3.11) | 0.37 (0.26 - 0.48) | <.001 |
| Personal Strength | 11.87 | 9.65 | 2.23 (1.59 - 2.86) | 0.37 (0.27 - 0.48) | <.001 |
| Spiritual Change | 5.30 | 4.34 | 0.96 (0.61 - 1.31) | 0.28 (0.18 - 0.38) | <.001 |
| Appreciation of Life | 9.58 | 8.56 | 1.02 (0.55 - 1.49) | 0.23 (0.12 - 0.34) | <.001 |
Effect Size = Standardized difference (i.e. Difference between groups divided by standard deviation of siblings).
Multivariable Modeling
Table 3 presents results of multivariable modeling (adjusted effect sizes) for survivors. As evidenced in the five subscale scores, endorsement of PPI was significantly more evident among female and non-white survivors, as well as among survivors exposed to at least one intense therapy or a second malignant neoplasm (SMN) or recurrence of cancer. The likelihood of reporting higher PPI increased significantly with age at diagnosis such that childhood survivors diagnosed at older ages were more likely to report PPI than those diagnosed at younger ages. In most cases, PPI decreased with years since diagnosis. Respondents with fewer years since diagnosis were more likely to endorse PPI. While the effect sizes appear small for each additional year, over several years the magnitude of change becomes more clinically meaningful. When compared to survivors of childhood leukemia, survivors of Hodgkin’s disease, brain and kidney (Wilm’s) tumors and neuroblastoma reported significantly less PPI. In contrast, survivors of bone cancer reported significantly more PPI when compared to leukemia survivors.
Table 3.
| Variables | Relating to Others subscale |
New Possibilities subscale |
Personal Strength subscale |
Spiritual Change subscale |
Appreciation of Life subscale |
|---|---|---|---|---|---|
| Diff (95% CI) | Diff (95% CI) | Diff (95% CI) | Diff (95% CI) | Diff (95% CI) | |
| Years since diagnosis | −0.01 (−0.02 to −0.01)** | −0.01 (−0.02 to −0.01)** | −0.01 (−0.02 to −0.01)** | ||
| Age at diagnosis | 0.01 (0.01 to 0.02)** | 0.02 (0.01 to 0.02)** | 0.01 (0.01 to 0.02)** | 0.01 (0.01 to 0.02)** | |
|
Race White non-Hispanic All others |
−0.10 (−0.18 to −0.03)** Ref |
−0.18 (−0.26 to −0.09)** Ref |
−0.13 (−0.20 to −0.05)** Ref |
−0.13 (−0.21 to −0.05)** Ref |
−0.09 (−0.17 to −0.02)* Ref |
|
Gender Male Female |
−0.24 (−0.29 to −0.19)** Ref |
−0.18 (−0.24 to −0.13)** Ref |
−0.20 (−0.24 to −0.15)** Ref |
−0.26 (−0.31 to −0.20)** Ref |
−0.25 (−0.30 to −0.20)** Ref |
|
Education <= High school grad Some college >= College graduate |
0.20 (0.12 to 0.27)** 0.06 (0.01 to 0.11)* Ref |
0.09 (0.01 to 0.17)* 0.02 (−0.04 to 0.08) Ref |
−0.09 (−0.17 to −0.02)* −0.06 (−0.11 to −0.01)* Ref |
||
|
Employed
$ Yes No |
|||||
|
Personal Income <$20,000 $20,000 |
−0.09 (−0.14 to −0.04)** Ref |
0.10 (0.04 to 0.16)** Ref |
|||
|
Ever Married Yes No |
0.12 (0.06 to 0.18)** Ref |
0.09 (0.04 to 0.15)** Ref |
|||
|
Year of Diagnosis 1970-1973 1974-1978 1979-1986 |
−0.15 (−0.22 to −0.07)** −0.04 (−0.10 to 0.01) Ref |
||||
|
SMN or Recurrence Yes No |
0.25 (0.18 to 0.31)** Ref |
0.25 (0.18 to 0.33)** Ref |
0.22 (0.15 to 0.28)** Ref |
0.23 (0.16 to 0.30)** Ref |
0.20 (0.13 to 0.26)** Ref |
|
At least one intense therapy Yes No |
0.20 (0.14 to 0.26)** Ref |
0.22 (0.15 to 0.29)** Ref |
0.21 (0.15 to 0.27)** Ref |
0.15 (0.08 to 0.22)** Ref |
0.18 (0.11 to 0.24)** Ref |
|
Diagnosis CNS HD NHL Kidney (Wilms) Neuroblastoma Soft tissue sarcoma Bone cancer Leukemia |
−0.12 (−0.20 to −0.03)** −0.11 (−0.20 to −0.02)* −0.02 (−0.12 to 0.08) −0.21 (−0.30 to −0.12)** −0.17 (−0.28 to −0.06)** −0.03 (−0.12 to 0.07) 0.14 (0.05 to 0.24)** Ref |
−0.14 (−0.23 to −0.04)** −0.06 (−0.15 to 0.02) −0.03 (−0.14 to 0.08) −0.29 (−0.39 to −0.19)** −0.23 (−0.35 to −0.11)** −0.08 (−0.18 to 0.02) 0.28 (0.18 to 0.38)** Ref |
