Abstract
Introduction
Diabetic ulcers are chronic non-healing ulcerations that despite the available medical tools still result in high amputation rates. Growing evidence suggests that alteration of the biochemical milieu of the chronic wound plays a significant role in diabetic wound healing impairment.
Areas covered
The basic pathophysiology and the conventional treatment strategy of diabetic foot ulcers have been reviewed in the first section. In the second part we describe the most up-to-date bench and translational research in the field. The third section focuses on the drugs currently under development and the ongoing clinical trials evaluating their safety and efficacy. Finally, we analyze the major drug development issues and the possible scientific approaches to overcome them.
Expert opinion
Significant strides in understanding the chronic wound development have led to the development of topical therapies to address aberrant expression of growth factors and overexpression of inflammatory cytokines. Current research in our lab suggests that in while decreased growth factor expression occurs at the local wound level, increased systemic serum levels of growth factors suggest growth factor resistance.
1. Background
15% of patients with diabetes mellitus (DM) are expected to develop a Diabetic Foot Ulcer (DFU) within their lifetime.1 With the expected increase of incidence of DM, DFUs will represent an even bigger burden for the health system as it will bear on the economy with an estimated total annual cost of 4 billion dollars in the United States alone.2
Three factors determine the onset of ulcerations: presence of peripheral neuropathy, foot deformities, and acute or chronic repetitive trauma. Once the ulceration develops, the main characteristic of DFUs is the inability to self-repair in a timely and orderly manner.3
2. Medical need
Despite all the available diagnostic and therapeutic tools, DFUs still result in lower extremity amputations in about 15% of the cases.1 Thus, there is a strong medical need in finding the underlying structural and functional abnormalities through which DM impairs wound healing. Basic science and translational investigation are intensively researching the key abnormalities responsible for altering the wound healing process in DM. The goals are to improve the current unsatisfactory clinical outcomes, to ameliorate the prognosis and the quality of life of subjects with DFUs. In order to achieve this, future therapies will probably have to target the impaired microvascular function, the diminished activity of growth factors, cytokines, neuropeptides, and the hypoxic tissue environment.
3. Existing Treatment
3.1 Debridement
Debridement consists in removal of the wound’s necrotic, dysvascular and nonviable tissue in order to obtain a red and granular bed. The removal of all nonviable tissue allows for greater visual assessment of the wound base, and also promotes the release of growth factors by “introducing” an acute wound in a chronic wound.4 There are several debridement techniques described, such as surgical, autolytic, chemical etc. Surgical debridement is the fastest way to debride a wound, but it is not selective because it removes viable tissue as well. Of note, the gold standard for DFU remains sharp debridement with a scalpel blade or a tissue nipper, and to debride to the level of bleeding tissue.5–7
3.2 Pressure off-loading
Reduction of pressures is essential for achieving healing of plantar DFUs since ulcerations occur in high-pressure areas. The most popular techniques are total contact casting (TCC), half shoes, short leg walkers, and felted foam dressings. Among these, TCC is the most effective as measured by the wound healing rate.8 Nevertheless, TCC is not very commonly used principally because of its intrinsic disadvantages (possible secondary skin lesions and inability to daily assess the wound). Other off-loading devices (such as the half shoe and short leg walker) are easier to apply and more accepted by the patient, even though pressure reduction is significantly less compared to TCC and the patient’s compliance cannot be assured. Felted foam dressing, another class of off-loading devices, offers customized pressure relief and, when combined to a surgical shoe or half-shoe, is more effective than a short leg walker or a half-shoe alone.9
3.3 Revascularization
Revascularization is fundamental to restore arterial blood flow to the foot in the presence of peripheral arterial disease (PAD). The treatment of peripheral arterial disease consists in first instance in lifestyle changes (weight loss, cessation of smoking, low fat diet), then in medical therapy (antiplatelet therapy, anticoagulants and low density cholesterol lowering drugs) and if necessary in surgery (angioplasty, endoarterectomy, grafting or by-pass). Of note, revascularization must be performed only after resolution of eventual infection.
3.4 Treatment of infection
In the presence of infection, drainage of purulent collections is a pivotal clinical maneuver. In addition to this, debridement remains imperative as in the non-infected chronic wounds. Broad-spectrum empirical antibiotic therapy should then be immediately started, but always after deep cultures have been performed. Antibiotics -that will then be modified according to the culture data- should be chosen based on the suspected bacterial pathogens, on their toxicity profiles and on their bioenvironmental-economic cost.
3.4 Wound care
Dressings are essential to a clean, moist wound-healing environment and thus preventing tissue dehydration and cell death, accelerating angiogenesis, and facilitating interaction of growth factors with the target cells.10 In this context, there is no randomized, controlled clinical trial that has evaluated the clinical effectiveness of antimicrobial wound products in preventing or eradicating infection.
3.5 Advanced wound care products
Growth factor signaling is altered in diabetic chronic non healing ulcerations. One of the first US Food and Drug Administration (FDA) approved products was Becaplermin, a platelet-derived growth factor-BB recombinant (rhPDGF-BB) capable of decreasing the time to complete wound healing.11 Since patients who use 3 or more tubes of Becaplermin present increased risk for cancer mortality, FDA added a black box warning to the safety labeling of this product.
Living Skin Equivalents (LSE) are another important class of advanced wound care products. Two products are currently approved and available for use in DFUs: composite graft, containing both epidermal and dermal components, named Apligraf (Organogenesis, Inc., Canton, MA, USA, distributed by Novartis Pharmaceutical Corp., East Hanover, NJ, USA) (Table 1) and a graft containing normal dermal matrix proteins and cytokines, named Dermagraft (Advanced Tissue Sciences Inc., La Jolla, CA, USA). Both products have shown to significantly increase the wound-healing rate and to decrease the time to complete wound closure.12, 13 The mechanism by which LSE seem to induce wound healing is by filling the wound, recruiting cells, and inducing the expression of growth factors and cytokines that facilitate extracellular cell matrix deposition. Compared to traditional skin grafting, LSE are noninvasive, do not require anesthesia, can be performed in an outpatient setting, and avoid potential-donor-site complications such as infection and scarring.14
Table 1.
The cytokine expression in Apligraf and human skin
| Human Keratinocytes | Human Dermal Fibroblasts | Apligraf | Human Skin | |
|---|---|---|---|---|
| FGF-1 | + | + | + | + |
| FGF-2 | − | + | + | + |
| FGF-7 | − | + | + | + |
| ECGF | − | + | + | + |
| IGF-1 | − | − | + | + |
| IGF-2 | − | + | + | + |
| PDGF-AB | + | + | + | + |
| TGF-α | + | − | + | + |
| IL-1α | + | − | + | + |
| IL-6 | − | + | + | + |
| IL-8 | − | − | + | + |
| IL-11 | − | + | + | + |
| TGF-β1 | − | + | + | + |
| TGF-β3 | − | + | + | + |
| VEGF | + | − | + | + |
FGF = fibroblast growth factor; ECGF = endothelial cell growth factor; IGF = insulin-like growth factor; PDGF = platelet-derived growth factor; TGF = transforming growth factor; IL = interleukin; VEGF = vascular endothelial growth factor.
