Abstract
Purpose of review
This review captures the existence, cause, and treatment challenges of residual cardiovascular risk (CVR) after aggressive low-density lipoprotein cholesterol (LDL-C) reduction.
Recent findings
Scientific evidence implicates low high-density lipoproteins cholesterol (HDL-C) and high triglycerides (TG) in the CVR observed after LDL-C lowering. However, the Action to Control Cardiovascular Risk in Diabetes (ACCORD) lipid trial with fenofibrate, the Investigation of Lipid Level Management to Understand its Impact in Atherosclerotic Events (ILLUMINATE) study with torcetrapib, and the recently terminated Atherothrombosis Intervention in Metabolic Syndrome with Low HDL Cholesterol/High Triglyceride and Impact on Global Health Outcomes (AIM-HIGH) study with niacin, do not clearly attribute risk reduction value to HDL-C/TG modulation.
Summary
The optimum approach to long-term lipid-modifying therapies for CVR reduction remains uncertain. Consequently, absolute risk modulation via lifestyle changes remains the centerpiece of a strategy addressing the physiological drivers of CVR associated with HDL-C/TG, especially in the context of diabetes/metabolic syndrome.
Keywords: Statins, LDL cholesterol, CV risk reduction, residual CV risk
Introduction
The deposition of atherogenic lipoproteins in the vessel wall is the major driver for atherosclerosis, a degenerative inflammatory disease that underpins most cardiovascular (CV) events. Consequently, CV disease treatment algorithms target low-density lipoprotein cholesterol (LDL-C) to prevent atherothrombosis and plaque rupture, which portend high CV morbidity and mortality. In this context, cumulative evidence from over two decades of clinical trials involving over 170,000 participants, have demonstrated the beneficial effects of statins, or 3-hydroxy-methylglutaryl coenzyme A (HMG-CoA) reductase inhibitors, in the reduction of CV event rates.[1] Thus, statins now stand as a bulwark in the frontier of therapeutic strategies for modulation of cholesterol levels and inflammation for primary and secondary prevention of atherosclerotic CV events, including stroke.[2–5] However, despite the chronicled successes from the use of statins, analyses of clinical trial data reveal significant residual CV risk in all patients treated with statins, even in the setting of optimal LDL-C reduction, thus highlighting the need to retool our CV risk reduction algorithms beyond the focus on LDL-C levels and/or the use of statins.
In this article, we discuss the large-scale placebo controlled and standard care-controlled trials of statin therapy on CV outcomes, which provide evidence for residual CV risk despite statin-induced optimal LDL-C reduction per existing treatment guidelines. Based on clinical and epidemiologic studies, we evaluate the potential underlying factors for residual CV risk, with a focus on the functional relationship between LDL-C and CV risk, other lipid culprits such as low high-density lipoprotein cholesterol (HDL-C) and high triglycerides (TG), as well as notable co-morbidities such as diabetes/metabolic syndrome and inflammation. We highlight the counterintuitive results from recent drug intervention trials designed to modulate HDL-C and/or TG levels and support the use of global CV risk assessment and lifestyle changes in the quest for maximal CV risk reduction.
Evidence for residual CV risk
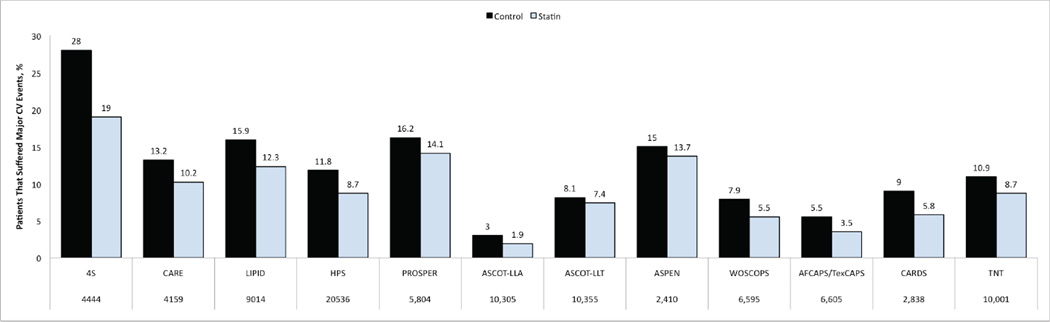
The Scandinavian Simvastatin Survival Study (4S) evaluated patients with known coronary heart disease and high levels of LDL-C. A significant reduction of CV events was observed with statin treatment compared to placebo, however, a 20% CV event rate was noted in statin treated patients.[6] Significant residual CV risk was also noted in other major trials of statin therapy, which prompted the focus on optimal statin use.[7–12] Consequently, additional studies have evaluated the incremental benefits of high-dose statin for intensive LDL-C lowering in high-risk patients. Three landmark trials that shaped our understanding in this regard include the Pravastatin or Atorvastatin Evaluation and Infection Therapy-Thrombolysis in Myocardial Infarction 22 (PROVE IT-TIMI) study, the Incremental Decrease in End Points Through Aggressive Lipid Lowering (IDEAL) study, and the Treating to New Targets (TNT) study.[13–15] These studies compared statin lowering of LDL-C to 100 mg/dl versus intensive reduction of LDL-C to 70 mg/dl using high dose statin. In all of these trials, intensive statin therapy with 80 mg atorvastatin led to greater CV risk reduction, but residual CV risk was still apparent in the intensive statin arms; 22.4% in the PROVE IT-TIMI study despite reduction of LDL-C to 62 mg/dl, 12% in the IDEAL study where LDL-C was reduced to 81 mg/dl, and 8.7% in the TNT study which reported LDL-C of 77 mg/dl.[13–15] In the A to Z Trial, early initiation of an intensive simvastatin regimen in patients with acute coronary syndrome (ACS) did not show a significant reduction in the composite primary CV endpoint compared to delayed initiation of a less intensive regimen.[16] However, a meta-analysis of these four studies that compared conventional statin therapy to more intensive treatment in patients with chronic stable coronary heart disease (TNT and IDEAL) or ACS (PROVE-IT and A to Z) showed a further 16% benefit in reducing coronary heart disease and a 18% further benefit in reducing stroke with more intensive treatment.[17] It is worth noting that the ACS trials (PROVE_IT and A/Z) were of shorter duration ~ 2 years, whereas TNT and IDEAL lasted 5 years. Generally, the aggregate of clinical studies indicate that treatment with statins reduces the risk of major CV events by 21% for every 39 mg/dl decrease in LDL-C, and that when LDL-C is lowered below 70 mg/dl further reduction in CV risk is accomplished.[18] However, despite aggressive LDL-C reduction, residual annual risk that approximates 9% in patients with established coronary artery disease remains a reality that has prompted questions about etiology and a therapeutic course of action. More recent reports corroborate the association between dyslipidemia and vascular events despite statin therapy, and therefore support the need for effective measures to combat residual CV risk.[19, 20] In general, it is important to note that residual CV risk remains after optimal reduction of LDL-C levels in major statin trials regardless of dose (Figure 1), in niacin and fibrate monotherapy trials, and in trials of combination therapies.[7–15, 21–23]
Figure 1. Presence of Residual CV Risk in Large Prospective Studies of Optimal Statin Therapy.
4S = Scandinavian Simvastatin Survival Study[6]; CARE = Cholesterol and Recurrent Events trial[10]; LIPID = Long-term Intervention with Pravastatin in Ischaemic Disease[7]; HPS = Heart Protection Study[8]; PROSPER = Prospective Study of Pravastatin in the Elderly at Risk[101]; ASCOT-LLA = Anglo-Scandinavian Cardiac Outcomes Trial–Lipid Lowering Arm[102]; ALLHAT-LLT = Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial[103]; ASPEN = Atorvastatin Study for Prevention of Coronary Heart Disease Endpoints in Non-Insulin-dependent Diabetes Mellitus[104]; WOSCOPS = West of Scotland Coronary Prevention Study[11]; AFCAPS/TexCAPS = Air Force/Texas Coronary Atherosclerosis Prevention Study[9]; CARDS = Collaborative Atorvastatin Diabetes Study[105]; TNT = Treating to New Targets study[14]
What is optimal LDL-C reduction?
