Abstract
Visual pigment proteins belong to the superfamily of G protein-coupled receptors and are the light-sensitive molecules in rod and cone photoreceptor cells. The protein moiety is known as opsin and the ligand in the dark is 11-cis retinal, which serves as both the photon detector and an inverse agonist. While much is known about properties of the rod pigment rhodopsin, much less is understood about cone visual pigments. Being able to identify ligands that effect opsins give an insight into structure–activity relationships. The action of some ligands indicates that there are differences between not only rod and cone opsins but also among the different classes of cone opsins. Furthermore, inverse agonists of cone opsins may have potential therapeutic uses under conditions when the native 11-cis retinal ligand is absent. A method for determining the effects of ligands on rod and cone opsin activity is described.
1. Introduction
Visual pigments are the light-sensitive components in rod and cone photoreceptor cells and are comprised of two parts: opsin, the G protein-coupled receptor (GPCR), and the 11-cis aldehyde form of vitamin A (11-cis retinal), the chromophore. Visual pigments differ from other GPCRs in that the ligand is covalently bound to the receptor via a Schiff base linkage to a strictly conserved lysine residue in the seventh transmem-brane helix (Bownds, 1967; Morton and Pitt, 1955; Wang et al., 1980). The opsins themselves are constitutively active (Cohen et al., 1993; Isayama et al., 2006; Kono, 2006; Melia et al., 1997; Surya et al., 1995). The native 11-cis retinal ligand is an inverse agonist, which limits spontaneous activation of the visual signaling cascade in the dark. The very fast response of visual pigments as GPCRs is that light isomerizes the 11–12 bond from cis to trans within 200 fs (Schoenlein et al., 1991). This is followed by conformational changes of the protein enabling it to activate its G protein transducin. Thus, light converts the bound ligand from an inverse agonist to an agonist. The all-trans ligand then dissociates from the opsin and is transported to the retinal pigment epithelium where it is converted back to 11-cis retinal and shuttled back to the photoreceptor to regenerate new pigment.
Rod photoreceptor cells contain the visual pigment rhodopsin and are used for dim light vision; cone photoreceptor cells contain cone visual pigments and are used for bright light and color vision. Although cone pigments are structurally and functionally highly homologous to rhodopsin, there are differences. Because the chromophore of both rod and cone opsins is 11-cis retinal, the specific interactions with the receptor result in tuning the absorption properties, resulting in sensitivity across the visible spectrum. Furthermore, cone pigments regenerate, activate, and inactivate faster than rhodopsin. The effects of retinal analogs on rod opsin’s activity have been studied (Bartl et al., 2005; Buczylko et al., 1996; Han et al., 1997); again, less is known about structure–activity relationships with cone opsins and ligands. One reason for this is the lack of methods and sources for pure cone opsins, whereas rod opsins in good purity are easily isolated. Another reason is the perceived instability of the protein (Ramon et al., 2009). Cone pigments have been shown to lose its chromophore or, in the presence of analogs, exchange chromophores in the dark (Crescitelli, 1984; Kefalov et al., 2005; Matsumoto et al., 1975), unlike the rod opsin where the pigment (rhodopsin) is quite stable even to hydroxylamine. However, the cone opsin proteins themselves are quite stable in a membrane environment, as pigments can be generated and regenerated repeatedly when fresh chromophore is supplied.