3.6 Preventive surgery
Surgery can be required for resolution of underlying infection (such as drainage of purulent collections and resection of osteomyelitis) or correction of biomechanical faults that cause increased pressure points (like Charcot deformities). Prophylactic surgery has been shown to represent a successful strategy in correcting foot deformities prior to the development of DFUs.15
3.7 Negative Pressure
Wound Therapy Negative pressure wound therapy (NPWT) provides an environment of sub-atmospheric pressure. The recommended negative pressure is −125 mmHg (corresponds to the maximum increase in blood flow).16–18 NPWT is safe and effective and its use results in a higher proportion of healed wounds and enhanced healing rates.19 NPWT probably exerts its positive effects on wound healing by reducing the perilesional edema, promoting the delivery of oxygen and nutrients, and by stimulating cellular proliferation.20
3.8 Hyperbaric Oxygen
The goal of Hyperbaric oxygen therapy (HBOT) is to increase the arterial PO2 to about 1500 mmHg through intermittent inhalation of 100% O2 in chambers pressurized to about 2 to 2.5 atmosphere absolute (ATA, 1 ATA is the atmospheric pressure at sea level, equivalent to 101.3 kiloPascals).21 Even though it increases tissue O2 levels, induces angiogenesis, stimulates fibroblasts’ activity and collagen synthesis, and possesses antimicrobial effect,22, 23 evidence on the clinical efficacy of HBOT is still weak. In fact, a randomized placebo-controlled clinical trial showed benefits only 9 to 12 months after treatment completion, raising some doubts on its actual benefits. 24
3.9 Extracorporeal shock wave therapy
Extracorporeal Shock Wave Therapy (ESWT) consists in the application of shock waves to the wound. Shock wave possess tissue regenerative properties on one hand, and both anti-inflammatory and pro-angiogenic effects (through VEGF and necrosis factor kB modulation) on the other.25 In animal models of wound healing ESWT had positive effects on wound size, angiogenesis and inflammation.26, 27 ESWT showed also to reduce wound size, decrease necrotic tissue and increase blood flow in human subjects with venous and diabetic chronic wounds.28
4. Market Review
There are currently three periodic national surveys tracking diabetes prevalence in the United States: the National Health Interview Survey, the National Health and Nutrition Examination Survey (NHANES) and the Behavioral Risk Factor Surveillance System. All three surveys use national population-based samples and in person interviews regarding diabetes diagnosis and history. The NHANES survey differs in its inclusion of laboratory testing to determine undiagnosed diabetes.
The longest running survey, the National Health Interview Survey, found that the diagnosis of diabetes increased from 1.6 million in 1958 to 12.1 million in 2000. This 8-fold increase in diabetes diagnoses occurred across all demographic categories, including sex, race and age. However, minority race and ethnic groups, including Hispanic, Native Americans and African Americans were disproportionately affected, with the prevalence generally 2 to 4 times higher in these groups. Finally the absolute extent of diabetes prevalence may be underestimated, given that the NHANES study found that approximately one third of all persons with diabetes were undiagnosed based on laboratory evaluation.29
Using a dynamic Markov model, Honeycutt et al generated diabetes prevalence in the United States through the year 2050.30 They projected that the number of people diagnosed with diabetes will rise from 12.0 million in 2000 to 39.0 million in 2050 based on data from the U.s. Census Bureau and inputs of estimated diagnosed diabetes prevalence and incidence. This estimate implies an increase prevalence of diabetes from 4.4% in 2000 to 9.7% in 2050.
As previously mentioned, an estimated 15% of patients with diabetes will develop a DFU in their lifetime. On average, patients with DFU are seen on an outpatient basis 14 times per year and hospitalized 1–2 times per year for this condition.31 The economic burden has been estimated to cost 33,000 US dollars per DFU incident with a total annual cost of over 4 billion in 2000. Given the estimated jump in diabetes prevalence from 4.4% in 2000 to 9.7% in 2050, a conservative extrapolation of the economic burden on the healthcare system of 8 billion dollars for the treatment of DFU by the year 2050 appears reasonable.
5. Current research goals
A search of “diabetic foot” on ClinicalTrials.gov reveals 275 studies listed for the treatment of DFU. These studies are varied and include evaluation of treatment of soft tissue infections and osteomyelitis in diabetes, the influence of sleep apnea on DFU, and modalities to assess and predict the development of DFU. However, the majority of studies listed involved the treatment of chronic DFU with novel preparations designed to address the altered biochemical composition of the chronic wound. These studies likely reflect the increasing evidence that deficiencies in secretion of neuropeptides and growth factors in DFU play a substantial role in their failure to heal. The majority of these studies are in the preliminary stages of investigation, evaluating for efficacy, safety and proof of concept regarding the effectiveness of these compounds.
5.1 Cell- based Therapies
Cell therapy is an emerging modality for enhancing DFUs. Even though stem cells, keratinocytes and fibroblasts have been widely studied for the treatment of chronic wounds, there is little data available on their potential role in diabetic wounds. Stem cells have been tested in animal models of diabetic wound healing. In a study, diabetic mice with wounds that were injected with CD34+ cells healed significantly faster and showed increased revascularization when compared to the non-diabetic controls.32 DM impairs Endothelial Progenitor Cells (EPCs) function, namely proliferation, adhesion, and incorporation into vascular structures.33 Since EPC play a pivotal role in wound healing, they have become an attractive stem cell candidate for the treatment of DFU.34
5.2 Gene Therapy
Gene therapy has not been clinically approved yet for the treatment of wound healing. Since growth factor therapy presents intrinsic obstacles (such as short half-life in the wound environment, large amounts of purified recombinant material needed, and toxicity associated with repetitive doses), a possible strategy is that of reprogramming somatic cells in order to induce the synthesis and secretion of growth factors necessary for mimicking the physiologic healing process. One possible methodology is to modify genetically cells in vitro (via viral vectors, liposomes, or naked DNA) and to subsequently transplant these autologous cells in to the host tissue. In a mouse model of diabetic wounding, lentiviral PDGF treated animals had enhanced angiogenesis and collagen deposition, even though re-epithelialization was not different among the study groups.35 In another mouse model, local injection of plasmid expressing TGF-beta-1 with subsequent electrical stimulation promoted wound healing.36 Another study showed that injecting an adenovirus encoding VEGF-C in the dermis surrounding the wound of diabetic mice accelerated healing and enhanced angiogenesis and lymphangiogenesis.37
5.4 Endothelial Progenitor Cells
Circulating Endothelial Progenitor Cells (EPCs) represent a biomarker of vascular function and cardiovascular risk.38 EPCs also contribute to neovascularization during atherosclerosis,39 postmyocardial infarction, 40 endothelialization of vascular grafts,41 wound healing, limb ischemia,42–44 and other clinical conditions.45, 46,47 Trauma or ischemia induce the bone marrow recruitment of EPCs, subsequently these cells are homed to the sites of neovascularization. The possible role of EPCs in DFUs is currently under intensive investigation.