Observational studies suggest a log-linear relationship between LDL-C concentrations and CV events.[24, 25] In this context, the Heart Protection Study (HPS) demonstrated a proportional produced by reduction of LDL-C concentration in the study participants. That is, a 1 mmol/dl reduction in LDL-C from 4 mmol/l to 3 mmol/l or from 3 mmol/l to 2 mmol/l led to the same one-quarter reduction in the risk of CV events.[8] This proportional reduction was consistent across the panorama of LDL-C concentrations among the study participants including concentrations below 2 mmol/l (77 mg/dl). The findings from the HPS put an end to the speculations in its era that a threshold of LDL-C might exist at 3.2 mmol/l (125mg/dl) below which further CV risk reduction would be futile.[10, 26] However, residual CV risk following optimal statin therapy has garnered attention, and precipitates the question as to what optimal reduction of LDL-C really is, given the log-linear association between LDL-C and vascular risk, and the fact that no clear threshold has been identified. Is further risk reduction feasible at LDL-C concentrations below the current target level of 70 mg/dL for high-risk CV patients? What impact will LDL-C of < 55 mg/dl have on CV risk reduction? In this context, what is the importance or clinical benefit of additional relative risk reduction when absolute risk is the clinically relevant parameter? Furthermore, a recent Cholesterol Treatment Trialists’ (CTT) meta-analyses of data from 170 000 participants in 26 randomized trials has substantiated the initial report from the HPS; essentially, there is no evidence, as of yet, of a threshold of LDL-C within cholesterol ranges studied, where additional CV risk reduction becomes futile.[1] Furthermore, this CTT meta-analysis suggested a 12% reduction in CV events per 1 mmol/L decrease in LDL during the first year of treatment with an additional 25% reduction for each subsequent year (mean CV event reduction of 22% per 1 mmol/L decrease in LDL), indicating that continued benefits in terms of CV event reduction accrue over time with persistent statin therapy. The question of what optimal LDL-C reduction should mean is worth contemplating as we grapple with the realities of residual risk reduction.
Beyond LDL-C
In the recent past, there has been increased recognition of the relationship between cardiovascular risk and other lipid parameters, most notably HDL-C and TG. Consequently, a lot of enthusiasm now surrounds the potential contribution of these non-LDL-C parameters to residual CV risk in optimally treated statin patients. This interest is further increased by the prospect of using existing and novel pharmacological agents in modulating the serum concentrations of HDL-C and TG with the aim to achieve further reduction in CV risk during statin therapy.
HDL-C
In a landmark report from the Framingham study cohort, Gordon et al. showed that HDL-C concentrations of <1.03 mmol/l (40 mg/dl) in men and <1.29 mmol/l (50 mg/dl) in women were associated with increased CV risk.[27] In another report, Gordon et al. demonstrated that CV risk decreases by 2 to 3% for every 0.03 mmol/l (1 mg/dl) increase in HDL-C.[28] Numerous population-based studies have confirmed the association between low HDL-C and CV risk in men and women.[29–32] Consequently, low HDL-C is accepted as a major independent risk factor and treatment target for coronary heart disease, according to guidelines of The National Cholesterol Education Program Adult Treatment Panel III (NCEP ATP III).[4] This independent association is maintained after adjusting for other risk parameters such as TG concentrations, obesity, and diabetes.[33] The magnitude of the problem is enormous, as recent studies have reported a 30 to 40% prevalence of low HDL-C in adult populations of men and women.[34, 35]
A recent meta-analysis of 20 randomized controlled trials that aggregated 543,210 person-years of follow-up indicated that the relationship between HDL-C and CV risk is not altered during statin use.[36] In this report by Jafri et al. there was an inverse relationship between on-treatment HDL-C levels and CV risk, which was statistically significant and independent of on-treatment LDL-C level, potency of the statin, age, hypertension, diabetes mellitus, and tobacco use.[36] A post hoc analysis of the TNT trial suggested that low levels of HDL were predictive of increased CV events for treatment with 10 mg of atorvastatin, but this relationship was attenuated when evaluated for 80 mg.[37]However, this trial also evaluated the subgroup with on-treatment LDL < 70 mg/dL (mean LDL 58 mg/dL) and suggested increased events in those with HDL < 42 mg/dl. The potential attenuation of low HDL in predicting CV events was also noted in PROVE-IT [38] and JUPITER [39] when on treatment LDL was < 70 mg/dL.
This report reinforces the prevailing notion that low HDL-C levels contribute to a significant portion of the residual CV risk in subjects on optimal statin therapy. Lifestyle changes that influence HDL-C levels include smoking cessation,[40, 41] weight loss and dietary manipulation,[42–48] and aerobic exercise.[49–51] Known pharmacologic strategies for raising HDL-C include fibrates,[23, 52–55] niacin,[21, 56–63] and agents under development, such as apoAI mimetics[64–68], apoAI expression stimulators, such as RVX208,[69, 70] and cholesteryl ester transfer protein (CETP) inhibitors, such anacetrapib and dalcetrapib.[71, 72] It is worth mentioning that the first CETP inhibitor, torcetrapib, failed to show CV risk reduction in the ILLUMINATE study, and indeed caused an excess of CV disease which warranted discontinuation of the trial only 10 months from its start. [73] The increase in CV events with torcetrapib has been attributed to off-target effects such as increase in blood pressure and aldosterone and electrolyte abnormalities.[74] Anacetrapib and dalcetrapib do not appear to have these off-target effects;[75] clinical outcomes trials of anacetrapib (REVEAL: Randomized Evaluation of the Effects of Anacetrapib through Lipid-modification) and dalcetrapib[70] are underway.
Among the currently approved agents for treatment of dyslipidemia, niacin is known as the most effective HDL-C raising agent. Furthermore, studies of niacin monotherapy,[76] as well as a recent meta-analysis supported its ability to reduce cardiovascular events.[77] Similarly, regression studies of niacin used in combination with a statin have shown the ability of this combination to promote regression of atherosclerosis.[21, 22] Therefore, niacin has been a strong candidate for reducing residual risk in statin-treated patients with low HDL-C. However, the utility of extended release niacin in reducing residual CV risk in statin-treated patients with established atherosclerotic cardiovascular disease with low HDL-C but optimally treated LDL-C is now questioned by the recent early termination of the AIM-HIGH study due to lack of treatment benefit. In AIM-HIGH, 3414 subjects with atherosclerotic cardiovascular disease, low HDL-C, and high TG were all treated with simvastatin, and randomized to either high-dose (2000 mg per day) extended-release niacin (n=1718) or placebo (n=1696). In addition, 515 were additional given the cholesterol absorption inhibitor ezetimibe in order to maintain LDL-C between 40 and 80 mg/dL. Mean baseline lipid levels in the 94% of participants taking a statin at baseline were LDL-C 71 mg/dL, HDL-C 35 mg/dL, and TG 161 mg/dL. Taken at face value, the results of AIM-HIGH indicate that the addition of niacin did not benefit this population with LDL-C well controlled by simvastatin during the 3 years of this study. Is it possible that in the setting of a low LDL-C it may take more than 3 years for the benefits of HDL-C raising with niacin to surface? In this regard, it is worth noting that the benefits of niacin in Coronary Drug Project on major cardiovascular events were noted in a follow up study 15 years later,[76] indeed suggesting delayed benefits from niacin therapy. Furthermore, it will be important to see if subjects with significant hypertriglyceridemia or with elevated Lp(a) may have benefited, although the relatively small size of AIM-HIGH may limit the power to detect differences in subgroup analyses. However, further insights on the relationship between niacin use and residual CV risk in statin treated patients will have to wait for the detailed analyses and publication of AIM-HIGH study results, and the completion of the larger international study of high-dose extended-release niacin in a population of patients with a panorama of HDL-C levels (HPS2-THRIVE: the Heart Protection Study 2 Treatment of HDL-C to Reduce the Incidence of Vascular Events). At this point, it would not be appropriate to extrapolate the results of AIM-HIGH to patients unable to achieve their LDL-C goal on statin therapy alone or to patients with very low HDL-C.