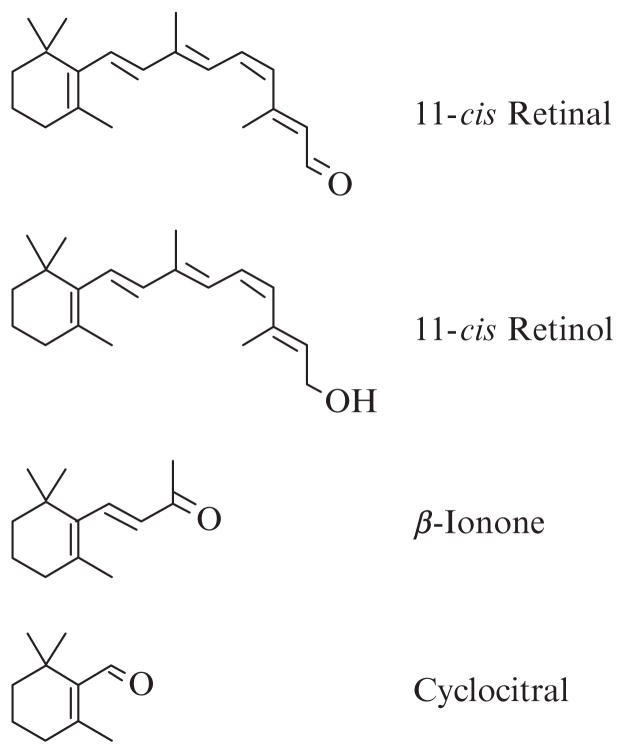
Furthermore, the importance of 11-cis retinal in vivo appears to extend beyond visual signaling for cones and less so with rods. When there is a limited supply of 11-cis retinal, as is the case with some forms of a childhood-onset blinding diseases Leber Congenital Amaurosis (LCA), cones appear to degenerate rapidly, while the rods survive for quite a bit longer, based on photoreceptor cell layer thicknesses in patients with LCA (Jacobson et al., 2007, 2009). Histological studies of mouse models of LCA clearly show rapid loss of cone cells preceded by opsins that are no longer localized solely in the outer segments of cones but rather distributed throughout (Rohrer et al., 2005; Znoiko et al., 2005). Supplementing mouse models of LCA with 11-cis retinal improved cone cell survival and cone opsin localization presumably by their abilities to form pigment (Rohrer et al., 2005). Thus, cone opsin inverse agonists have the potential as therapeutic agents in preserving cone photoreceptor cells in LCA. A practical problem with using the native ligand as treatment is that light abolishes any benefits of 11-cis retinal in mouse models (manuscript in preparation). Thus, our recent interests in identifying compounds that can inactivate cone opsins have extended beyond structure–function characterization of cone opsins. We have begun to characterize the effects of analogs of retinal on the abilities of cone opsins to activate transducin. A partial list of retinoids and analogs that are inverse agonists to long wavelength-sensitive cone opsins is shown in Fig. 12.1. We have found that 11-cis retinol, however, is an agonist to rod opsin (Ala-Laurila et al., 2009; Kono et al., 2008). Also, β-ionone is an agonist to rod opsin and a class of short wavelength-sensitive cone opsins (Buczylko et al., 1996; Isayama et al., 2006). Thus, not all opsins respond to ligands in the same manner, and generalizations about which ones act as inverse agonists or agonists should be made with caution in the absence of data.
Figure 12.1.
Compounds that have been found to act as inverse agonists to human red cone opsins. 11-cis retinal is the native ligand in the eye and was obtained from the National Eye Institute and Dr Rosalie Crouch of the Medical University of South Carolina; 11-cis retinol was obtained from Dr Crouch; β-ionone and cyclocitral were purchased from Sigma-Aldrich (St. Louis, MO). Even though these compounds were found to be inverse agonists to the red cone opsin from our in vitro assays, 11-cis retinol and β-ionone are agonists to rod opsin (Isayama et al., 2006; Kono, 2006; Kono et al., 2008), and β-ionone is also an agonist to a short wavelength-sensitive class of cone opsins (Isayama et al., 2006).
1.1. Assay considerations
There are a few techniques that can be and have been used to study ligand effects on rod and cone opsins and cells with strengths and limitations of each one. Perhaps the most sensitive is single cell electrophysiology where single photoreceptor cells are monitored for light- and/or ligand-dependent changes to ion permeability (Estevez et al., 2006; Jin et al., 1993; Jones et al., 1989; Kefalov et al., 1999, 2001). Advantages include the high sensitivity, fast time resolution, and physiological environment of the opsins. However, potential complications could arise from the presence of the native ligand, which needs to be washed out; the test ligand affecting ion channel properties directly (Dean et al., 2002; McCabe et al., 2004); the presence of multiple types of opsins within the same photoreceptor cell (Applebury et al., 2000; Makino and Dodd, 1996); and modification of ligand by the cell (e.g., 11-cis retinol is oxidized to 11-cis retinal in cone cells and regenerate cone visual pigments; Ala-Laurila et al., 2009; Jones et al., 1989).
In vitro studies with heterologously expressed opsins have their own set of advantages. There is only the single opsin that is expressed; there is no need to wash out native chromophore because it was never present; mutants can be easily constructed and expressed. The ability of testing mutant opsins demonstrated that some rhodopsin mutants associated with retinitis pigmentosa are constitutively active which could be deactivated with either 11-cis retinal or 11-cis retinyl Schiff base (Cohen et al., 1992; Robinson et al., 1992; Zhukovsky et al., 1991). Limitations include the fact that the opsins are not in their native in vivo environment and assays may not be as sensitive as electrophysiological measurements (Melia et al., 1997).