5.5 Protein Tyrosine Phosphatase (PTP) 1B
PTP1B is ubiquitously expressed and localizes to the endoplasmic reticulum.48 Intense research over the last decade by us and others has shown PTP1B negatively regulates insulin and leptin signaling and sensitivity, as a phosphatase of insulin receptor and leptin receptor-associated kinase JAK2.49–51 Though a role for PTP1B in DFU has not been reported, PTP1B is abundantly expressed in many cell types and tissues affected by DFU, namely neurons,50 skin,52 vascular smooth muscle53 and endothelial progenitor cells. 54 Beyond regulating insulin and leptin, PTP1B plays a detrimental role in the signaling regulation of a broad network of growth factors that mediate wound healing, including vascular endothelial growth factor (VEGF), epidermal growth factor (EGF), platelet derived growth factor (PDGF), nerve growth factor (NGF), and TGFβ, among others. 55–59 Importantly, PTP1B is identified as a negative regulator of vascular remodeling after injury, 60 corneal wound healing, 61 and matrix remodeling, 62 suggesting PTP1B may regulate multiple aspects of wound healing.
6. Scientific rationale
6.1 Neuropeptides
Peripheral nerves and cutaneous neurobiology contribute to normal wound healing by sustaining a bidirectional connection between the nervous and immune systems. Diabetic peripheral neuropathy (DPN) is known to impair these signaling pathways, contributing to chronic wounds and ulcers.63 DPN is believed to play a fundamental role in the pathogenesis of DFU. Moreover, it is thought that peripheral neuropathy causes the onset of wounds by allowing prolonged tissue injury secondary to pain insensitivity. Our unit and other groups have showed that the impaired secretion of neuropeptides by the C-nociceptive fibers, secondary to neuropathy, influences impairment of wound healing. This could represent the major reason that leads to the development of chronic DFU. The neuropeptides involved in wound healing include SP, NPY, Calcitonin gene related peptide (CGRP), corticotropin-releasing factor (CSF), a- Melanocyte Stimulating Hormone (MSH) and NT.64–69 SP is broadly distributed in the central and peripheral nervous system systems (CNS and PNS, respectively)70–72 and noxious stimuli determine its release from neurons. SP promotes vasodilatation, leukocyte chemotaxis and leukocyte–endothelial-cell adhesion, and therefore subsequent leukocyte extravasation, migration and accumulation at sites of injury.73 Diabetes reduces SP-positive nerve fibers74 and through this impairs the wound repairing process. NPY stimulates angiogenesis through EC proliferation and migration,75 and plays an important role in the inflammatory and angiogenic phases of wound healing. Diabetes exerts a detrimental effect on NPY levels in the skin.76 In an animal model of diabetes in fact the deletion of one of its receptors (Y2) results in blockage of NPY-induced angiogenesis and delayed wound healing.66 CGRP derives from the alternative splicing of the calcitonin gene. CGRP exerts both vasolidatatory and angiogeneic effects.77 Diabetes decreases expression, release and action of CGRP in humans. It has been observed that CGRP-mediated vasodilation is significantly reduced in diabetic rats with improved vascular and neural function following treatment with a vasopeptidase inhibitor.78
6.2 Chronic Inflammation
Type 1 and type 2 diabetes present dysregulation of pro- and anti-inflammatory cytokines that result in impaired tissue repair and weakened cellular and humoral immune mechanisms.79, 80 Human and experimental diabetes, are characterized by high susceptibility and severity of infections due in part to defects of chemotactic, phagocytic and microbiocidal activities of neutrophils.81, 82 Patients with DFU infections present impaired immunological responses, such as neutrophil dysfunction and IL-1b dysregulation,83 decreased immune cell infiltration, and persistence of neutrophils and macrophages.84 Moreover, recently a large prospective study in our unit has demonstrated that the diabetes-induced proinflammatory state predisposes neuropathic patients to impaired wound healing despite increased levels of circulating growth factors.85
6.3 Peripheral arterial disease
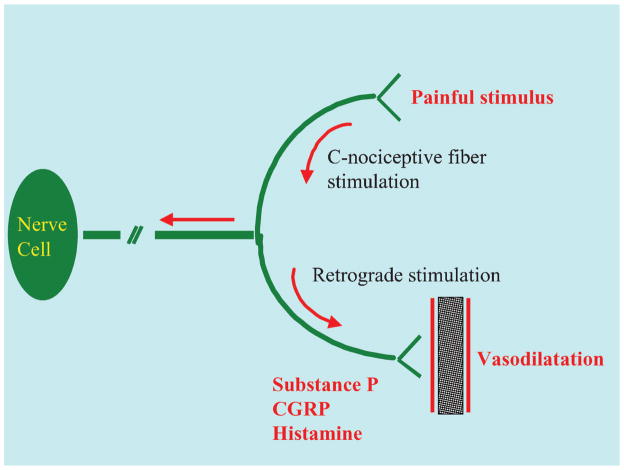
DM is characterized by micro and macro-circulatory alterations. In microvascular disease, DM determines basement membrane thickening and impairs the function of the microcirculation. DM induces in fact dysfunction of endothelium, smooth muscle cells and of the Lewis triple-flare response (also known as Nerve Axon Reflex, NARV) (Figure 1). Peripheral neuropathy reduces the vessel dilatatory response to stress and trauma, producing functional ischemia and impaired wound healing in diabetes. 86, 87
Figure 1.
The nerve–axon reflex (also known as Lewis triple-flare response). Injury or inflammation stimulate the C-nociceptive fibers and cause retrograde stimulation of the adjacent fibers that release active vasodilators (such as histamine, SP and CGRP). This results finally in hyperemic reaction during stress.
Macrovascular disease in diabetes is characterized by the anatomic location. In DM in fact, macrovascular disease tends to be located at the posterior and anterior tibial and the peroneal arteries.88 Revascularization of the large vessels is commonly performed to address non-healing DFUs with good success. However, DM patients with DFU and neuropathy may still fail to heal their ulcer since the microcirculation is not completely restored by revascularization.89
6.4 Muscle metabolism
DM induces tissue hypoxia even in the absence of PAD and this may be related to impaired wound healing. In the muscle adenosine triphosphate (ATP) is maintained at a steady state level through the creatine kinase reaction, as long as the supply of oxygen and other nutrients are sufficient to maintain the Phosphocreatine (PCr) reserve.90 Our group has also demonstrated that alterations in muscle metabolism and tissue hypoxia are present in the foot muscles of diabetic patients with or without neuropathy in the absence of PAD.90–94
6.5 Skin Oxygenation
Skin oxygenation of diabetic patients with neuropathy has been shown to be reduced and has been implicated as a potential cause of impaired wound healing. Hyperspectral Imaging (MHSI) is a spectroscopic technique that is capable of measuring wound oxygenation as well as possessing the potential to predict future areas of DFU.91 Our group found changes in oxygen delivery or extraction and lower oxygen saturation levels in the foot skin of diabetic patients with neuropathy.91
7. Competitive environment
Growing evidence suggests that alteration of the biochemical milieu of the chronic wound plays a significant role in delayed wound healing. A number of studies have evaluated the influence of growth factor supplementation on wound healing, in conjunction with good wound care practices. As a result of observed accelerated wound healing with growth factor treatment, continued investigation into modulation of the chronic wound environment on healing remains a strong focus of current research efforts.