−0.19 (−0.28 to −0.10)** −0.06 (−0.14 to 0.03) 0.01 (−0.08 to 0.11) −0.21 (−0.30 to −0.12)** −0.13 (−0.24 to −0.02)* 0.03 (−0.06 to 0.12) 0.23 (0.13 to 0.32)** Ref |
−0.18 (−0.27 to −0.08)** −0.12 (−0.22 to −0.02)* 0.07 (−0.04 to 0.18) −0.21 (−0.30 to −0.11)** −0.10 (−0.22 to 0.02) −0.01 (−0.11 to 0.09) 0.09 (−0.02 to 0.20) Ref |
−0.30 (−0.39 to −0.21)** −0.04 (−0.13 to 0.05) 0.03 (−0.07 to 0.13) −0.17 (−0.26 to −0.08)** −0.04 (−0.14 to 0.07) −0.01 (−0.11 to 0.08) 0.16 (0.06 to 0.25)** Ref |
p<0.05,
p<0.01; blank cells indicate that the standardized mean difference was not statistically significant at p<0.05.
Effect Size = Standardized mean difference (i.e. Differences in mean compared to referent, divided by standard deviation)
For each subscale, all variables whose effect-size estimates are shown in the table were included in the model and adjusted for each other. Negative scores reflect relatively less growth, while positive scores reflect more growth.
Employment status (“Employed”) was one of the candidate variables in the backward selection, but it was not selected in any of the models.
Sociodemographic and cancer-related variables appeared to have varied relationships to PPI depending upon the subscale domain, although the small effect sizes suggest that these significant relationships may simply be a function of the power of the analysis and not clinically meaningful, thus subject to further research. For example, those without a college degree reported significantly greater PPI with regard to relating to others and perceiving new life possibilities. In contrast, college graduates were more likely than those without a college degree to report greater appreciation for life. Survivors who were married or in long-term relationships reported more spiritual change and appreciation of life than those not married or in committed relationships. Survivors reporting personal incomes of <$20,000/year reported less personal strength but more spiritual change compared to those earning more. Employment status was not observed to be associated with any subscale.
DISCUSSION
The findings reported here suggest that long-term post-cancer perceptions of positive impact are greater among survivors than siblings. It may be that childhood survivors’ experiences of exposures to invasive medical procedures, treatments and long-term complications contribute to a greater likelihood of reporting PPI when compared to siblings, for whom the experience of childhood cancer in the family often involves disruptions in daily routines and relationships with parents and not necessarily physical debilitation or trauma (Zeltzer, et al., 1996; Zeltzer, et al., 2009). The experience of physical symptoms, debilitation, or therapy-related late effects may be of relatively greater salience, in contrast to social life disruption, when it comes to predicting PPI.
The existence and variability of PPI in young adult survivors in this study suggests that survivor characteristics, inclusive of race, gender, cancer type, intensity of treatment, age at diagnosis and time since diagnosis, have unique and specific associations with different aspects of PPI. For instance, the positive relationship observed between age at diagnosis and PPI suggests that the cognitive capacity to acknowledge the severity of the life disruption caused by cancer may be a necessary antecedent to later perceiving some positive effect. This finding supports theories of posttraumatic growth and resilience that are predicated on a subject perceiving an experience as traumatic in order to derive positive meaning or growth (Carver, 1998; Tedeschi & Calhoun, 2004). However, relevant and competing theories (e.g., event centrality) do not require a subject to acknowledge an experience as traumatic in order to grow from it (Boals & Schuettler, 2010; Phipps, et al., 2007).