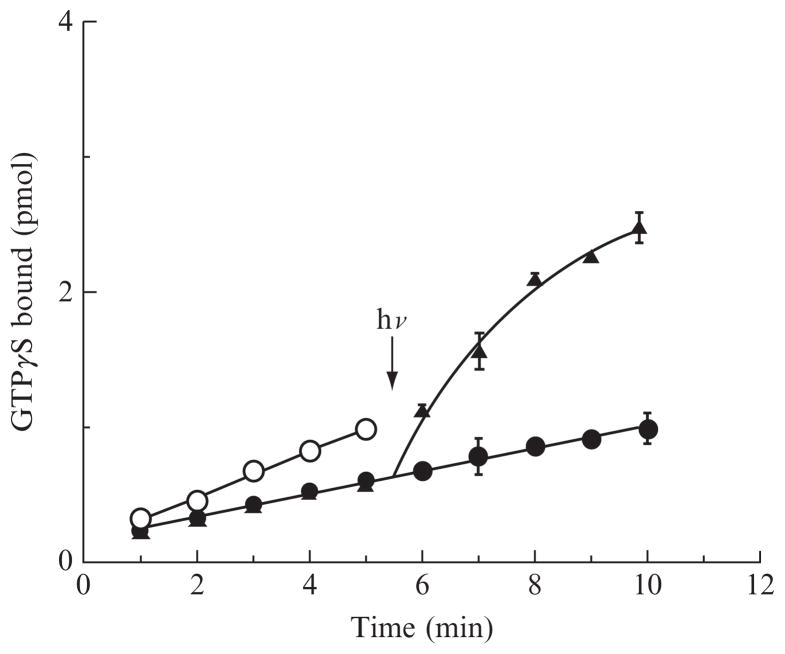
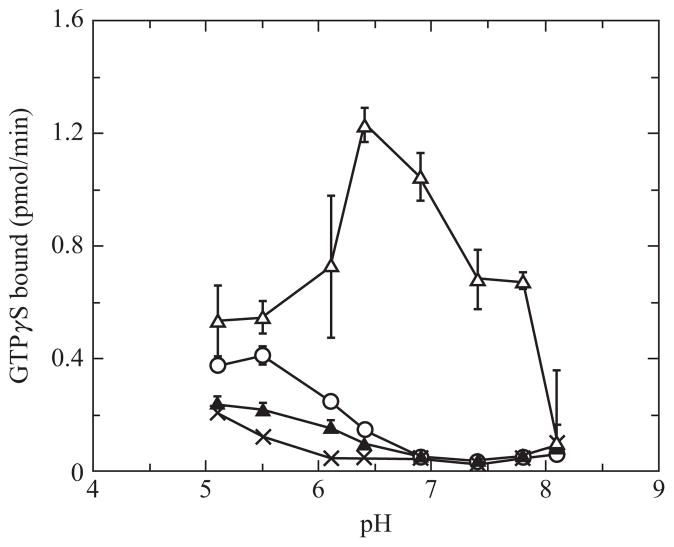
We use a radioactive filter binding assay to assess the abilities of the opsins to activate transducin in the absence and presence of ligand (Fig. 12.2). Opsins are expressed in COS-1 cells, and membranes isolated. Binding of the native ligand is easy to demonstrate as the receptor becomes able to activate G proteins in a light-dependent manner. To determine inverse agonist behavior, we need to demonstrate that activation with ligand is lower than without ligand. The opsins are maintained in a lipid environment as the purified apoprotein is not stable. The constitutive activity of the apoprotein is difficult to distinguish at physiological pH values. Figure 12.3 illustrates the pH dependence of transducin activation by the human red cone opsin and pigment in the dark and light. This follows a similar pattern as with the bovine rod opsin pH dependence of transducin activation (Cohen et al., 1992). Thus, we assay at a slightly acidic pH of 6.4 where we can differentiate a change in activity between opsin and opsin with an inverse agonist. In order to carry out these assays, we use bovine rod transducin as our G protein for both rod and cone opsins because of the availability of bovine retinas and relative ease of purifying large quantities in a cost-effective manner. Both preparations are briefly described here, followed by the radioactive filter binding assay.