7.1 Growth Factors
PDGF is the most widely studied growth factor in wound healing and is currently approved for clinical use in a recombinant DNA technology. The effectiveness of PDGF was initially studied in chronic diabetic foot ulcers with ulcers treated with the highest dose of PDGF showing the greatest amount of wound closure.95 However, it was also noted that the addition of PDGF to ulcer care only accelerated wound healing when good wound care was also present. Namely, debridement appeared to be instrumental in the healing process.
The success of PDGF treated wounds resulted in exploration of other growth factors on wound healing. These factors have included VEGF, EGF, FGF, KGF and IGF. Some of these factors have showed promise in preliminary studies in wound healing, but their development into the clinical market it still in the early stages as evidenced by the Competitive Environment table.
Despite early positive effects of growth factor treatment of diabetic foot ulcers, recent evidence from our lab showed serum levels of growth factors to be equivalent in patients with foot ulcers that healed and patients with foot ulcers that failed to heal.85 Furthermore, growth factor serum levels were actually lower in patients with foot ulcers that went on to heal, suggesting that growth factor resistance may play in failure of wounds to heal even when supplemented with exogenous growth factors.
In the same study, the main factors associated with failure to heal an ulcer included increased expression of inflammatory cytokines such as TNFα, G-SF, GRO, MCP-1 and leptin. It should also be noted that these increases were present in the serum samples collected well before the development of the foot ulcer. This finding suggests that higher serum concentrations are not secondary to the healing process of an existing ulcer, suggesting that a pre-existing low-grade proinflammatory state, known to be pivotal in metabolic and cardiovascular disease, exerts a major role in the healing impairment of DFUs.
7.2 Platelet Rich Plasma (PRP)
PRP is a bioactive component of whole blood with an enhanced concentration of platelets compared to baseline blood. The abundance of platelets allows for the release of large quantities of growth factors when stimulated by thrombin or calcium. The benefits of PRP have been observed and explored in a number of pathologies, including tendon injuries, bone healing and wound healing.
In a systematic review in 2010, Villela et al examined the use of PRP on chronic leg wounds. Of the 18 studies identified, 7 were clinical and randomized, with 5 of those studies evaluating PRP in chronic diabetic foot ulcers 96. Meta-analysis of the results showed favorable healing with the PRP therapy. When PRP treated diabetic foot ulcers were compared directly with those treated with PPP (Platelet Poor Plasma), the PRP treated group demonstrated significantly increased wound healing 97.
It is important to bear in mind that not all growth factors confer healing properties. Instead, some growth factors will signal an end to wound healing. It is unclear if PRP concentrates growth factors that potentially stimulate healing or incorporate growth factors that will terminate the would healing process. Thus, the role of PRP in wound healing remains indeterminate.
7.3 Neuropeptides
A link between wound healing and the nervous system is evident by the peripheral neuropathy connecting the two and has emerged as a focal point in new treatments. Neuropeptides and the cytokines released from nerve fibers, immune cells, and cutaneous cells have emerged as a significant player in the wound healing process. Alterations in neuropeptide levels have been observed in patients with diabetes, with increased levels noted in the hypothalamus and decreased levels in skin.
Recently, investigation into a topically applied angiotensin analog demonstrated increased wound healing in patients with diabetic foot ulcers compared to control.98 Furthermore, the topical treatment showed no significant side effects with increased rate of healing. This improved healing appears to be dose dependent with the higher 0.03% concentration showed greater healing when compared to the 0.01% formulation.
Competitive Environment Table
| Compound | Company | Structure | Indication | Stage of development | Mechanism of action |
|---|---|---|---|---|---|
| Connexin43 | CoDa Therapeutics | Cx43-specific antisense oligodeoxynucleotide | Venous ulcers, DFU | Phase 2 | downregulation of Cx43 protein |
| DSC127 | Derma Sciences Inc. | Angiotensin analog | Venous ulcers, pressure ulcers, DFU | Phase 2 | Up-regulation of mesenchymal stem cells |
| Oral BBR-012 | Bridge BioResearch Ltd. | Isoniazide | DFU | Phase 2 | P450 inhibitor |
| TRAfermin | Olympus Biotech Corporation | bFGF | DFU | Phase 3 | Growth factor |
| Platelet Rich Fibrin(PRF) | Vivostat | Platelet concentrate in fibrin sealant | DFU | Phase 4 | Vascular ingrowth promotion |
| Controlled nitric oxide releasing patch | Fundación Cardiovascular de Colombia | Topical nitric oxide | DFU | Phase 3 | Collagen synthesis, angiogenesis, |
| rhEGF(recombinant human Epidermal Growth Factor) | Daewoong Pharmaceutical Co. | rhEGF | DFU | Phase 3 | Growth factor |
| KUR-211 | Kuros Biosurgery AG | modified variant of platelet-derived growth factor (PDGF) | DFU | Phase 2 | Growth factor |
| Galnobax | Novalead Pharma Private Limited | Esmolol hydrochloride | DFU | Phase 1 | short-acting beta-1 adrenergic receptor blocker |
| LeucoPatch | Reapplix | autologous platelet- rich fibrin | Chronic wounds | Phase 4 | Growth factors |
| HO/03/03 | HealOr | PKC-modulating agents | Chronic wounds | Phase 3 | PKCa activation and PKCd inhibition |
| MRE0094 | King Pharmaceuticals | Adenosine A2A receptor agonist | DFU | Phase 2 | Inflammatory cell mediator |
| AMD3100 | Genzyme | Plerixafor | DFU | Phase 1 | Mobilization of endothelial progenitor cells |
| Woulgan biogel | Biotec Pharmacon ASA | Soluble beta-glucan | DFU | Phase 3 | Wound immunomodulating properties |
8. Potential development issues
8.1 Complex molecular events
Understanding the complex molecular events underlying diabetic foot ulcer healing remains in its infancy. Through animal models and skin samples, we have been able to understand the key molecules involved as well as their over or under-expression in impaired wound healing. However, the complexity of their interactions, and the key elements necessary for successful wound healing has limited the effectiveness of current treatments.
8.2 Delivery of therapeutic agents
The sustained delivery of therapeutically active agents topically to the ulcer site has proven to be a hurdle since growth factors are rapidly degraded once secreted. For example, the biologic half-lives of PDGF and VEGF are 2 minutes and 50 minutes, respectively 99, 100. As a result, therapeutic application of growth factors at sustained levels may be difficult to achieve. It has been proposed that failure of some clinical trials may have been the result of failure of the delivery systems used to allow the peptides to reach their target cells and tissues. Furthermore, it has been speculated that growth factor treatment is in part impaired by the inflammatory environment of the wound,, resulting in breakdown of these peptides before they may act.