Educational attainment and marital/relationship status also appeared to be positively associated with reporting PPI. The successful attainment of life goals and dreams such as finishing school, having a significant, meaningful and intimate relationship, or starting a family may contribute to survivors feeling like their lives are normal after having had cancer. These achievements perhaps forge a more positive conception of benefits derived from once having had cancer. Provocative is the converse notion that acknowledgement or recognition of positive impacts of cancer may somehow increase the likelihood of achieving these or other normative developmental tasks. This cross-sectional study cannot determine the direction of this relationship; however, the question of whether or not cognitive behavioral therapies can incur a re-framing of one’s cancer experience and subsequently improve mental or occupational health outcomes for childhood cancer survivors is worthy of future investigation.
The gender and race differences observed here suggest that PPI may be a socially and culturally influenced coping process whereby men and women, and Whites and non-Whites, differ. These differences may reflect a greater coping ability among women and individuals from racial minority groups that results from being primed to deal with adversity related to the subtle but sometimes explicit challenges of experiencing race- or gender-based discrimination. This speculative interpretation of race and gender-based differences may be examined further in the context of stress-based theories of allostasis, which suggest that there exists a threshold up to which people are resilient and can manage stress (McEwen & Seeman, 1999; Seeman, Singer, Rowe, Horwitz, & McEwen, 1997). However, over-exposures to stress in general, and discrimination in particular, prohibit possibilities of resilience or growth, and instead result in multiple and varied deleterious health outcomes (James, Hartnett, & Kalsbeek, 1983; Schulz, et al., 2006; Banks, Kohn-Wood, & Spencer, 2006).
If we are to understand PPI in the context of event centrality, than we must look for evidence to the effect that a cancer history has somehow shaped and/or continues to shape individuals’ senses of themselves or the world around them. For example, survivors of bone cancers were significantly more likely to report PPI than survivors of any other cancer types (Table 3). It is possible that bone cancer, with its attendant loss of limb or body disfigurement, is perceived as a most salient aspect of a childhood cancer survivor’s identity due to its constant physical reminders (e.g., amputation, limb salvage). In turn, PPI was significantly greater among leukemia survivors compared to all other cancer types (except bone cancers), perhaps due to the relatively longer length of time spent in treatment (upward of 2-3 years). Survivors who reported more intense therapies or a recurrence of cancer or second malignancy also were more likely to report PPI. One can assume that more intense treatments are accompanied by more severe, sickening or debilitating side effects (e.g. nausea, vomiting, hair loss, diarrhea, constipation, mouth sores) and thus may contribute to a greater likelihood that survivors recall the cancer experience as traumatic, or at least as having a greater impact on their life at the time. Also, consistent with prior research (Barakat, et al., 2006), reporting PPI in this study sample decreased over time. Perhaps in the absence of reminders, any perceived growth or potential positive life influence attributable to cancer is forgotten, or becomes less central to the identity of the survivor over time. Without cognitive or behavioral reinforcements, survivors may be less likely to maintain new values, attitudes, beliefs or behaviors that may have emerged in the first years following cancer treatment.
CNS/brain tumor survivors may be an exception, in that the centrality of on-going cognitive, behavioral, and physically debilitating effects contributes to a greater likelihood of perceiving a more negative long-term impact of cancer. Indeed, brain tumor survivors experience disproportionate rates of learning disabilities, unemployment and other social life disruptions when compared to survivors of other childhood cancer types (Gurney, et al., 2009; Ness, Gurney, Zeltzer, & al., 2008). Thus, cognitive and behavioral limitations may preclude PPI.
A lack of empirical data and underdeveloped theories of posttraumatic growth, thriving, and perceived positive impact limits our ability to fully understand the findings reported here. Evaluating the influence of cancer in a cross-sectional study involving subjects who, on average, are 24 years beyond their cancer therapy potentially confounds our ability to definitively conclude that reports of PPI are actually attributable to cancer. Yet, they do not negate the possibility that one’s cancer experience can and does become central to one’s identity and sense of the world, at least for some period of time in one’s life. By changing the question stem of the PTGI to assess “influence” of cancer on one’s life as opposed to change attributable to cancer, we may have altered the validity and reliability of the PTGI as a measure of “post-traumatic growth,” although psychometric analyses supported the instrument’s theoretically-derived factor structure in this study sample.