Figure 12.2.
Transducin activation by human red cone opsin at pH 6.4 as a function of time. Activation by opsin (open circles), opsin with 11-cis retinol (filled circles), and opsin with 11-cis retinal (triangles) were determined in the dark for the first 5.5 min, at which time the samples were illuminated with a 6 s pulse of light from a 300 W slide projector passed through a 530 nm long pass filter. Both 11-cis retinoids act as inverse agonists in the dark, but only the sample with 11-cis retinal forms a pigment that absorbs visible light and results in photoactivation of the pigment. The figure is adapted from Ala-Laurila et al. (2009). © The American Society for Biochemistry and Molecular Biology.
Figure 12.3.
pH dependence of transducin activation by human red cone opsin in the absence and presence of 11-cis retinal and light. Human cone opsins were incubated with ethanol for opsin alone (open circles), and 11-cis retinal in the dark (filled triangles), 11-cis retinal followed by photobleaching (open triangles). Membranes from mock transfected COS-1 cells were used to measure activity of transducin in the absence of opsin and ligand (X). The following buffers were used to set the pH: MES for pH 5.1–6.4 and HEPES for pH 6.8–8.1.
2. Opsin Preparation
For a quick preparation of opsins, we isolate the membrane fractions from transfected COS-1 cells. The methods are straightforward and relatively inexpensive. The opsin genes generally have the last codons for the last eight amino acid residues of bovine rhodopsin, the 1D4 epitope (Molday and MacKenzie, 1983), to help with quantification on slot or western blots, although it is not absolutely necessary. Typically, 2 μg of plasmid is transfected for each 10-cm plate of confluent COS-1 cells using the DEAE-dextran method described previously (Oprian, 1993), although other transfection methods work without problems. After 3 days, the cells are harvested and up to 10 plates are pooled into 15 mL conical tubes at a volume of 1 mL per 10-cm plate, then pelleted and stored at −80 °C until needed. Cells are resuspended in a hypotonic buffer of 10 mM Tris–HCl (pH 7.4) buffer with 30 μM phenylmethanesulfonyl fluoride (PMSF) and passed through a 25 G needle attached to a 10–20 mL syringe four times. The homogenate is then layered on top of a 20 mL 37.7% (w/v) sucrose cushion in a 25 × 89 mm polyallomer tube (Beckman-Coulter, Palo Alto, CA) and spun in a Beckman SW32 rotor at 15,000 rpm for 30 min (Kono, 2006; Kono et al., 2005; Robinson, 2000). The membrane fraction at the interface is collected by piercing the polyallomer tube with an 18 G needle attached to a 5 mL syringe and sucrose diluted with 10 mM Tris–HCl (pH 7.4) and recentrifuged. The pellet is resuspended in a buffer containing 150 mM NaCl, 1 mM MgCl2, 1 mM CaCl2, 0.1 mM EDTA, 10 mM Tris–HCl (pH 7.4) at a volume of 25 μL per plate of COS-1 cells used and stored at −80 °C in 25 μL aliquots. The amount of opsin in the membrane preparations are determined by slot blot analysis (Kono et al., 2005) using known amounts of bovine rhodopsin as reference and probed with the rhodopsin 1D4 antibody (available through a number of vendors such as catalog number MA1-722 from Affinity BioReagents/Thermo Fisher Scientific, Rockford, IL).
3. Transducin Preparation
Transducin is purified from 100 bovine retinas (W. L. Lawson, Lincoln, NE). The procedure is essentially a rod outer segment (ROS) preparation using a discontinuous sucrose gradient (Wessling-Resnick and Johnson, 1987) conducted in the light such that transducin is bound to the bleached rhodopsin and will pellet with the membranes. Transducin is released into solution by incubating the ROS with 40 μM GTP on ice for 30 min. After centrifugation, the supernatant containing transducin is subjected to further purification by DEAE-cellulose anion exchange chromo-tography (Baehr et al., 1982). Transducin fractions are pooled and dialyzed against and stored at −20 °C in 50% glycerol, 10 mM Tris–HCl (pH 7.4), 2 mM MgCl2, and 1 mM DTT at a concentration of 50 μM (Yu et al., 1995).
4. Transducin Activation Assay
4.1. Stock solutions
Reaction/wash buffer (10×): 100 mM 2-(N-morpholino)ethanesufonic acid (MES), pH 6.5 (final pH will be 6.4 in reaction), 1 M NaCl, 50 mM MgCl2.