Clinical and epidemiologic evidence that supports the association between levels of HDL-C and CV risk; thus, it is puzzling that recent effort at modulating HDL-C levels have failed to reduce residual CV risk. Given recent evidence that HDL-C function can contribute to CV risk independent of HDL-C levels,[78] it is possible that heterogeneity of HDL-C function in the study populations may have confounded the results. Alternatively, it is possible that the functionality of HDL raised by pharmacologic means may fall short of the functional integrity of elevated particle levels naturally achieved by lifestyle changes. The development of clinical assays for HDL-C function may allow targeting of appropriate HDL therapies to populations with high residual risk.
TG and non-HDL-C
Numerous studies have demonstrated an association between high TG level and CV risk. This is captured in the meta-analyses of data from 262,525 participants in 29 prospective studies, showing that the TG level is a strong and independent predictor of CV risk.[79] In their report, Sarwar et al noted that the association between high TG levels and CV risk is independent of duration of follow-up, gender, or fasting.[79] Adjustment for HDL-C level attenuated, but did not eliminate, the strength of the association between high TG level and CV risk.[79] The meta-analyses by Sarwar et al predated the completion of the PROVE IT-TIMI 22 study. Analyses of the data from the latter revealed that TG levels, independent of LDL-C levels, had a substantial impact on the CV outcomes in patients with acute coronary syndromes.[80] Notably, among statin treated patients, on-treatment TG level <150 mg/dl was associated with reduced CV risk independent of LDL-C concentration. Furthermore, the beneficial effect of reducing LDL-C to <70 mg/dl was maximal in subjects with TG levels <150 mg/dl.[80] Our knowledge of the impact of TG lowering therapies on cardiovascular events is limited by the fact that no major outcomes trials have been done in patients with moderate to severe hypertriglyceridemia, as these patients were excluded from statin and fibrate outcomes trials. Fibric acid derivatives are very effective at lowering plasma triglyceride levels, moderately effective in raising HDL-C, but have only modest LDL-C lowering effects. The mean baseline TG levels in the 5 major fibrate outcomes trials ranged from 175 mg/dL in Helsinki Heart Study (HHS)[55] to 149 mg/dL in the Bezafibrate Infarction Prevention (BIP) Study [52] (Table I). In general, fibrates are not used in clinical practice to treat individuals with TG so mildly elevated. Gemfibrozil has been shown to be effective in both primary and secondary cardiovascular risk reduction.[23, 55] In the Veterans Affairs High-Density Lipoprotein Cholesterol Intervention Trial (VA-HIT), gemfibrozil was effective in reducing cardiovascular events in the absence of significant changes in LDL-C (and with a baseline LDL-C level of 111 mg/dL).[23] In contrast, two major trials with fenofibrate in patients with Type 2 Diabetes Mellitus have failed to demonstrate reductions in major cardiovascular events (Table I). The Fenofibrate Intervention and Event Lowering in Diabetes (FIELD) study randomized 9795 diabetic patients with mean levels of TG 154mg/dL, HDL-C 43mg/dL, and LDL-C 119mg/dL to placebo and fenofibrate over a 5-year study period, and did not demonstrate a significant reduction in the primary combined endpoint for CV risk between study groups.[54] A higher rate of statin therapy commencement in the placebo group (17% versus 8%) may have masked the treatment benefit of fenofibrate. FIELD did provide support for potential safety of combination therapy (fibrate + statin).[54] In addition, post hoc analysis of data from the FIELD study demonstrates that patients with elevated TG (>200mg/dl) or low HDL-C (< 40 mg/dl in men and < 50 mg/dl in women) derived greater CV risk reduction with fenofibrate.[81] The efficacy and safety of using a statin and fibrate in combination was recently evaluated in the Action to Control Cardiovascular Risk in Diabetes lipid trial (ACCORD Lipid), which reported no effects on the primary composite outcome (nonfatal myocardial infarction, nonfatal stroke, or CV death) of fenofibrate added to simvastatin; however there was an overall trend toward increased risk in women as compared to men.[82] Of note, in a prespecified subgroup analysis, there was a trend towards benefit of fenofibrate in patients with TG level of ≥ 204 mg/dl (2.30 mmol/l) or HDL-C of ≤ 34 mg/dl (0.88 mmol/l).[82] Indeed, post hoc analyses of all of the fibrate trials have shown reductions in cardiovascular events in subgroups with features of the metabolic syndrome, including overweight participants with high TG and low HDL-C levels (Table I).[83, 84] Therefore, the hypothesis that fibrate therapy may reduce residual CV risk in patients with T2DM with elevated TG and low HDL-C has not been adequately tested and should be investigated in a major cardiovascular outcome trial.
Table 1.
Fibrates Outcomes Studies
| Trial | TC | TG | HDL-C | LDL-C | Non- HDL |
CVD Reduction Entire Cohort |
Lipid Subgroup Criteria | CVD Reduction Subgroup |
|---|---|---|---|---|---|---|---|---|
| HHS[55] | 269 | 175 | 47 | 189 | 222 | −34% (0.02) | TG>204; HDL-C<42* | −78%(0.002) |
| VA-HIT[23] | 175 | 161 | 32 | 111 | 143 | −22% (0.006) | TG>180 | −28%(<0.05) |
| BIP[52] | 212 | 149 | 35 | 148 | 177 | −7.3% (0.24) | TG>200 | −39.5(0.02) |
| FIELD[54] | 195 | 154 | 43 | 119 | 152 | −11% (0.16) | TG>204; HDL-C<40** | −27%(0.005) |
| ACCORD[82] | 175 | 162 | 38 | 100 | 137 | −8% (0.32) | TG>204; HDL-C<34 | −31%(0.03) |
HHS = Helsinki Heart Study; VA-HIT = Veterans Affairs High-Density Lipoprotein Cholesterol Intervention Trial; BIP = Bezafibrate Infarction Prevention; FIELD = Fenofibrate Intervention and Event Lowering in Diabetes; ACCORD = Action to Control Cardiovascular Risk in Diabetes.