Despite the large sample size and the large geographic representation of survivors, there are other limitations to this study. The survey was completed by mail, and thus is self-report and expected to be less sensitive than an interview. Additionally, not all of those contacted completed this survey, which raises the potential for some self-selection in the respondents. Although there could be a number of reasons for such self-selection, it is possible that those who were most distressed by the questions failed to complete the forms.
Future research is needed to examine the extent to which PPI is related to, and distinguishable from, health-related quality of life and psychological health and well-being. Future challenges also will involve differentiating the experiences of cancer survivors from variously-affected (siblings, survivors of other medical, physical or sexual trauma) or non-affected (e.g same age peers, population norms) populations. In addition, future studies utilizing longitudinal study designs would have the potential for assessing cancer-related life changes within the context of rapid social and emotional changes that commonly occur in this younger aged population. Finally, investigation into the relationship of PPI to posttraumatic stress symptoms will be an important effort in better understanding the long-term affect of cancer on young adult survivors. This information can guide the development of future interventions that aim not only to prevent or treat distress reactions among some survivors but also promote survivors’ interpretations of their cancer experience in a manner that gives meaning and continuity to their sense of self and life story (McAdams et al., 2006).
FUNDING/ACKNOWLEDGEMENTS
The Childhood Cancer Survivor Study (CCSS) is a collaborative, multi-institutional study currently funded by a U24 resource grant (National Cancer Institute grant # U24 CA55727; L.L. Robison, Principal Investigator). Support to investigators at St. Jude Children’s Research Hospital provided by the Cancer Center Support (CORE) grant (CA 21765) and the American Lebanese-Syrian Associated Charities (ALSAC). This work was also supported by a grant from the Children’s Cancer Fund of the University of Michigan Comprehensive Cancer Center (G009323).
Contributor Information
Brad J. Zebrack, School of Social Work, University of Michigan and University of Michigan Comprehensive Cancer Center, 1080 S. University, Ann Arbor, Michigan.
Margaret L. Stuber, Semel Institute at UCLA, 740 Westwood Plaza, Los Angeles, CA, 90024-1759
Kathleen A. Meeske, Childrens Center for Cancer and Blood Diseases, Childrens Hospital Los Angeles, 4650 Sunset Blvd. MS #54, Los Angeles, CA 90027
Sean Phipps, Department of Behavioral Medicine, St. Jude Children’s’ Research Hospital, 262 Danny Thomas Place, MS 735, Memphis, TN 38105-3678
Kevin R. Krull, Department of Epidemiology and Cancer Control, St. Jude Children’s’ Research Hospital, 262 Danny Thomas Place, MS 735, Memphis, TN 38105-3678
Qi Liu, Department of Public Health Sciences, University of Alberta, Canada, T6G 2T4 Yutaka Yasui, Ph.D., Department of Public Health Sciences, University of Alberta, Canada, T6G 2T4
Carla Parry, University of Colorado, School of Medicine, Division of Health Care Policy and Research. 13611 East Colfax Avenue, Aurora, CO 80045.