Development of systems such as gene delivery and polymers, may allow for sustained and effective delivery of therapeutically active agents to the chronic wound. Additionally, major advances in tissue engineering hold the promise of delivering matrix materials and live cells that may well provide the right combination and concentration of growth factors necessary for wound healing.
9. Conclusions
By the year 2050, it is predicted that as much as 10% of the general population will have been diagnosed with DM and 15% will be expected to develop a DFU in their lifetime. The cost of treating DFU weighs heavily on the US medical system, with an annual cost of 4 billion USD. In addition to the cost, the fact that 85% of lower extremity amputations are preceeded by a DFU is an alarming call to action.
Current treatment of DFU includes periodic debridement, infection treatment, pressure-offloading and good wound care. This gold standard has been successful in the management of most DFU, however, there is a subset of patients who fail to heal their DFU despite this gold standard model of wound care. In these patients, basic science and translational investigation has revealed impaired microvascular function, diminished activity of growth factors, neuropeptides, and the hypoxic tissue environment as possible obstacles to wound healing.
Continued research has lead to the development of wound care therapies to address the altered biochemical milieu of the chronic wound. Currently, single growth factor supplementation (PDGF) applied topically to the wound is the only FDA approved therapy for DFU and has been met with only modest success. Additionally, clinical trials with other growth factors have showed variable success rates, suggesting that problems may exist with the delivery systems, or questioning the need for better modulation of the wound environment with multiple growth factors or cytokine inhibitors.
10. Expert Opinion
The startling rise of type 2 Diabetes makes research into secondary complications such as DFU all the more essential given that the cost to the medical system in the US alone is expected to reach 8 billion by 2050. Regarding lower extremity problems, the key findings in impaired healing of DFU include decreased expression of growth factors and increased levels of inflammatory cytokines. This altered molecular environment of DFU represents a pro-inflammatory state that may be responsible for ulcers that fail to heal.
Weaknesses in DFU wound healing research include the still evolving understanding of the pathophysiology of the chronic wound. While the research is progressing at a rapid clip, a clear understanding of the exact barriers in wound healing in DFU remains elusive. Another area of weakness includes the delivery of topical treatments in an effective manner. For example, the application of a single growth factor (PDGF) to chronic wounds has increased healing DFU, but only to a modest degree. However, there is no tangible measurement of whether the growth factor is reaching the target cells and in a timely fashion.
The ultimate goal in the field of DFU research is to understand fully the mechanisms behind impaired wound healing. Once this altered process is fully delineated and the hurdles recognized, proper assessment and treatment can be provided. As the collaborative efforts between the fields of bioengineering and medicine advance, new therapies using technology such as gene therapy, novel polymer delivery systems, and tissue matrixes will play an essential role in ensuring adequate and efficient delivery of topical therapies.
The recent work from our lab showing increased serum growth factors suggesting growth factor resistance reveals that even with sufficient levels for wound healing, there are other underlying mechanisms that require further investigation. In particular, the role of PTP1B is of considerable interest to our group and the research into PTP1B inhibition treatment of type 2 DM is being closely monitored.
Acknowledgments
This work was supported by National Institutes of Health Grants R01-NS046710, R01-DK076937 and R01 NS066205 to AV.
Contributor Information
Francesco Tecilazich, Research Fellow, Microcirculation Lab. and Joslin-Beth Israel Deaconess Foot Center, Harvard Medical School, Boston, MA, USA.
Thanh L. Dinh, Division of Podiatry, Beth Israel Deaconess Medical Center, Assistant Professor in Surgery, Harvard Medical School, Boston, MA, USA
Aristidis Veves, Research Director, Microcirculation Lab. and Joslin-Beth Israel Deaconess Foot Center, Professor of Surgery, Harvard Medical School, Boston, MA, USA
References
- 1.Ramsey SD, Newton K, Blough D, McCulloch DK, et al. Incidence, outcomes, and cost of foot ulcers in patients with diabetes. Diabetes Care. 1999;22:382–387. doi: 10.2337/diacare.22.3.382. [DOI] [PubMed] [Google Scholar]
- 2.Apelqvist J, Bakker K, van Houtum WH, Nabuurs-Franssen MH, Schaper NC. International consensus and practical guidelines on the management and the prevention of the diabetic foot. International working group on the diabetic foot. Diabetes Metab Res Rev. 2000;16 (Suppl 1):S84–92. doi: 10.1002/1520-7560(200009/10)16:1+<::aid-dmrr113>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 3.Lazarus GS, Cooper DM, Knighton DR, Margolis DJ, et al. Definitions and guidelines for assessment of wounds and evaluation of healing. Arch Dermatol. 1994;130:489–493. [PubMed] [Google Scholar]
- 4.Panuncialman J, Falanga V. The science of wound bed preparation. Surg Clin North Am. 2009;89:611–626. doi: 10.1016/j.suc.2009.03.009. [DOI] [PubMed] [Google Scholar]
- *5.Steed DL, Donohoe D, Webster MW, Lindsley L. Effect of extensive debridement and treatment on the healing of diabetic foot ulcers. Diabetic ulcer study group. J Am Coll Surg. 1996;183:61–64. This landmark study established the importance of aggressive debridement in the wound healing of diabetic foot ulcers. [PubMed] [Google Scholar]
- 6.Falanga V. Wound healing and its impairment in the diabetic foot. Lancet. 2005;366:1736–1743. doi: 10.1016/S0140-6736(05)67700-8. [DOI] [PubMed] [Google Scholar]
- 7.Falanga V, Sabolinski M. A bilayered living skin construct (apligraf) accelerates complete closure of hard-to-heal venous ulcers. Wound Repair Regen. 1999;7:201–207. doi: 10.1046/j.1524-475x.1999.00201.x. [DOI] [PubMed] [Google Scholar]