DTT (50 mM) in milli-Q water.
Bovine rod transducin (50 μM, see above).
Opsin in membranes (see above). The opsin concentration is typically 10–50 nM.
Test ligand dissolved in ethanol. As a starting point, we often make stock concentrations of 2 mM or 200 μM.
150 μM GTPγS. A mixture of cold GTPγS with GTPγS35 at ~0.25 mCi/ mL is prepared as follows: to 100 μL of a 150 μM solution of GTPγS from ~3 mM stock solution is added 2 μL (25 μCi) GTPγS-35. (GTPγS-35: catalog number NEG030H250UC, PerkinElmer Life and Analytical Sciences, Waltham, MA).
4.2. Measurement
The ability of the specific opsin to activate bovine rod transducin is determined using a radioactive filter binding assay with membrane preparations of opsin expressed in COS-1 cells essentially as described previously with a few modifications (Kono, 2006; Robinson, 2000). We use a Millipore 1225 sampling vacuum manifold (Millipore, Billerica, MA) with 25 mm diameter Millipore mixed cellulose ester membranes (HAWP 02500, Millipore, Bill-erica, MA). The filter membranes are first moistened in water and then placed in the vacuum manifold.
The reaction is set up in 1.5 mL microcentrifuge tube without retinoid and GTPγS as follows:
| Volume (μL) | |
|---|---|
| 70 | Deionized water |
| 10 | 10× buffer (100 mM Tris buffer, pH 7.5, 1 M NaCl, 50 mM MgCl2) |
| 2 | 50 mM DTT |
| 5 | Transducin (50 μM stock) |
| 10 | Opsin/visual pigment (typically, 10–50 nM stock concentration) in membranes |
If the test ligand is a full-length or light-sensitive retinoid, then at this point the room lights are turned off and dim red light (photographic dark room filters such as Kodak number 2 or GBX2) conditions are used. One microliter of ligand solution (or ethanol for as a control) is added and mixed with a micropipettor. 1 min later, 2 μL of 150 μM GTPγS solution is added and mixed, and a timer is started. At 1 min, a 10 μL aliquot of the reaction mixture is transferred to the vacuum manifold. Filters are immediately rinsed three times with 4 mL rinse buffer (10 mM Tris, pH 6.4, 100 mM NaCl, 5 mM MgCl2) with a repeating pipettor, which leaves proteins including transducin and bound GTPγS on the filter and unbound GTPγS to flow through. This process is repeated at typically 1 min intervals. After 3–6 time points, the filter membranes are placed into scintillation vials and filled with 10 mL Amersham BCS scintillation cocktail (catalog number: NBCS104, GE Healthcare, Piscataway, NJ). An additional vial is filled with 10 mL scintillant, into which 10 μL of the reaction mixture is pipetted. These counts will be used to convert counts per minute (cpm) to mol GTPγS. The vials are shaken for at least 1 h and often overnight for convenience and measured in a scintillation counter (usually 1–5 min counts).
The cpm from the 10 μL reaction mixture pipetted directly into the scintillation fluid is used to convert cpm to pmol GTPγS taken up as a result of G protein activation. If aliquots from more than one reaction were measured, they can be averaged. Ten microliters of the reaction mixture contains 30 pmol of GTPγS. Thus, 30 pmol divided by these counts is used to determine the pmol GTPγS bound to transducin. The number of moles GTPγS bound is plotted against time, and the activity is the slope from this plot. If the concentration of opsin has been quantified, then the specific activity can be reported.
Because GTPγS is a nonhydrolyzable analog of GTP, the data represents an accumulation of transducin activated with time. This assay can, of course, be used to assay agonists as well. If light-dependent activity is to be determined, then the total reaction mixture is scaled to 150 μL total volume, and dark points first taken (i.e., see Fig. 12.2). A brief pulse of bright light is then given at a convenient time such as at 5.5 min after initiation of the reaction and measurement continued. A change in activity before and after light will indicate light-dependent activity. If assaying light activity of cone pigments, then one should take care to consider that the lifetime of the active intermediate of wild-type cone pigments is on the order of tens of seconds and a long photobleach will result in a large fraction of decayed product (Das et al., 2004; Kono, 2006). Furthermore, if starting with cone opsins in membranes and adding an excess of chromophore such as 11-cis retinal according to the protocol described in this chapter, then it is best to keep the room lights off except during the photobleach as cone pigments can be regenerated after the rapid decay of the active species; thus with continued light, the activity would result from a steady state of light activated cone pigments which would give an overestimate of activity.