Rachel Hamilton, School of Social Work, University of Michigan, 1080 S. University, Ann Arbor, Michigan
Leslie L. Robison, Department of Epidemiology and Cancer Control, St. Jude Children’s’ Research Hospital, 262 Danny Thomas Place, MS 735, Memphis, TN 38105-3678
Lonnie K. Zeltzer, Division of Cancer Prevention and Control Research, UCLA Jonsson Comprehensive Cancer Center, 22-464 MDCC, 10833 Le Conte Ave., L.A., CA 90095
References
- Banks KH, Kohn-Wood LP, Spencer M. An examination of the African American experience of everyday discrimination and symptoms of psychological distress. Community Mental Health Journal. 2006;42(6):555–570. doi: 10.1007/s10597-006-9052-9. [DOI] [PubMed] [Google Scholar]
- Barakat LP, Alderfer MA, Kazak AE. Posttraumatic growth in adolescent survivors of cancer and their mothers and fathers. Journal of Pediatric Psychology. 2006;31(4):413–419. doi: 10.1093/jpepsy/jsj058. [DOI] [PubMed] [Google Scholar]
- Berntsen D, Rubin D. The centrality of event scale: A measure of integrating a trauma into one’s identity and its relation to post-traumatic stress disorder symptoms. Behavior Research and Therapy. 2006;44:219–231. doi: 10.1016/j.brat.2005.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boals A, Schuettler D. A double-edged sword: Event centrality, PTSD and posttraumatic growth. Applied Cognitive Psychology. 2010 [Google Scholar]
- Buchbinder D, Casillas J, Krull K, Goodman P, Leisenring W, Recklitis C, et al. Psychological and Psychosocial Outcomes in Siblings of Cancer Survivors: A Report from the Childhood Cancer Survivor Study. Psycho-Oncology. doi: 10.1002/pon.1848. (In Press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calhoun LG, Tedeschi RG. Beyond Recovery From Trauma: Implications for Clinical Practice and Research. Journal of Social Issues. 1998;54(2):357–371. [Google Scholar]
- Carver CS. Resilience and thriving: Issues, models, and linkages. Journal of Social Issues. 1998;54(2):245–266. [Google Scholar]
- Chesler M. Some survivors of childhood cancer are “thriving”--illusion or reality? A synthetic review of the literature and our empirical work. University of Michigan; 2000. [Google Scholar]
- Chesler M, Parry C. Thematic evidence of psychosocial thriving in survivors of childhood cancer. Qualitative Health Research. 2005;15(8):1055–1073. doi: 10.1177/1049732305277860. [DOI] [PubMed] [Google Scholar]
- Cordova MJ, Andrykowski MA. Responses to cancer diagnosis and treatment: Posttraumatic stress and posttraumatic growth. Seminars in Clinical Neuropsychiatry. 2003;8(4):286–296. [PubMed] [Google Scholar]
- Cordova MJ, Cunningham LLC, Carlson CR, Andrykowski MA. Posttraumatic growth following breast cancer: A controlled comparison study. Health Psychology. 2001;20(3):176–185. [PubMed] [Google Scholar]
- Fleer J, Sleijfer D, Hoekstra H, Tuinman M, Klip E, Hoekstra-Weebers J. Objective and subjective predictors of cancer-related stress symptoms in testicular cancer survivors. Patient Education and Counseling. 2006;64:142–150. doi: 10.1016/j.pec.2005.12.009. [DOI] [PubMed] [Google Scholar]
- Floyd FJ, Widaman KF. Factor analysis in the development and refinement of clinical assessment instruments. Psychological Assessment. 1995;7:286–299. [Google Scholar]
- Gurney JG, Krull KR, Kadan-Lottick N, Nicholson HS, Nathan PC, Zebrack B, et al. Social outcomes in long-term survivors of childhood cancer. Journal of Clinical Oncology. 2009;27(14):2390–2395. doi: 10.1200/JCO.2008.21.1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrell FE. Regression Modeling Strategies: With Applications to Linear Models, Logistic Regression, and Survival Analysis. Springer; New York: 2001. [Google Scholar]
- Hobbie WL, Stuber M, Meeske K, Wissler K, Rourke MT, Ruccione K, et al. Symptoms of posttraumatic stress in young adult survivors of childhood cancer. Journal of Clinical Oncology. 2000;18(24):4060–4066. doi: 10.1200/JCO.2000.18.24.4060. [DOI] [PubMed] [Google Scholar]