BMI > 26 Kg/M2;
HDL-C < 50 mg/dl in women. Adapted from Elam MB and colleagues.[84]
Non-HDL-C has been shown to predict CV risk as well as correlate with most lipid parameters associated with CV risk.[85–88] Evidence for the association between non-HDL-C and CV risk has also come from epidemiologic data reported by Liu et al.[89] In their analyses of data from 2,693 men and 3,101 women in the Framingham study cohort, a strong association between non-HDL-C and CV risk was noted within all strata of LDL-C values.[89] In this study, non-HDL-C was apparently a stronger predictor of CV risk than LDL-C, and this finding was independent of whether the TG level was < 200 mg/dl or > 200 mg/dl.[89]
A recent report from the INTERHEART study shows that the ApoB/ApoAI ratio was strongly associated with risk of myocardial infarction,[90] more so in patients with type 2 diabetes and metabolic syndrome.[91] Residual CV risk in patients treated with statins is particularly high among diabetics. Kearney et al. in their meta-analyses of data from 18,686 patients with diabetes in 14 randomized trials of statin therapy demonstrated a 9% proportional reduction in all-cause mortality per 1 mmol/L reduction in LDL-C in participants with diabetes (rate ratio [RR] 0.91, 99% CI 0.82–1.01; p=0.02), similar to the 13% reduction in those without diabetes (0.87, 0.82–0.92; p<0.0001).[92] However, residual CV risk in diabetic patients treated with statins was higher than in non-diabetic patients randomized to placebo treatment.[92]
Beyond Lipids
Recent evidence indicates that regardless of the lipid endpoint adopted, a low systemic burden of inflammation, as determined by the concentration of high sensitivity C reactive protein (hsCRP), confers a better prognosis in statin treated patients.[93, 94] Justification for the Use of Statins in Primary Prevention: an intervention Trial Evaluating Rosuvastatin (JUPITER),[94] provides important experimental data that inflammation, and its modulation, is critical to the benefits of statin therapy in a population of patients with normal levels of LDL-C but elevated hsCRP. The finding from JUPITER is supported by a prior report of the importance of other lipid parameters such as the cholesterol to HDL-C ratio when combined with hsCRP.[88] Taken together, these studies add to the body of evidence that supports the notion of inflammation as a unifying hypothesis in the pathogenesis of CV disease.[95] The evidence for the independent contribution of inflammation to CV disease provides the rationale and justification for the recently launched cardiovascular inflammation reduction trial (CIRT), which will allocate 7000 stable coronary artery disease patients with persistent elevations of hsCRP to placebo or very-low-dose-methotrexate (10mg), a widely utilized anti-inflammatory agent which lowers levels of tumor necrosis alpha, interleukin-6, and CRP.[96]
Lifestyle changes
Regardless of the recent disappointments from drug trials of established and novel agents for HDL-C and TG modulation, it is important to retain the important perspective that for many individuals high TG and low HDL-C are features of the metabolic syndrome, which is highly responsive to lifestyle modification. A paramount action for overall CV risk reduction is smoking cessation, which has been reported to raise HDL-C levels by 4 mg/dl,[41] with reversion to HDL-C levels seen in non-smokers.[40] Other recommended modifications include weight reduction, dietary control, and increased physical activity. A 10-kilogram weight loss can result in a 20% increase in HDL-C level.[45] Diets rich in fruits, vegetables, low-fat dairy products, with low simple carbohydrates, and reduced content of saturated and total fat can reduce blood pressure, LDL-C, and raise HDL-C levels.[42, 43, 47, 48, 97, 98] Aerobic physical activity such as brisk walking at least 30 minutes per day, more than three days of the week can result in a 4–9 mm Hg blood pressure reduction, and an elevation of HDL-C levels by 5 to 10%. [49, 50, 99, 100]
Conclusions
The use of statins to modulate levels of cholesterol and inflammation has led to remarkable reduction in CV endpoints and consequently their ubiquitous employment in contemporary approaches for primary and secondary prevention of atherosclerotic CV disease. However, residual CV risk remains a major concern, and highlights room for additional improvement. One approach to residual CV risk reduction implicates low HDL-C and high TG levels. Consequently, the quest to modulate HDL-C and TG levels via pharmacologic approaches has led to clinical trials of novel agents as well as studies of long-term combination therapy of statins with niacin or fibrates. On both fronts, we have been plagued by disappointments, which may reflect the complexities of HDL-C and TG as biomarkers and the challenges of clinical trial design when the goal is to show incremental benefits. The recent termination of the AIM-HIGH study due to lack of treatment benefit implies that the jury on combination pharmacotherapy for dyslipidemia will remain in deliberation pending detailed analyses and publication of the study results, and the completion of the Heart Protection Study 2 Treatment of HDL-C to Reduce the Incidence of Vascular Events (HPS-2-THRIVE), a large international study of high-dose, extended-release niacin in a population of patients with a panorama of HDL-C levels. All the major fibrate outcomes trials have shown reduced cardiovascular events in subgroups with elevated triglycerides and low HDL-C with features of the metabolic syndrome or with diabetes, supporting the need for a major outcomes trial of statin and fibrate combination therapy in a population with T2DM or metabolic syndrome and significant hypertriglyceridemia and low HDL-C cholesterol. Until then, lifestyle interventions will remain the best tool for aggressively managing residual risk in statin-treated patients.
Key points.
Significant residual cardiovascular risk after optimum reduction of LDL-C has been clearly documented in numerous statin, non-statin, and combination therapy trials
The most notable lipid parameters implicated in residual CV risk are HDL-C and TG.
It is harder than previously thought to clearly attribute value to HDL-C and TG modulation; however, these parameters are still viable targets.
Attention to global risk assessment and therapeutic lifestyle modification for primary prevention and optimal CV risk reduction is an attractive approach.
Acknowledgements
Funding support for Dr. Sampson was provided in part by the Harold Amos Medical Faculty Development Award of the Robert Wood Johnson Foundation and the Vanderbilt Clinical and Translational Scholars Award. Drs. Fazio and Linton were partially supported by NIH grants HL65709, HL086988, and HL105375. The final publication is available at www.springerlink.com http://link.springer.com/content/pdf/10.1007%2Fs11883-011-0219-7.pdf
Footnotes
Disclosure
U.K. Sampson: none. S. Fazio is a scientific advisory board member of Merck, Sharp, and Dohme; is a consultant for Merck, Takeda, Kowa, and Pfizer; has received grants (paid to his institution) from the National Institutes of Health, Merck, and Pfizer; and has had travel expenses covered by Merck. M.F. Linton is a consultant for Merck and Kowa and has received grants from ISIS, Genzyme, and Merck.
Contributor Information
Uchechukwu K. Sampson, Email: u.sampson@vanderbilt.edu.
Sergio Fazio, Email: sergio.fazio@vanderbilt.edu.
MacRae F. Linton, Email: macrae.linton@vanderbilt.edu.
References
- 1. Baigent C, Blackwell L, Emberson J, et al. Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet. 2010;376:1670–1681. doi: 10.1016/S0140-6736(10)61350-5. ** The strongest available meta-analysis on the value of intensive LDL-C lowering in reducing cardiovascular risk.