Other opsin preparations such as immunoaffinity purification after solubilizing in a detergent such as CHAPS with lipid can also be used, and with dilution or removal of CHAPS, purified opsins will be in vesicles (Rim and Oprian, 1995; Rim et al., 1997). If pigments are formed from incubation of COS-1 cell suspensions with 11-cis retinal, then they can similarly be immunopurified. We use the 1D4 antibody conjugated to sepharose routinely to isolate and purify pigments (Das et al., 2004; Kono, 2006). If detergent-solubilized and purified pigment is to be used, then care must be taken with detergent type and concentration for the transducin activation assay. For example, we try to keep the final concentration of dodecyl maltoside to be about 0.01%; otherwise, activity decreases precipitously.
Acknowledgments
This work was supported in part by NIH grant R01-EY019515 and an unrestricted grant to the Department of Ophthalmology at MUSC from the Research to Prevent Blindness. 11-cis retinal and 11-cis retinol were gifts from Rosalie Crouch of the Medical University of South Carolina.
References
- Ala-Laurila P, Cornwall MC, Crouch RK, Kono M. The action of 11-cis-retinol on cone opsins and intact cone photoreceptors. J Biol Chem. 2009;284:16492–16500. doi: 10.1074/jbc.M109.004697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Applebury ML, Antoch MP, Baxter LC, Chun LLY, Falk JD, Farhangfar F, Kage K, Krzystolik MG, Lyass LA, Robbins JT. The murine cone photoreceptor: A single cone type expresses both S and M opsins with retinal spatial patterning. Neuron. 2000;27:513–523. doi: 10.1016/s0896-6273(00)00062-3. [DOI] [PubMed] [Google Scholar]
- Baehr W, Morita EA, Swanson RJ, Applebury ML. Characterization of bovine rod outer segment G-protein. J Biol Chem. 1982;257:6452–6460. [PubMed] [Google Scholar]
- Bartl FJ, Fritze O, Ritter E, Herrmann R, Kuksa V, Palczewski K, Hofmann KP, Ernst OP. Partial agonism in a G protein-coupled receptor. Role of the retinal ring structure in rhodopsin activation. J Biol Chem. 2005;280:34259–34267. doi: 10.1074/jbc.M505260200. [DOI] [PubMed] [Google Scholar]
- Bownds D. Site of attachment of retinal in rhodopsin. Nature. 1967;216:1178–1181. doi: 10.1038/2161178a0. [DOI] [PubMed] [Google Scholar]
- Buczylko J, Saari JC, Crouch RK, Palczewski K. Mechanisms of opsin activation. J Biol Chem. 1996;271:20621–20630. doi: 10.1074/jbc.271.34.20621. [DOI] [PubMed] [Google Scholar]
- Cohen GB, Oprian DD, Robinson PR. Mechanism of activation and inactivation of opsin: Role of Glu113 and Lys296. Biochemistry. 1992;31:12592–12601. doi: 10.1021/bi00165a008. [DOI] [PubMed] [Google Scholar]
- Cohen GB, Yang T, Robinson PR, Oprian DD. Constitutive activation of opsin: Influence of charge at position 134 and size at position 296. Biochemistry. 1993;32:6111–6115. doi: 10.1021/bi00074a024. [DOI] [PubMed] [Google Scholar]
- Crescitelli F. The gecko visual pigment: The dark exchange of chromopohore. Vision Res. 1984;24:1551–1553. doi: 10.1016/s0042-6989(84)80004-8. [DOI] [PubMed] [Google Scholar]
- Das J, Crouch RK, Ma JX, Oprian DD, Kono M. Role of the 9-methyl group of retinal in cone visual pigments. Biochemistry. 2004;43:5532–5538. doi: 10.1021/bi036097u. [DOI] [PubMed] [Google Scholar]