- James S, Hartnett S, Kalsbeek W. John Henryism and blood pressure differences among black men. Journal of Behavioral Medicine. 1983;6(3):259–278. doi: 10.1007/BF01315113. [DOI] [PubMed] [Google Scholar]
- Kallay E. Possible positive posttraumatic reactions in cancer patients: Meaning making, benefit finding, and religiosity. Cognition, Brain, Behavior. 2006;1:133–150. [Google Scholar]
- Kamibeppu K, Sato I, Honda M, Ozono S, Sakamoto N, Iwai T, et al. Mental health among young adult survivors of childhood cancer and their siblings including posttraumatic growth. Journal of Cancer Survivorship. 2010;4:303–312. doi: 10.1007/s11764-010-0124-z. [DOI] [PubMed] [Google Scholar]
- Kleim B, Ehlers A. Evidence for a curvilinear relationship between posttraumatic growth and posttrauma depression and PTSD in assault survivors. Journal of Traumatic Stress. 2009;22(1):45–52. doi: 10.1002/jts.20378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang KY, Zeger S. Longitudinal data analysis using generalized linear models. Biometrika. 1986;73:13–22. [Google Scholar]
- McAdams DP, Josselson R, Lieblich A, editors. Identity and story: Creating self in narrative. American Psychological Association; Washington, DC: 2006. [Google Scholar]
- McEwen B, Seeman T. Protective and damaging effects of mediators of stress: Elaborating and testing the concepts of allostasis and allostatic load. Annals of the New York Academy of Sciences. 1999;896:30–47. doi: 10.1111/j.1749-6632.1999.tb08103.x. [DOI] [PubMed] [Google Scholar]
- Meeske K, Ruccione K, Globe DR, Stuber ML. Posttraumatic stress, quality of life, and psychological distress in young adult survivors of childhood cancer. Oncology Nursing Forum. 2001;28(3):481–489. [PubMed] [Google Scholar]
- Mertens AC, Walls RS, Taylor L. Characteristics of childhood cancer survivors predicted their successful tracing. Journal of Clinical Epidemiology. 2004;57:933–944. doi: 10.1016/j.jclinepi.2004.01.005. al., e. [DOI] [PubMed] [Google Scholar]
- Ness KK, Gurney JG, Zeltzer LK. The impact of limitations in physical, executive, and emotional function on health-related quality of life among adult survivors of childhood cancer: A report from the Childhood Cancer Survivor Study. Archives of Physical and Medical Rehabilitation. 2008;89:128–136. doi: 10.1016/j.apmr.2007.08.123. al., e. [DOI] [PubMed] [Google Scholar]
- Orbuch T, Parry C, Chesler M, Fritz J, Repetto P. Parent-Child Relationships and Quality of Life: Resilience Among Childhood Cancer Survivors. Family Relations. 2005;54(2):171–183. [Google Scholar]
- Park CL. Stress-related growth and thriving through coping: The roles of personality and cognitive processes. Journal of Social Issues. 1998;54(2):267–277. [Google Scholar]
- Parry C, Chesler MA. Thematic evidence of psychosocial thriving in childhood cancer survivors. Qualitative Health Research. 2005;15:1055–1073. doi: 10.1177/1049732305277860. [DOI] [PubMed] [Google Scholar]
- Pemberger S, Jagsch R, Frey E, Felder-Puig R, Gadner H, Kryspin-Exner I, et al. Quality of life in long-term childhood cancer survivors and the relation of late effects and subjective well-being. Supportive Care in Cancer. 2005;13:49–56. doi: 10.1007/s00520-004-0724-0. [DOI] [PubMed] [Google Scholar]
- Phipps S, Long AM, Ogden J. Benefit finding scale for children: Preliminary findings from a childhood cancer population. Journal of Pediatric Psychology. 2007;32(10):1264–1271. doi: 10.1093/jpepsy/jsl052. [DOI] [PubMed] [Google Scholar]
- Robison LL, Armstrong GT, Boice JD, Chow RJ, Davies SM, Donaldson SS, et al. The Childhood Cancer Survivor Study: A National Cancer Institute–Supported Resource for Outcome and Intervention Research. Journal of Clinical Oncology. 2009;27(14):2308–2318. doi: 10.1200/JCO.2009.22.3339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAS Institute, I . SAS System for Mixed Mod. SAS Institute, Inc; Chapel Hill: 1996. [Google Scholar]