- 8.Armstrong DG, Nguyen HC, Lavery LA, van Schie CH, et al. Off-loading the diabetic foot wound: A randomized clinical trial. Diabetes Care. 2001;24:1019–1022. doi: 10.2337/diacare.24.6.1019. [DOI] [PubMed] [Google Scholar]
- 9.Birke JAFB, Krieger LA, Sliman K. The effectiveness of an accommodative dressing in offloading pressure over areas of previous metatarsal head ulceration. Wounds. 2003;15:33–39. [Google Scholar]
- 10.Field FK, Kerstein MD. Overview of wound healing in a moist environment. Am J Surg. 1994;167:2S–6S. doi: 10.1016/0002-9610(94)90002-7. [DOI] [PubMed] [Google Scholar]
- 11.Wieman TJ, Smiell JM, Su Y. Efficacy and safety of a topical gel formulation of recombinant human platelet-derived growth factor-bb (becaplermin) in patients with chronic neuropathic diabetic ulcers. A phase iii randomized placebo-controlled double-blind study. Diabetes Care. 1998;21:822–827. doi: 10.2337/diacare.21.5.822. [DOI] [PubMed] [Google Scholar]
- **12.Veves A, Falanga V, Armstrong DG, Sabolinski ML. Graftskin, a human skin equivalent, is effective in the management of noninfected neuropathic diabetic foot ulcers: A prospective randomized multicenter clinical trial. Diabetes Care. 2001;24:290–295. doi: 10.2337/diacare.24.2.290. This study established the effectiveness of bioengineered skin tissue as an adjunct to established diabetic foot wound management. [DOI] [PubMed] [Google Scholar]
- 13.Gentzkow GD, Iwasaki SD, Hershon KS, Mengel M, et al. Use of dermagraft, a cultured human dermis, to treat diabetic foot ulcers. Diabetes Care. 1996;19:350–354. doi: 10.2337/diacare.19.4.350. [DOI] [PubMed] [Google Scholar]
- 14.Muhart M, McFalls S, Kirsner RS, Elgart GW, et al. Behavior of tissue-engineered skin: A comparison of a living skin equivalent, autograft, and occlusive dressing in human donor sites. Arch Dermatol. 1999;135:913–918. doi: 10.1001/archderm.135.8.913. [DOI] [PubMed] [Google Scholar]
- 15.Mueller MJ, Sinacore DR, Hastings MK, Strube MJ, et al. Effect of achilles tendon lengthening on neuropathic plantar ulcers. A randomized clinical trial. J Bone Joint Surg Am. 2003;85-A:1436–1445. [PubMed] [Google Scholar]
- 16.Morykwas MJ, Faler BJ, Pearce DJ, Argenta LC. Effects of varying levels of subatmospheric pressure on the rate of granulation tissue formation in experimental wounds in swine. Ann Plast Surg. 2001;47:547–551. doi: 10.1097/00000637-200111000-00013. [DOI] [PubMed] [Google Scholar]
- 17.Nather A, Chionh SB, Han AY, Chan PP, et al. Effectiveness of vacuum-assisted closure (vac) therapy in the healing of chronic diabetic foot ulcers. Ann Acad Med Singapore. 2010;39:353–358. [PubMed] [Google Scholar]
- 18.Eginton MT, Brown KR, Seabrook GR, Towne JB, et al. A prospective randomized evaluation of negative-pressure wound dressings for diabetic foot wounds. Ann Vasc Surg. 2003;17:645–649. doi: 10.1007/s10016-003-0065-3. [DOI] [PubMed] [Google Scholar]
- 19.Armstrong DG, Lavery LA. Negative pressure wound therapy after partial diabetic foot amputation: A multicentre, randomised controlled trial. Lancet. 2005;366:1704–1710. doi: 10.1016/S0140-6736(05)67695-7. [DOI] [PubMed] [Google Scholar]
- 20.Ubbink DT, Westerbos SJ, Evans D, Land L, et al. Topical negative pressure for treating chronic wounds. Cochrane Database Syst Rev. 2008:CD001898. doi: 10.1002/14651858.CD001898.pub2. [DOI] [PubMed] [Google Scholar]
- 21.Wang C, Schwaitzberg S, Berliner E, Zarin DA, et al. Hyperbaric oxygen for treating wounds: A systematic review of the literature. Arch Surg. 2003;138:272–279. doi: 10.1001/archsurg.138.3.272. discussion 280. [DOI] [PubMed] [Google Scholar]
- 22.Knighton DR, Halliday B, Hunt TK. Oxygen as an antibiotic. A comparison of the effects of inspired oxygen concentration and antibiotic administration on in vivo bacterial clearance. Arch Surg. 1986;121:191–195. doi: 10.1001/archsurg.1986.01400020077009. [DOI] [PubMed] [Google Scholar]
- 23.Hunt TK, Pai MP. The effect of varying ambient oxygen tensions on wound metabolism and collagen synthesis. Surg Gynecol Obstet. 1972;135:561–567. [PubMed] [Google Scholar]
- **24.Londahl M, Katzman P, Nilsson A, Hammarlund C. Hyperbaric oxygen therapy facilitates healing of chronic foot ulcers in patients with diabetes. Diabetes Care. 2010;33:998–1003. doi: 10.2337/dc09-1754. This was the first double-blinded, controlled, prospective, randomized study on the effects of hyperbaric oxygen therapy on chronic diabetic foot ulcers. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang FS, Wang CJ, Chen YJ, Chang PR, et al. Ras induction of superoxide activates erk-dependent angiogenic transcription factor hif-1alpha and vegf-a expression in shock wave-stimulated osteoblasts. J Biol Chem. 2004;279:10331–10337. doi: 10.1074/jbc.M308013200. [DOI] [PubMed] [Google Scholar]
- 26.Stojadinovic A, Elster EA, Anam K, Tadaki DA, et al. Angiogenic response to extracorporeal shock wave treatment in murine skin isografts. Angiogenesis. 2008;11:369–380. doi: 10.1007/s10456-008-9120-6. [DOI] [PubMed] [Google Scholar]
- 27.Oi K, Fukumoto Y, Ito K, Uwatoku T, Abe K, et al. Extracorporeal shock wave therapy ameliorates hindlimb ischemia in rabbits. Tohoku J Exp Med. 2008;214:151–158. doi: 10.1620/tjem.214.151. [DOI] [PubMed] [Google Scholar]
- 28.Saggini R, Figus A, Troccola A, Cocco V, et al. Extracorporeal shock wave therapy for management of chronic ulcers in the lower extremities. Ultrasound Med Biol. 2008;34:1261–1271. doi: 10.1016/j.ultrasmedbio.2008.01.010. [DOI] [PubMed] [Google Scholar]
- 29.Harris MI, Flegal KM, Cowie CC, Eberhardt MS, et al. Prevalence of diabetes, impaired fasting glucose, and impaired glucose tolerance in u.S. Adults. The third national health and nutrition examination survey, 1988–1994. Diabetes Care. 1998;21:518–524. doi: 10.2337/diacare.21.4.518. [DOI] [PubMed] [Google Scholar]
- 30.Honeycutt AA, Boyle JP, Broglio KR, Thompson TJ, et al. A dynamic markov model for forecasting diabetes prevalence in the united states through 2050. Health Care Manag Sci. 2003;6:155–164. doi: 10.1023/a:1024467522972. [DOI] [PubMed] [Google Scholar]
- 31.Margolis DJ, Malay DS, Hoffstad OJ, Leonard CE, et al. Data points publication series. Rockville (MD): 2011. Economic burden of diabetic foot ulcers and amputations: Data points #3. [PubMed] [Google Scholar]