- 2.Smith SC, Jr, Allen J, Blair SN, et al. AHA/ACC guidelines for secondary prevention for patients with coronary and other atherosclerotic vascular disease: 2006 update endorsed by the National Heart, Lung, and Blood Institute. J Am Coll Cardiol. 2006;47:2130–2139. doi: 10.1016/j.jacc.2006.04.026. [DOI] [PubMed] [Google Scholar]
- 3.Grundy SM, Cleeman JI, Merz CN, et al. Implications of recent clinical trials for the National Cholesterol Education Program Adult Treatment Panel III guidelines. Circulation. 2004;110:227–239. doi: 10.1161/01.CIR.0000133317.49796.0E. [DOI] [PubMed] [Google Scholar]
- 4.Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III) JAMA. 2001;285:2486–2497. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 5.Graham I, Atar D, Borch-Johnsen K, et al. European guidelines on cardiovascular disease prevention in clinical practice: executive summary: Fourth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (Constituted by representatives of nine societies and by invited experts) Eur Heart J. 2007;28:2375–2414. doi: 10.1093/eurheartj/ehm316. [DOI] [PubMed] [Google Scholar]
- 6.Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian Simvastatin Survival Study (4S) Lancet. 1994;344:1383–1389. [PubMed] [Google Scholar]
- 7.Prevention of cardiovascular events and death with pravastatin in patients with coronary heart disease and a broad range of initial cholesterol levels. The Long-Term Intervention with Pravastatin in Ischaemic Disease (LIPID) Study Group. N Engl J Med. 1998;339:1349–1357. doi: 10.1056/NEJM199811053391902. [DOI] [PubMed] [Google Scholar]
- 8.MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20,536 high-risk individuals: a randomised placebo-controlled trial. Lancet. 2002;360:7–22. doi: 10.1016/S0140-6736(02)09327-3. [DOI] [PubMed] [Google Scholar]
- 9.Downs JR, Clearfield M, Weis S, et al. Primary prevention of acute coronary events with lovastatin in men and women with average cholesterol levels: results of AFCAPS/TexCAPS. Air Force/Texas Coronary Atherosclerosis Prevention Study. JAMA. 1998;279:1615–1622. doi: 10.1001/jama.279.20.1615. [DOI] [PubMed] [Google Scholar]
- 10.Sacks FM, Pfeffer MA, Moye LA, et al. The effect of pravastatin on coronary events after myocardial infarction in patients with average cholesterol levels. Cholesterol and Recurrent Events Trial investigators. N Engl J Med. 1996;335:1001–1009. doi: 10.1056/NEJM199610033351401. [DOI] [PubMed] [Google Scholar]
- 11.Shepherd J, Cobbe SM, Ford I, et al. Prevention of coronary heart disease with pravastatin in men with hypercholesterolemia. West of Scotland Coronary Prevention Study Group. N Engl J Med. 1995;333:1301–1307. doi: 10.1056/NEJM199511163332001. [DOI] [PubMed] [Google Scholar]
- 12.Libby P. The forgotten majority: unfinished business in cardiovascular risk reduction. J Am Coll Cardiol. 2005;46:1225–1228. doi: 10.1016/j.jacc.2005.07.006. [DOI] [PubMed] [Google Scholar]
- 13.Cannon CP, Braunwald E, McCabe CH, et al. Intensive versus moderate lipid lowering with statins after acute coronary syndromes. N Engl J Med. 2004;350:1495–1504. doi: 10.1056/NEJMoa040583. [DOI] [PubMed] [Google Scholar]
- 14.LaRosa JC, Grundy SM, Waters DD, et al. Intensive lipid lowering with atorvastatin in patients with stable coronary disease. N Engl J Med. 2005;352:1425–1435. doi: 10.1056/NEJMoa050461. [DOI] [PubMed] [Google Scholar]
- 15.Pedersen TR, Faergeman O, Kastelein JJ, et al. High-dose atorvastatin vs usual-dose simvastatin for secondary prevention after myocardial infarction: the IDEAL study: a randomized controlled trial. JAMA. 2005;294:2437–2445. doi: 10.1001/jama.294.19.2437. [DOI] [PubMed] [Google Scholar]
- 16.de Lemos JA, Blazing MA, Wiviott SD, et al. Early intensive vs a delayed conservative simvastatin strategy in patients with acute coronary syndromes: phase Z of the A to Z trial. JAMA. 2004;292:1307–1316. doi: 10.1001/jama.292.11.1307. [DOI] [PubMed] [Google Scholar]
- 17.Cannon CP, Steinberg BA, Murphy SA, et al. Meta-analysis of cardiovascular outcomes trials comparing intensive versus moderate statin therapy. J Am Coll Cardiol. 2006;48:438–445. doi: 10.1016/j.jacc.2006.04.070. [DOI] [PubMed] [Google Scholar]
- 18.Baigent C, Keech A, Kearney PM, et al. Efficacy and safety of cholesterol-lowering treatment: prospective meta-analysis of data from 90,056 participants in 14 randomised trials of statins. Lancet. 2005;366:1267–1278. doi: 10.1016/S0140-6736(05)67394-1. [DOI] [PubMed] [Google Scholar]
- 19. Sazonov V, Beetsch J, Phatak H, et al. Association between dyslipidemia and vascular events in patients treated with statins: report from the UK General Practice Research Database. Atherosclerosis. 2010;208:210–216. doi: 10.1016/j.atherosclerosis.2009.07.021.* A retrospective cohort study that demonstrated the association between low HDL-C and/or elevated triglycerides (TG) and cardiovascular events despite statin treatment for LDL-C reduction.
- 20.Arsenault BJ, Barter P, DeMicco DA, et al. Prediction of cardiovascular events in statin-treated stable coronary patients by lipid and nonlipid biomarkers. J Am Coll Cardiol. 2011;57:63–69. doi: 10.1016/j.jacc.2010.06.052. [DOI] [PubMed] [Google Scholar]
- 21.Brown BG, Zhao XQ, Chait A, et al. Simvastatin and niacin, antioxidant vitamins, or the combination for the prevention of coronary disease. N Engl J Med. 2001;345:1583–1592. doi: 10.1056/NEJMoa011090. [DOI] [PubMed] [Google Scholar]
- 22.Brown G, Albers JJ, Fisher LD, et al. Regression of coronary artery disease as a result of intensive lipid-lowering therapy in men with high levels of apolipoprotein B [see comments] N Engl J Med. 1990;323:1289–1298. doi: 10.1056/NEJM199011083231901. [DOI] [PubMed] [Google Scholar]
- 23.Rubins HB, Robins SJ, Collins D, et al. Gemfibrozil for the secondary prevention of coronary heart disease in men with low levels of high-density lipoprotein cholesterol. Veterans Affairs High-Density Lipoprotein Cholesterol Intervention Trial Study Group. N Engl J Med. 1999;341:410–418. doi: 10.1056/NEJM199908053410604. [DOI] [PubMed] [Google Scholar]
- 24.Chen Z, Peto R, Collins R, et al. Serum cholesterol concentration and coronary heart disease in population with low cholesterol concentrations. BMJ. 1991;303:276–282. doi: 10.1136/bmj.303.6797.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stamler J, Vaccaro O, Neaton JD, Wentworth D. Diabetes, other risk factors, and 12-yr cardiovascular mortality for men screened in the Multiple Risk Factor Intervention Trial. Diabetes Care. 1993;16:434–444. doi: 10.2337/diacare.16.2.434. [DOI] [PubMed] [Google Scholar]
- 26.Sacks FM, Tonkin AM, Shepherd J, et al. Effect of pravastatin on coronary disease events in subgroups defined by coronary risk factors: the Prospective Pravastatin Pooling Project. Circulation. 2000;102:1893–1900. doi: 10.1161/01.cir.102.16.1893. [DOI] [PubMed] [Google Scholar]
- 27.Gordon T, Castelli WP, Hjortland MC, et al. High density lipoprotein as a protective factor against coronary heart disease. The Framingham Study. Am J Med. 1977;62:707–714. doi: 10.1016/0002-9343(77)90874-9. [DOI] [PubMed] [Google Scholar]
- 28.Gordon DJ, Probstfield JL, Garrison RJ, et al. High-density lipoprotein cholesterol and cardiovascular disease. Four prospective American studies. Circulation. 1989;79:8–15. doi: 10.1161/01.cir.79.1.8. [DOI] [PubMed] [Google Scholar]
- 29.Genest JJ, Jr, Martin-Munley SS, McNamara JR, et al. Familial lipoprotein disorders in patients with premature coronary artery disease. Circulation. 1992;85:2025–2033. doi: 10.1161/01.cir.85.6.2025. [DOI] [PubMed] [Google Scholar]
- 30.Genest JJ, McNamara JR, Salem DN, Schaefer EJ. Prevalence of risk factors in men with premature coronary artery disease. Am J Cardiol. 1991;67:1185–1189. doi: 10.1016/0002-9149(91)90924-a. [DOI] [PubMed] [Google Scholar]
- 31.Assmann G, Schulte H, von Eckardstein A, Huang Y. High-density lipoprotein cholesterol as a predictor of coronary heart disease risk. The PROCAM experience and pathophysiological implications for reverse cholesterol transport. Atherosclerosis. 1996;124(Suppl):S11–S20. doi: 10.1016/0021-9150(96)05852-2. [DOI] [PubMed] [Google Scholar]
- 32.Sharrett AR, Ballantyne CM, Coady SA, et al. Coronary heart disease prediction from lipoprotein cholesterol levels, triglycerides, lipoprotein(a), apolipoproteins A-I and B HDL density subfractions: The Atherosclerosis Risk in Communities (ARIC) Study. Circulation. 2001;104:1108–1113. doi: 10.1161/hc3501.095214. [DOI] [PubMed] [Google Scholar]
- 33.Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002;106:3143–3421. [PubMed] [Google Scholar]
- 34.Bruckert E, Baccara-Dinet M, McCoy F, Chapman J. High prevalence of low HDL-cholesterol in a pan-European survey of 8545 dyslipidaemic patients. Curr Med Res Opin. 2005;21:1927–1934. doi: 10.1185/030079905X74871. [DOI] [PubMed] [Google Scholar]
- 35.Ford ES, Giles WH, Dietz WH. Prevalence of the metabolic syndrome among US adults: findings from the third National Health and Nutrition Examination Survey. JAMA. 2002;287:356–359. doi: 10.1001/jama.287.3.356. [DOI] [PubMed] [Google Scholar]
- 36. Jafri H, Alsheikh-Ali AA, Karas RH. Meta-analysis: statin therapy does not alter the association between low levels of high-density lipoprotein cholesterol and increased cardiovascular risk. Ann Intern Med. 2010;153:800–808. doi: 10.7326/0003-4819-153-12-201012210-00006. **The strongest available meta-analysis showing the persistent relationship between low HDL-C and cardiovascular risk despite statin therapy.