- Dean DM, Nguitragool W, Miri A, McCabe SL, Zimmerman AL. All-trans-retinal shuts down rod cyclic nucleotide-gated ion channels: A novel role for photoreceptor retinoids in the response to bright light? Proc Natl Acad Sci USA. 2002;99:8372–8377. doi: 10.1073/pnas.122681899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estevez ME, Ala-Laurila P, Crouch RK, Cornwall MC. Turning cones off: The role of the 9-methyl group of retinal in red cones. J Gen Physiol. 2006;128:671–685. doi: 10.1085/jgp.200609630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han M, Groesbeek M, Sakmar TP, Smith SO. The C9 methyl group of retinal interacts with glycine-121 in rhodopsin. Proc Natl Acad Sci USA. 1997;94:13442–13447. doi: 10.1073/pnas.94.25.13442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isayama T, Chen Y, Kono M, DeGrip WJ, Ma JX, Crouch RK, Makino CL. Differences in the pharmacological activation of visual opsins. Vis Neurosci. 2006;23:899–908. doi: 10.1017/S0952523806230256. [DOI] [PubMed] [Google Scholar]
- Jacobson SG, Aleman TS, Cideciyan AV, Heon E, Golczak M, Beltran WA, Sumaroka A, Schwartz SB, Roman AJ, Windsor EAM, Wilson JM, Aguirre GD, et al. Human cone photoreceptor dependence on RPE65 isomerase. Proc Natl Acad Sci USA. 2007;104:15123–15128. doi: 10.1073/pnas.0706367104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson SG, Aleman TS, Cideciyan AV, Roman AJ, Sumaroka A, Windsor EAM, Schwartz SB, Heon E, Stone EM. Defining the residual vision in Leber congenital amaurosis caused by RPE65 mutations. Invest Ophthalmol Vis Sci. 2009;50:2368–2375. doi: 10.1167/iovs.08-2696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin J, Crouch RK, Corson DW, Katz BM, MacNichol EF, Cornwall MC. Noncovalent occupancy of the retinal-binding pocket of opsin diminishes bleaching adaptation of retinal cones. Neuron. 1993;11:513–522. doi: 10.1016/0896-6273(93)90155-k. [DOI] [PubMed] [Google Scholar]
- Jones GJ, Crouch RK, Wiggert B, Cornwall MC, Chader GJ. Retinoid requirements for recovery of sensitivity after visual-pigment bleaching in isolated photoreceptors. Proc Natl Acad Sci USA. 1989;86:9606–9610. doi: 10.1073/pnas.86.23.9606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kefalov VJ, Cornwall MC, Crouch RK. Occupancy of the chromophore binding site of opsin activates visual transduction in rod photoreceptors. J Gen Physiol. 1999;113:491–503. doi: 10.1085/jgp.113.3.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kefalov VJ, Crouch RK, Cornwall MC. Role of noncovalent binding of 11-cis-retinal to opsin in dark adaptation of rod and cone photoreceptors. Neuron. 2001;29:749–755. doi: 10.1016/s0896-6273(01)00249-5. [DOI] [PubMed] [Google Scholar]
- Kefalov VJ, Estevez ME, Kono M, Goletz PW, Crouch RK, Cornwall MC, Yau KW. Breaking the covalent bond—A pigment property that contributes to desensitization in cones. Neuron. 2005;46:879–890. doi: 10.1016/j.neuron.2005.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kono M. Constitutive activity of a UV cone opsin. FEBS Lett. 2006;580:229–232. doi: 10.1016/j.febslet.2005.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kono M, Crouch RK, Oprian DD. A dark and constitutively active mutant of the tiger salamander UV pigment. Biochemistry. 2005;44:799–804. doi: 10.1021/bi047898f. [DOI] [PubMed] [Google Scholar]
- Kono M, Goletz PW, Crouch RK. 11-cis and all-trans retinols can activate rod opsin: Rational design of the visual cycle. Biochemistry. 2008;47:7567–7571. doi: 10.1021/bi800357b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makino CL, Dodd RL. Multiple visual pigments in a photoreceptor of the salamander retina. J Gen Physiol. 1996;108:27–34. doi: 10.1085/jgp.108.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto H, Tokunaga F, Yoshizawa T. Accessibility of the iodopsin chromophore. Biochim Biophys Acta. 1975;404:300–308. doi: 10.1016/0304-4165(75)90337-2. [DOI] [PubMed] [Google Scholar]