- *32.Sivan-Loukianova E, Awad OA, Stepanovic V, Bickenbach J, Schatteman GC. Cd34+ blood cells accelerate vascularization and healing of diabetic mouse skin wounds. J Vasc Res. 2003;40:368–377. doi: 10.1159/000072701. This study established the role of endothelial progenitor cells in revascularization of wounds and enhanced wound healing. [DOI] [PubMed] [Google Scholar]
- 33.Tepper OM, Galiano RD, Capla JM, Kalka C, et al. Human endothelial progenitor cells from type ii diabetics exhibit impaired proliferation, adhesion, and incorporation into vascular structures. Circulation. 2002;106:2781–2786. doi: 10.1161/01.cir.0000039526.42991.93. [DOI] [PubMed] [Google Scholar]
- 34.Bauer SM, Goldstein LJ, Bauer RJ, Chen H, et al. The bone marrow-derived endothelial progenitor cell response is impaired in delayed wound healing from ischemia. J Vasc Surg. 2006;43:134–141. doi: 10.1016/j.jvs.2005.08.038. [DOI] [PubMed] [Google Scholar]
- 35.Lee JA, Conejero JA, Mason JM, Parrett BM, et al. Lentiviral transfection with the pdgf-b gene improves diabetic wound healing. Plast Reconstr Surg. 2005;116:532–538. doi: 10.1097/01.prs.0000172892.78964.49. [DOI] [PubMed] [Google Scholar]
- 36.Lee PY, Chesnoy S, Huang L. Electroporatic delivery of tgf-beta1 gene works synergistically with electric therapy to enhance diabetic wound healing in db/db mice. J Invest Dermatol. 2004;123:791–798. doi: 10.1111/j.0022-202X.2004.23309.x. [DOI] [PubMed] [Google Scholar]
- 37.Saaristo A, Tammela T, Farkkila A, Karkkainen M, et al. Vascular endothelial growth factor-c accelerates diabetic wound healing. Am J Pathol. 2006;169:1080–1087. doi: 10.2353/ajpath.2006.051251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Werner N, Kosiol S, Schiegl T, Ahlers P, et al. Circulating endothelial progenitor cells and cardiovascular outcomes. N Engl J Med. 2005;353:999–1007. doi: 10.1056/NEJMoa043814. [DOI] [PubMed] [Google Scholar]
- 39.Sata M, Saiura A, Kunisato A, Tojo A, et al. Hematopoietic stem cells differentiate into vascular cells that participate in the pathogenesis of atherosclerosis. Nat Med. 2002;8:403–409. doi: 10.1038/nm0402-403. [DOI] [PubMed] [Google Scholar]
- 40.Orlic D, Kajstura J, Chimenti S, Jakoniuk I, et al. Bone marrow cells regenerate infarcted myocardium. Nature. 2001;410:701–705. doi: 10.1038/35070587. [DOI] [PubMed] [Google Scholar]
- 41.Kaushal S, Amiel GE, Guleserian KJ, Shapira OM, et al. Functional small-diameter neovessels created using endothelial progenitor cells expanded ex vivo. Nat Med. 2001;7:1035–1040. doi: 10.1038/nm0901-1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kalka C, Masuda H, Takahashi T, Kalka-Moll WM, et al. Transplantation of ex vivo expanded endothelial progenitor cells for therapeutic neovascularization. Proc Natl Acad Sci U S A. 2000;97:3422–3427. doi: 10.1073/pnas.070046397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Majka SM, Jackson KA, Kienstra KA, Majesky MW, et al. Distinct progenitor populations in skeletal muscle are bone marrow derived and exhibit different cell fates during vascular regeneration. J Clin Invest. 2003;111:71–79. doi: 10.1172/JCI16157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Takahashi T, Kalka C, Masuda H, Chen D, et al. Ischemia- and cytokine-induced mobilization of bone marrow-derived endothelial progenitor cells for neovascularization. Nat Med. 1999;5:434–438. doi: 10.1038/7434. [DOI] [PubMed] [Google Scholar]
- 45.Grant MB, May WS, Caballero S, Brown GA, et al. Adult hematopoietic stem cells provide functional hemangioblast activity during retinal neovascularization. Nat Med. 2002;8:607–612. doi: 10.1038/nm0602-607. [DOI] [PubMed] [Google Scholar]
- 46.Otani A, Kinder K, Ewalt K, Otero FJ, et al. Bone marrow-derived stem cells target retinal astrocytes and can promote or inhibit retinal angiogenesis. Nat Med. 2002;8:1004–1010. doi: 10.1038/nm744. [DOI] [PubMed] [Google Scholar]
- 47.Lyden D, Hattori K, Dias S, Costa C, et al. Impaired recruitment of bone-marrow-derived endothelial and hematopoietic precursor cells blocks tumor angiogenesis and growth. Nat Med. 2001;7:1194–1201. doi: 10.1038/nm1101-1194. [DOI] [PubMed] [Google Scholar]
- 48.Frangioni JV, Beahm PH, Shifrin V, Jost CA, et al. The nontransmembrane tyrosine phosphatase ptp-1b localizes to the endoplasmic reticulum via its 35 amino acid c-terminal sequence. Cell. 1992;68:545–560. doi: 10.1016/0092-8674(92)90190-n. [DOI] [PubMed] [Google Scholar]
- 49.Dube N, Tremblay ML. Involvement of the small protein tyrosine phosphatases tc-ptp and ptp1b in signal transduction and diseases: From diabetes, obesity to cell cycle, and cancer. Biochim Biophys Acta. 2005;1754:108–117. doi: 10.1016/j.bbapap.2005.07.030. [DOI] [PubMed] [Google Scholar]
- 50.Zabolotny JM, Bence-Hanulec KK, Stricker-Krongrad A, Haj F, et al. Ptp1b regulates leptin signal transduction in vivo. Dev Cell. 2002;2:489–495. doi: 10.1016/s1534-5807(02)00148-x. [DOI] [PubMed] [Google Scholar]
- 51.Cheng A, Uetani N, Simoncic PD, Chaubey VP, et al. Attenuation of leptin action and regulation of obesity by protein tyrosine phosphatase 1b. Dev Cell. 2002;2:497–503. doi: 10.1016/s1534-5807(02)00149-1. [DOI] [PubMed] [Google Scholar]
- 52.Gunaratne P, Stoscheck C, Gates RE, Li L, et al. Protein tyrosyl phosphatase-1b is expressed by normal human epidermis, keratinocytes, and a-431 cells and dephosphorylates substrates of the epidermal growth factor receptor. J Invest Dermatol. 1994;103:701–706. doi: 10.1111/1523-1747.ep12398566. [DOI] [PubMed] [Google Scholar]
- 53.Sreejayan N, Lin Y, Hassid A. No attenuates insulin signaling and motility in aortic smooth muscle cells via protein tyrosine phosphatase 1b-mediated mechanism. Arterioscler Thromb Vasc Biol. 2002;22:1086–1092. doi: 10.1161/01.atv.0000020550.65963.e9. [DOI] [PubMed] [Google Scholar]
- 54.Trop S, Tremblay ML, Bourdeau A. Modulation of bone marrow-derived endothelial progenitor cell activity by protein tyrosine phosphatases. Trends Cardiovasc Med. 2008;18:180–186. doi: 10.1016/j.tcm.2008.07.001. [DOI] [PubMed] [Google Scholar]