- 37.Barter P, Gotto AM, LaRosa JC, et al. HDL cholesterol, very low levels of LDL cholesterol, and cardiovascular events. N Engl J Med. 2007;357:1301–1310. doi: 10.1056/NEJMoa064278. [DOI] [PubMed] [Google Scholar]
- 38.Ray KK, Cannon CP, Cairns R, et al. Prognostic utility of apoB/AI, total cholesterol/HDL, non-HDL cholesterol, or hs-CRP as predictors of clinical risk in patients receiving statin therapy after acute coronary syndromes: results from PROVE IT-TIMI 22. Arterioscler Thromb Vasc Biol. 2009;29:424–430. doi: 10.1161/ATVBAHA.108.181735. [DOI] [PubMed] [Google Scholar]
- 39.Ridker PM, Genest J, Boekholdt SM, et al. HDL cholesterol and residual risk of first cardiovascular events after treatment with potent statin therapy: an analysis from the JUPITER trial. Lancet. 2010;376:333–339. doi: 10.1016/S0140-6736(10)60713-1. [DOI] [PubMed] [Google Scholar]
- 40.Garrison RJ, Kannel WB, Feinleib M, et al. Cigarette smoking and HDL cholesterol: the Framingham offspring study. Atherosclerosis. 1978;30:17–25. doi: 10.1016/0021-9150(78)90149-1. [DOI] [PubMed] [Google Scholar]
- 41.Maeda K, Noguchi Y, Fukui T. The effects of cessation from cigarette smoking on the lipid and lipoprotein profiles: a meta-analysis. Prev Med. 2003;37:283–290. doi: 10.1016/s0091-7435(03)00110-5. [DOI] [PubMed] [Google Scholar]
- 42.Carlson LA, Hamsten A, Asplund A. Pronounced lowering of serum levels of lipoprotein Lp(a) in hyperlipidaemic subjects treated with nicotinic acid. J Intern Med. 1989;226:271–276. doi: 10.1111/j.1365-2796.1989.tb01393.x. [DOI] [PubMed] [Google Scholar]
- 43.Dattilo AM, Kris-Etherton PM. Effects of weight reduction on blood lipids and lipoproteins: a meta-analysis. Am J Clin Nutr. 1992;56:320–328. doi: 10.1093/ajcn/56.2.320. [DOI] [PubMed] [Google Scholar]
- 44.Ellison RC, Zhang Y, Qureshi MM, et al. Lifestyle determinants of high-density lipoprotein cholesterol: the National Heart, Lung, and Blood Institute Family Heart Study. Am Heart J. 2004;147:529–535. doi: 10.1016/j.ahj.2003.10.033. [DOI] [PubMed] [Google Scholar]
- 45.James WP, Astrup A, Finer N, et al. Effect of sibutramine on weight maintenance after weight loss: a randomised trial. STORM Study Group. Sibutramine Trial of Obesity Reduction and Maintenance. Lancet. 2000;356:2119–2125. doi: 10.1016/s0140-6736(00)03491-7. [DOI] [PubMed] [Google Scholar]
- 46.Karpe F, Frayn KN. The nicotinic acid receptor--a new mechanism for an old drug. Lancet. 2004;363:1892–1894. doi: 10.1016/S0140-6736(04)16359-9. [DOI] [PubMed] [Google Scholar]
- 47.Lichtenstein AH. Dietary fat and cardiovascular disease risk: quantity or quality? J Womens Health (Larchmt) 2003;12:109–114. doi: 10.1089/154099903321576493. [DOI] [PubMed] [Google Scholar]
- 48.Meksawan K, Pendergast DR, Leddy JJ, et al. Effect of low and high fat diets on nutrient intakes and selected cardiovascular risk factors in sedentary men and women. J Am Coll Nutr. 2004;23:131–140. doi: 10.1080/07315724.2004.10719353. [DOI] [PubMed] [Google Scholar]
- 49.Couillard C, Despres JP, Lamarche B, et al. Effects of endurance exercise training on plasma HDL cholesterol levels depend on levels of triglycerides: evidence from men of the Health, Risk Factors, Exercise Training and Genetics (HERITAGE) Family Study. Arterioscler Thromb Vasc Biol. 2001;21:1226–1232. doi: 10.1161/hq0701.092137. [DOI] [PubMed] [Google Scholar]
- 50.King AC, Haskell WL, Young DR, et al. Long-term effects of varying intensities and formats of physical activity on participation rates, fitness, and lipoproteins in men and women aged 50 to 65 years. Circulation. 1995;91:2596–2604. doi: 10.1161/01.cir.91.10.2596. [DOI] [PubMed] [Google Scholar]
- 51.Thompson PD. What do muscles have to do with lipoproteins? Circulation. 1990;81:1428–1430. doi: 10.1161/01.cir.81.4.1428. [DOI] [PubMed] [Google Scholar]
- 52.Secondary prevention by raising HDL cholesterol and reducing triglycerides in patients with coronary artery disease: the Bezafibrate Infarction Prevention (BIP) study. Circulation. 2000;102:21–27. doi: 10.1161/01.cir.102.1.21. [DOI] [PubMed] [Google Scholar]
- 53.Effect of fenofibrate on progression of coronary-artery disease in type 2 diabetes: the Diabetes Atherosclerosis Intervention Study, a randomised study. Lancet. 2001;357:905–910. [PubMed] [Google Scholar]
- 54.Keech A, Simes RJ, Barter P, et al. Effects of long-term fenofibrate therapy on cardiovascular events in 9795 people with type 2 diabetes mellitus (the FIELD study): randomised controlled trial. Lancet. 2005;366:1849–1861. doi: 10.1016/S0140-6736(05)67667-2. [DOI] [PubMed] [Google Scholar]
- 55.Manninen V, Elo MO, Frick MH, et al. Lipid alterations and decline in the incidence of coronary heart disease in the Helsinki Heart Study. JAMA. 1988;260:641–651. [PubMed] [Google Scholar]
- 56.Jacobson TA, Chin MM, Fromell GJ, et al. Fluvastatin with and without niacin for hypercholesterolemia. Am J Cardiol. 1994;74:149–154. doi: 10.1016/0002-9149(94)90088-4. [DOI] [PubMed] [Google Scholar]
- 57.O'Keefe JH, Jr, Harris WS, Nelson J, Windsor SL. Effects of pravastatin with niacin or magnesium on lipid levels and postprandial lipemia. Am J Cardiol. 1995;76:480–484. doi: 10.1016/s0002-9149(99)80134-9. [DOI] [PubMed] [Google Scholar]
- 58.Gardner SF, Schneider EF, Granberry MC, Carter IR. Combination therapy with low-dose lovastatin and niacin is as effective as higher-dose lovastatin. Pharmacotherapy. 1996;16:419–423. [PubMed] [Google Scholar]