- Schulz A, Gravlee C, Williams D, Israel B, Mentz G, Rowe Z. Discrimination, symptoms of depression, and self-rated health among African American women in Detroit: Results from a longitudinal analysis. American Journal of Public Health. 2006;96(7):1265–1270. doi: 10.2105/AJPH.2005.064543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeman T, Singer B, Rowe J, Horwitz R, McEwen B. Price of adaptation-- allostatic load and its health consequences. Archives of Internal Medicine. 1997;157(19):2259–2268. [PubMed] [Google Scholar]
- Stanton AL, Bower JE, Low CA. Posttraumatic growth after cancer. In: Calhoun L, Tedeschi R, editors. Handbook of Posttraumatic Growth: Research and Practice. Lawrence Erlbaum Associates, Inc; Mahwah: 2006. pp. 138–175. [Google Scholar]
- Stuber M, Meeske K, Gonzalez S, Houskamp BM, Pynoos R. Post-traumatic stress after childhood cancer I: The role of appraisal. Psycho-Oncology. 1994;3(4):305–312. [Google Scholar]
- Stuber ML, Meeske KA, Krull KR, Leisenring W, Stratton K, Kazak AE, et al. Prevalence and Predictors of Posttraumatic Stress Disorder in Adult Survivors of Childhood Cancer. Pediatrics. 2010;125(5):e1124–e1134. doi: 10.1542/peds.2009-2308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumalla EC, Ochoa C, Blanco I. Posttraumatic growth in cancer: Reality or illusion? Clinical Psychology Review. 2009;29:24–33. doi: 10.1016/j.cpr.2008.09.006. [DOI] [PubMed] [Google Scholar]
- Taieb O, Moro MR, Baubet T, Revah-Levy A, Flament MF. Posttraumatic stress symptoms after childhood cancer. European Child and Adolescent Psychiatry. 2003;12(6):255–264. doi: 10.1007/s00787-003-0352-0. [DOI] [PubMed] [Google Scholar]
- Taku K, Cann A, Calhoun LG, Tedeschi RG. The factor structure of the Posttraumatic Growth Inventory: A comparison of five models using confirmatory factor analysis. Journal of Traumatic Stress. 2008;21(2):158–164. doi: 10.1002/jts.20305. [DOI] [PubMed] [Google Scholar]
- Tedeschi RG, Calhoun LG. The Posttraumatic Growth Inventory: Measuring the positive legacy of trauma. Journal of Traumatic Stress. 1996;9(3):455–471. doi: 10.1007/BF02103658. [DOI] [PubMed] [Google Scholar]
- Tedeschi RG, Calhoun LG. Posttraumatic growth: Conceptual foundations and empirical evidence. Psychological Inquiry. 2004;15(1):1–18. [Google Scholar]
- Tedeschi RG, Calhoun LG, Cann A. Evaluating resource gain: Understanding and misunderstanding posttraumatic growth. Applied Psychology: An International Review. 2007;56(3):396–406. [Google Scholar]
- Thornton AA. Perceiving benefits in the cancer experience. Journal of Clinical Psychology in Medical Settings. 2002;9(2):153–165. doi: 10.1007/s10880-011-9270-3. [DOI] [PubMed] [Google Scholar]
- Thornton AA, Perez MA. Posttraumatic growth in prostate cancer survivors and their partners. Psycho-Oncology. 2006;15(4):285–296. doi: 10.1002/pon.953. [DOI] [PubMed] [Google Scholar]
- Woodgate RL. Conceptual understanding of resilience in the adolescent with cancer: Part 1. Journal of Pediatric Oncology Nursing. 1999;16(1):35–43. doi: 10.1177/104345429901600105. [DOI] [PubMed] [Google Scholar]
- Zebrack B, Zeltzer LK, Whitton J, Berkow R, Chesler MA. Survivors of childhood cancer: Using siblings as a control group (Letter to the Editor) Pediatrics. 2003 Dec;112:1454–1455. doi: 10.1542/peds.112.6.1454. [DOI] [PubMed] [Google Scholar]
- Zebrack BJ, Yi J, Petersen L, Ganz PA. The impact of cancer and quality of life for long-term survivors. Psycho-Oncology. 2008;17:891–900. doi: 10.1002/pon.1300. [DOI] [PubMed] [Google Scholar]
- Zeltzer LK, Dolgin MJ, Sahler OJZ, Roghmann K, Barbarin OA, Carpenter PJ, et al. Sibling adaptation to childhood cancer collaborative study: Outcomes of siblings of children with cancer. Medical and Pediatric Oncology. 1996;27:98–107. doi: 10.1002/(SICI)1096-911X(199608)27:2<98::AID-MPO6>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Zeltzer LK, Recklitis C, Buchbinder D, Zebrack B, Casillas J, Tsao JCI, et al. Psychological status in childhood cancer survivors: A report from the Childhood Cancer Survivor Study. Journal of Clinical Oncology. 2009;27(14):2396–2404. doi: 10.1200/JCO.2008.21.1433. [DOI] [PMC free article] [PubMed] [Google Scholar]