- McCabe SL, Pelosi DM, Tetreault M, Miri A, Nguitragool W, Kovithvathanaphong P, Mahajan R, Zimmerman AL. All-trans-retinal is a closed-state inhibitor of rod cyclic nucleotide-gated ion channels. J Gen Physiol. 2004;123:521–531. doi: 10.1085/jgp.200409011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melia TJ, Jr, Cowan CW, Angleson JK, Wensel TG. A comparison of the efficiency of G protein activation by ligand-free and light-activated forms of rhodopsin. Biophys J. 1997;73:3182–3191. doi: 10.1016/S0006-3495(97)78344-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molday RS, MacKenzie D. Monoclonal antibodies to rhodopsin: Characterization, cross-reactivity, and application as structural probes. Biochemistry. 1983;22:653–660. doi: 10.1021/bi00272a020. [DOI] [PubMed] [Google Scholar]
- Morton RA, Pitt GAJ. Studies on rhodopsin. 9 pH and the hydrolysis of indicator yellow. Biochem J. 1955;59:128–134. doi: 10.1042/bj0590128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oprian DD. Expression of opsin genes in COS cells. Methods Neurosci. 1993;15:301–306. [Google Scholar]
- Ramon E, Mao X, Ridge KD. Studies on the stability of the human cone visual pigments. Photochem Photobiol. 2009;85:509–516. doi: 10.1111/j.1751-1097.2008.00504.x. [DOI] [PubMed] [Google Scholar]
- Rim J, Oprian DD. Constitutive activation of opsin: Interaction of mutants with rhodopsin kinase and arrestin. Biochemistry. 1995;34:11938–11945. doi: 10.1021/bi00037a035. [DOI] [PubMed] [Google Scholar]
- Rim J, Faurobert E, Hurley JB, Oprian DD. In vitro assay for trans-phosphorylation of rhodopsin by rhodopsin kinase. Biochemistry. 1997;36:7064–7070. doi: 10.1021/bi970470e. [DOI] [PubMed] [Google Scholar]
- Robinson PR. Assays for the detection of constitutively active opsins. Methods Enzymol. 2000;315:207–218. doi: 10.1016/s0076-6879(00)15845-8. [DOI] [PubMed] [Google Scholar]
- Robinson PR, Cohen GB, Zhukovsky EA, Oprian DD. Constitutively active mutants of rhodopsin. Neuron. 1992;9:719–725. doi: 10.1016/0896-6273(92)90034-b. [DOI] [PubMed] [Google Scholar]
- Rohrer B, Lohr HR, Humphries P, Redmond TM, Seeliger MW, Crouch RK. Cone opsin mislocalization in Rpe65−/− mice: A defect that can be corrected by 11-cis retinal. Invest Ophthalmol Vis Sci. 2005;46:3876–3882. doi: 10.1167/iovs.05-0533. [DOI] [PubMed] [Google Scholar]
- Schoenlein RW, Peteanu LA, Mathies RA, Shank CV. The first step in vision: Femtosecond isomerization of rhodopsin. Science. 1991;254:412–415. doi: 10.1126/science.1925597. [DOI] [PubMed] [Google Scholar]
- Surya A, Foster KW, Knox BE. Transducin activation by the bovine opsin apoprotein. J Biol Chem. 1995;270:5024–5031. doi: 10.1074/jbc.270.10.5024. [DOI] [PubMed] [Google Scholar]
- Wang JK, McDowell JH, Hargrave PA. Site of attachement of 11-cis-retinal in bovine rhodopsin. Biochemistry. 1980;19:5111–5117. doi: 10.1021/bi00563a027. [DOI] [PubMed] [Google Scholar]
- Wessling-Resnick M, Johnson GL. Allosteric behavior in transducin activation mediated by rhodopsin. J Biol Chem. 1987;262:3697–3705. [PubMed] [Google Scholar]
- Yu H, Kono M, McKee TD, Oprian DD. A general method for mapping tertiary contacts between amino acid residues in membrane-embedded proteins. Biochemistry. 1995;34:14963–14969. doi: 10.1021/bi00046a002. [DOI] [PubMed] [Google Scholar]
- Zhukovsky EA, Robinson PR, Oprian DD. Transducin activation by rhodopsin without a covalent bond to the 11-cis-retinal chromophore. Science. 1991;251:558–560. doi: 10.1126/science.1990431. [DOI] [PubMed] [Google Scholar]
- Znoiko SL, Rohrer B, Lu K, Lohr HR, Crouch RK, Ma J-x. Downregulation of cone-specific gene expression and degeneration of cone photore-ceptors in the rpe65−/− mouse at early ages. Invest Ophthalmol Vis Sci. 2005;46:1473–1479. doi: 10.1167/iovs.04-0653. [DOI] [PubMed] [Google Scholar]