- 59.Capuzzi DM, Morgan JM, Weiss RJ, et al. Beneficial effects of rosuvastatin alone and in combination with extended-release niacin in patients with a combined hyperlipidemia and low high-density lipoprotein cholesterol levels. Am J Cardiol. 2003;91:1304–1310. doi: 10.1016/s0002-9149(03)00318-7. [DOI] [PubMed] [Google Scholar]
- 60.Insull W, Jr, McGovern ME, Schrott H, et al. Efficacy of extended-release niacin with lovastatin for hypercholesterolemia: assessing all reasonable doses with innovative surface graph analysis. Arch Intern Med. 2004;164:1121–1127. doi: 10.1001/archinte.164.10.1121. [DOI] [PubMed] [Google Scholar]
- 61.Hunninghake DB, McGovern ME, Koren M, et al. A dose-ranging study of a new, once-daily, dual-component drug product containing niacin extended-release and lovastatin. Clin Cardiol. 2003;26:112–118. doi: 10.1002/clc.4960260304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Taylor AJ, Sullenberger LE, Lee HJ, et al. Arterial Biology for the Investigation of the Treatment Effects of Reducing Cholesterol (ARBITER) 2: a double-blind, placebo-controlled study of extended-release niacin on atherosclerosis progression in secondary prevention patients treated with statins. Circulation. 2004;110:3512–3517. doi: 10.1161/01.CIR.0000148955.19792.8D. [DOI] [PubMed] [Google Scholar]
- 63.Taylor AJ, Lee HJ, Sullenberger LE. The effect of 24 months of combination statin and extended-release niacin on carotid intima-media thickness: ARBITER 3. Curr Med Res Opin. 2006;22:2243–2250. doi: 10.1185/030079906x148508. [DOI] [PubMed] [Google Scholar]
- 64.Navab M, Anantharamaiah GM, Reddy ST, Fogelman AM. Apolipoprotein A-I mimetic peptides and their role in atherosclerosis prevention. Nat Clin Pract Cardiovasc Med. 2006;3:540–547. doi: 10.1038/ncpcardio0661. [DOI] [PubMed] [Google Scholar]
- 65.Navab M, Anantharamaiah GM, Reddy ST, et al. Oral D-4F causes formation of pre-beta high-density lipoprotein and improves high-density lipoprotein-mediated cholesterol efflux and reverse cholesterol transport from macrophages in apolipoprotein E-null mice. Circulation. 2004;109:3215–3220. doi: 10.1161/01.CIR.0000134275.90823.87. [DOI] [PubMed] [Google Scholar]
- 66.Navab M, Anantharamaiah GM, Hama S, et al. Oral administration of an Apo A-I mimetic Peptide synthesized from D-amino acids dramatically reduces atherosclerosis in mice independent of plasma cholesterol. Circulation. 2002;105:290–292. doi: 10.1161/hc0302.103711. [DOI] [PubMed] [Google Scholar]
- 67.Garber DW, Datta G, Chaddha M, et al. A new synthetic class A amphipathic peptide analogue protects mice from diet-induced atherosclerosis. J Lipid Res. 2001;42:545–552. [PubMed] [Google Scholar]
- 68.Nissen SE, Tsunoda T, Tuzcu EM, et al. Effect of recombinant ApoA-I Milano on coronary atherosclerosis in patients with acute coronary syndromes: a randomized controlled trial. JAMA. 2003;290:2292–2300. doi: 10.1001/jama.290.17.2292. [DOI] [PubMed] [Google Scholar]
- 69.Davidson MH. Apolipoprotein A-I therapy promise, challenges, and disappointment. J Am Coll Cardiol. 2011;57:1120–1121. doi: 10.1016/j.jacc.2010.11.025. [DOI] [PubMed] [Google Scholar]
- 70.Schwartz GG, Olsson AG, Ballantyne CM, et al. Rationale and design of the dal-OUTCOMES trial: efficacy and safety of dalcetrapib in patients with recent acute coronary syndrome. Am Heart J. 2009;158:896 e3–901 e3. doi: 10.1016/j.ahj.2009.09.017. [DOI] [PubMed] [Google Scholar]
- 71.Brousseau ME, Schaefer EJ, Wolfe ML, et al. Effects of an inhibitor of cholesteryl ester transfer protein on HDL cholesterol. N Engl J Med. 2004;350:1505–1515. doi: 10.1056/NEJMoa031766. [DOI] [PubMed] [Google Scholar]
- 72.de Grooth GJ, Kuivenhoven JA, Stalenhoef AF, et al. Efficacy and safety of a novel cholesteryl ester transfer protein inhibitor, JTT-705, in humans: a randomized phase II dose-response study. Circulation. 2002;105:2159–2165. doi: 10.1161/01.cir.0000015857.31889.7b. [DOI] [PubMed] [Google Scholar]
- 73.Barter PJ, Caulfield M, Eriksson M, et al. Effects of torcetrapib in patients at high risk for coronary events. N Engl J Med. 2007;357:2109–2122. doi: 10.1056/NEJMoa0706628. [DOI] [PubMed] [Google Scholar]
- 74.Vergeer M, Bots ML, van Leuven SI, et al. Cholesteryl ester transfer protein inhibitor torcetrapib and off-target toxicity: a pooled analysis of the rating atherosclerotic disease change by imaging with a new CETP inhibitor (RADIANCE) trials. Circulation. 2008;118:2515–2522. doi: 10.1161/CIRCULATIONAHA.108.772665. [DOI] [PubMed] [Google Scholar]
- 75.Miyares MA. Anacetrapib and dalcetrapib: two novel cholesteryl ester transfer protein inhibitors. Ann Pharmacother. 2011;45:84–94. doi: 10.1345/aph.1P446. [DOI] [PubMed] [Google Scholar]
- 76.Canner PL, Furberg CD, Terrin ML, McGovern ME. Benefits of niacin by glycemic status in patients with healed myocardial infarction (from the Coronary Drug Project) Am J Cardiol. 2005;95:254–257. doi: 10.1016/j.amjcard.2004.09.013. [DOI] [PubMed] [Google Scholar]
- 77. Bruckert E, Labreuche J, Amarenco P. Meta-analysis of the effect of nicotinic acid alone or in combination on cardiovascular events and atherosclerosis. Atherosclerosis. 2010;210:353–361. doi: 10.1016/j.atherosclerosis.2009.12.023. **The strongest available metaanalysis on the beneficial effects of nicotinic acid in reducing cardiovascular risk.
- 78. Khera AV, Cuchel M, de la Llera-Moya M, et al. Cholesterol efflux capacity, high-density lipoprotein function, and atherosclerosis. N Engl J Med. 2011;364:127–135. doi: 10.1056/NEJMoa1001689. **Strong evidence that the functionality of HDL, independent of HDL-C level, has a strong inverse association with atherosclerotic cardiovascular disease.
- 79.Sarwar N, Danesh J, Eiriksdottir G, et al. Triglycerides and the risk of coronary heart disease: 10,158 incident cases among 262,525 participants in 29 Western prospective studies. Circulation. 2007;115:450–458. doi: 10.1161/CIRCULATIONAHA.106.637793. [DOI] [PubMed] [Google Scholar]
- 80.Miller M, Cannon CP, Murphy SA, et al. Impact of triglyceride levels beyond low-density lipoprotein cholesterol after acute coronary syndrome in the PROVE IT-TIMI 22 trial. J Am Coll Cardiol. 2008;51:724–730. doi: 10.1016/j.jacc.2007.10.038. [DOI] [PubMed] [Google Scholar]
- 81.Sacks FM. After the Fenofibrate Intervention and Event Lowering in Diabetes (FIELD) study: implications for fenofibrate. Am J Cardiol. 2008;102:34L–40L. doi: 10.1016/j.amjcard.2008.09.073. [DOI] [PubMed] [Google Scholar]
- 82.Ginsberg HN, Elam MB, Lovato LC, et al. Effects of combination lipid therapy in type 2 diabetes mellitus. N Engl J Med. 2010;362:1563–1574. doi: 10.1056/NEJMoa1001282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Barter PJ, Rye KA. Is there a role for fibrates in the management of dyslipidemia in the metabolic syndrome? Arterioscler Thromb Vasc Biol. 2008;28:39–46. doi: 10.1161/ATVBAHA.107.148817. [DOI] [PubMed] [Google Scholar]
- 84.Elam M, Lovato LC, Ginsberg H. Role of fibrates in cardiovascular disease prevention, the ACCORD-Lipid perspective. Curr Opin Lipidol. 2011;22:55–61. doi: 10.1097/MOL.0b013e328341a5a8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Cui Y, Blumenthal RS, Flaws JA, et al. Non-high-density lipoprotein cholesterol level as a predictor of cardiovascular disease mortality. Arch Intern Med. 2001;161:1413–1419. doi: 10.1001/archinte.161.11.1413. [DOI] [PubMed] [Google Scholar]
- 86.Jiang R, Schulze MB, Li T, et al. Non-HDL cholesterol and apolipoprotein B predict cardiovascular disease events among men with type 2 diabetes. Diabetes Care. 2004;27:1991–1997. doi: 10.2337/diacare.27.8.1991. [DOI] [PubMed] [Google Scholar]
- 87.Liu J, Sempos C, Donahue RP, et al. Joint distribution of non-HDL and LDL cholesterol and coronary heart disease risk prediction among individuals with and without diabetes. Diabetes Care. 2005;28:1916–1921. doi: 10.2337/diacare.28.8.1916. [DOI] [PubMed] [Google Scholar]
- 88.Ridker PM, Rifai N, Cook NR, et al. Non-HDL cholesterol, apolipoproteins A-I and B100, standard lipid measures, lipid ratios, and CRP as risk factors for cardiovascular disease in women. Jama. 2005;294:326–333. doi: 10.1001/jama.294.3.326. [DOI] [PubMed] [Google Scholar]
- 89.Liu J, Sempos CT, Donahue RP, et al. Non-high-density lipoprotein and very-low-density lipoprotein cholesterol and their risk predictive values in coronary heart disease. Am J Cardiol. 2006;98:1363–1368. doi: 10.1016/j.amjcard.2006.06.032. [DOI] [PubMed] [Google Scholar]
- 90.Yusuf S, Hawken S, Ounpuu S, et al. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): case-control study. Lancet. 2004;364:937–952. doi: 10.1016/S0140-6736(04)17018-9. [DOI] [PubMed] [Google Scholar]
- 91.Shepherd J, Betteridge J, Van Gaal L. Nicotinic acid in the management of dyslipidaemia associated with diabetes and metabolic syndrome: a position paper developed by a European Consensus Panel. Curr Med Res Opin. 2005;21:665–682. doi: 10.1185/030079905x43677. [DOI] [PubMed] [Google Scholar]
- 92.Kearney PM, Blackwell L, Collins R, et al. Efficacy of cholesterol-lowering therapy in 18,686 people with diabetes in 14 randomised trials of statins: a meta-analysis. Lancet. 2008;371:117–125. doi: 10.1016/S0140-6736(08)60104-X. [DOI] [PubMed] [Google Scholar]
- 93.Ridker PM, Danielson E, Fonseca FA, et al. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med. 2008;359:2195–2207. doi: 10.1056/NEJMoa0807646. [DOI] [PubMed] [Google Scholar]
- 94. Ridker PM, Danielson E, Fonseca FA, et al. Reduction in C-reactive protein and LDL cholesterol and cardiovascular event rates after initiation of rosuvastatin: a prospective study of the JUPITER trial. Lancet. 2009;373:1175–1182. doi: 10.1016/S0140-6736(09)60447-5. **Clinical trial evidence for the relationship between inflammation, LDL-C, and cardiovascular events in patients with low LDL-C but elevated hs-CRP.
- 95.Libby P, Ridker PM, Maseri A. Inflammation and atherosclerosis. Circulation. 2002;105:1135–1143. doi: 10.1161/hc0902.104353. [DOI] [PubMed] [Google Scholar]
- 96.Ridker PM. Testing the inflammatory hypothesis of atherothrombosis: scientific rationale for the cardiovascular inflammation reduction trial (CIRT) J Thromb Haemost. 2009;7(Suppl 1):332–339. doi: 10.1111/j.1538-7836.2009.03404.x. [DOI] [PubMed] [Google Scholar]
- 97.Vollmer WM, Sacks FM, Ard J, et al. Effects of diet and sodium intake on blood pressure: subgroup analysis of the DASH-sodium trial. Ann Intern Med. 2001;135:1019–1028. doi: 10.7326/0003-4819-135-12-200112180-00005. [DOI] [PubMed] [Google Scholar]
- 98.Sacks FM, Svetkey LP, Vollmer WM, et al. Effects on blood pressure of reduced dietary sodium and the Dietary Approaches to Stop Hypertension (DASH) diet. DASH-Sodium Collaborative Research Group. N Engl J Med. 2001;344:3–10. doi: 10.1056/NEJM200101043440101. [DOI] [PubMed] [Google Scholar]
- 99.Kelley GA, Kelley KS. Progressive resistance exercise and resting blood pressure : A meta-analysis of randomized controlled trials. Hypertension. 2000;35:838–843. doi: 10.1161/01.hyp.35.3.838. [DOI] [PubMed] [Google Scholar]
- 100.Whelton SP, Chin A, Xin X, He J. Effect of aerobic exercise on blood pressure: a meta-analysis of randomized, controlled trials. Ann Intern Med. 2002;136:493–503. doi: 10.7326/0003-4819-136-7-200204020-00006. [DOI] [PubMed] [Google Scholar]
- 101.Shepherd J, Blauw GJ, Murphy MB, et al. Pravastatin in elderly individuals at risk of vascular disease (PROSPER): a randomised controlled trial. Lancet. 2002;360:1623–1630. doi: 10.1016/s0140-6736(02)11600-x. [DOI] [PubMed] [Google Scholar]
- 102.Sever PS, Dahlof B, Poulter NR, et al. Prevention of coronary and stroke events with atorvastatin in hypertensive patients who have average or lower-than-average cholesterol concentrations, in the Anglo-Scandinavian Cardiac Outcomes Trial--Lipid Lowering Arm (ASCOT-LLA): a multicentre randomised controlled trial. Lancet. 2003;361:1149–1158. doi: 10.1016/S0140-6736(03)12948-0. [DOI] [PubMed] [Google Scholar]
- 103.Major outcomes in moderately hypercholesterolemic, hypertensive patients randomized to pravastatin vs usual care: The Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT-LLT) JAMA. 2002;288:2998–3007. doi: 10.1001/jama.288.23.2998. [DOI] [PubMed] [Google Scholar]
- 104.Knopp RH, d'Emden M, Smilde JG, Pocock SJ. Efficacy and safety of atorvastatin in the prevention of cardiovascular end points in subjects with type 2 diabetes: the Atorvastatin Study for Prevention of Coronary Heart Disease Endpoints in non-insulin-dependent diabetes mellitus (ASPEN) Diabetes Care. 2006;29:1478–1485. doi: 10.2337/dc05-2415. [DOI] [PubMed] [Google Scholar]
- 105.Colhoun HM, Betteridge DJ, Durrington PN, et al. Primary prevention of cardiovascular disease with atorvastatin in type 2 diabetes in the Collaborative Atorvastatin Diabetes Study (CARDS): multicentre randomised placebo-controlled trial. Lancet. 2004;364:685–696. doi: 10.1016/S0140-6736(04)16895-5. [DOI] [PubMed] [Google Scholar]