- 55.Nakamura Y, Patrushev N, Inomata H, Mehta D, et al. Role of protein tyrosine phosphatase 1b in vascular endothelial growth factor signaling and cell-cell adhesions in endothelial cells. Circ Res. 2008;102:1182–1191. doi: 10.1161/CIRCRESAHA.107.167080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Stenzinger A, Schreiner D, Pfeiffer T, Tag C, et al. Epidermal growth factor-, transforming growth factor-beta-, retinoic acid- and 1,25-dihydroxyvitamin d3-regulated expression of the novel protein ptpip51 in keratinocytes. Cells Tissues Organs. 2006;184:76–87. doi: 10.1159/000098949. [DOI] [PubMed] [Google Scholar]
- 57.Pu Q, Chang Y, Zhang C, Cai Y, et al. Chronic insulin treatment suppresses ptp1b function, induces increased pdgf signaling, and amplifies neointima formation in the balloon-injured rat artery. Am J Physiol Heart Circ Physiol. 2009;296:H132–139. doi: 10.1152/ajpheart.00370.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lanahan AA, Hermans K, Claes F, Kerley-Hamilton JS, et al. Vegf receptor 2 endocytic trafficking regulates arterial morphogenesis. Dev Cell. 2010;18:713–724. doi: 10.1016/j.devcel.2010.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Oshikawa J, Urao N, Kim HW, Kaplan N, et al. Extracellular sod-derived h2o2 promotes vegf signaling in caveolae/lipid rafts and post-ischemic angiogenesis in mice. PloS one. 2010;5:e10189. doi: 10.1371/journal.pone.0010189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chang Y, Ceacareanu B, Zhuang D, Zhang C, et al. Counter-regulatory function of protein tyrosine phosphatase 1b in platelet-derived growth factor- or fibroblast growth factor-induced motility and proliferation of cultured smooth muscle cells and in neointima formation. Arterioscler Thromb Vasc Biol. 2006;26:501–507. doi: 10.1161/01.ATV.0000201070.71787.b8. [DOI] [PubMed] [Google Scholar]
- 61.Kakazu A, Sharma G, Bazan HE. Association of protein tyrosine phosphatases (ptps)-1b with c-met receptor and modulation of corneal epithelial wound healing. Invest Ophthalmol Vis Sci. 2008;49:2927–2935. doi: 10.1167/iovs.07-0709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.El Sayegh TY, Kapus A, McCulloch CA. Beyond the epithelium: Cadherin function in fibrous connective tissues. FEBS Lett. 2007;581:167–174. doi: 10.1016/j.febslet.2006.12.029. [DOI] [PubMed] [Google Scholar]
- 63.Quattrini C, Jeziorska M, Malik RA. Small fiber neuropathy in diabetes: Clinical consequence and assessment. Int J Low Extrem Wounds. 2004;3:16–21. doi: 10.1177/1534734603262483. [DOI] [PubMed] [Google Scholar]
- 64.Nakamura M, Kawahara M, Morishige N, Chikama T, et al. Promotion of corneal epithelial wound healing in diabetic rats by the combination of a substance p-derived peptide (fglm-nh2) and insulin-like growth factor-1. Diabetologia. 2003;46:839–842. doi: 10.1007/s00125-003-1105-9. [DOI] [PubMed] [Google Scholar]
- 65.Movafagh S, Hobson JP, Spiegel S, Kleinman HK, et al. Neuropeptide y induces migration, proliferation, and tube formation of endothelial cells bimodally via y1, y2, and y5 receptors. FASEB J. 2006;20:1924–1926. doi: 10.1096/fj.05-4770fje. [DOI] [PubMed] [Google Scholar]
- 66.Ekstrand AJ, Cao R, Bjorndahl M, Nystrom S, et al. Deletion of neuropeptide y (npy) 2 receptor in mice results in blockage of npy-induced angiogenesis and delayed wound healing. Proc Natl Acad Sci U S A. 2003;100:6033–6038. doi: 10.1073/pnas.1135965100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kuo LE, Abe K, Zukowska Z. Stress, npy and vascular remodeling: Implications for stress-related diseases. Peptides. 2007;28:435–440. doi: 10.1016/j.peptides.2006.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Delgado AV, McManus AT, Chambers JP. Exogenous administration of substance p enhances wound healing in a novel skin-injury model. Exp Biol Med (Maywood) 2005;230:271–280. doi: 10.1177/153537020523000407. [DOI] [PubMed] [Google Scholar]
- 69.Zukowska Z, Grant DS, Lee EW. Neuropeptide y: A novel mechanism for ischemic angiogenesis. Trends Cardiovasc Med. 2003;13:86–92. doi: 10.1016/s1050-1738(02)00232-3. [DOI] [PubMed] [Google Scholar]
- 70.Hokfelt T, Kellerth JO, Nilsson G, Pernow B. Substance p: Localization in the central nervous system and in some primary sensory neurons. Science. 1975;190:889–890. doi: 10.1126/science.242075. [DOI] [PubMed] [Google Scholar]
- 71.Harrison S, Geppetti P. Substance p. Int J Biochem Cell Biol. 2001;33:555–576. doi: 10.1016/s1357-2725(01)00031-0. [DOI] [PubMed] [Google Scholar]
- 72.Khawaja AM, Rogers DF. Tachykinins: Receptor to effector. Int J Biochem Cell Biol. 1996;28:721–738. doi: 10.1016/1357-2725(96)00017-9. [DOI] [PubMed] [Google Scholar]
- 73.Pernow B. Substance p. Pharmacol Rev. 1983;35:85–141. [PubMed] [Google Scholar]
- 74.Lindberger M, Schroder HD, Schultzberg M, Kristensson K, et al. Nerve fibre studies in skin biopsies in peripheral neuropathies. I. Immunohistochemical analysis of neuropeptides in diabetes mellitus. J Neurol Sci. 1989;93:289–296. doi: 10.1016/0022-510x(89)90198-6. [DOI] [PubMed] [Google Scholar]
- 75.Zukowska-Grojec Z, Karwatowska-Prokopczuk E, Rose W, Rone J, et al. Neuropeptide y: A novel angiogenic factor from the sympathetic nerves and endothelium. Circ Res. 1998;83:187–195. doi: 10.1161/01.res.83.2.187. [DOI] [PubMed] [Google Scholar]
- 76.Wallengren J, Badendick K, Sundler F, Hakanson R, et al. Innervation of the skin of the forearm in diabetic patients: Relation to nerve function. Acta Derm Venereol. 1995;75:37–42. doi: 10.2340/00015555753742. [DOI] [PubMed] [Google Scholar]
- 77.Toda M, Suzuki T, Hosono K, Kurihara Y, et al. Roles of calcitonin gene-related peptide in facilitation of wound healing and angiogenesis. Biomed Pharmacother. 2008;62:352–359. doi: 10.1016/j.biopha.2008.02.003. [DOI] [PubMed] [Google Scholar]
- 78.Oltman CL, Davidson EP, Coppey LJ, Kleinschmidt TL, et al. Treatment of zucker diabetic fatty rats with ave7688 improves vascular and neural dysfunction. Diabetes Obes Metab. 2009;11:223–233. doi: 10.1111/j.1463-1326.2008.00924.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hatanaka E, Monteagudo PT, Marrocos MS, Campa A. Neutrophils and monocytes as potentially important sources of proinflammatory cytokines in diabetes. Clin Exp Immunol. 2006;146:443–447. doi: 10.1111/j.1365-2249.2006.03229.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Fisman EZ, Adler Y, Tenenbaum A. Biomarkers in cardiovascular diabetology: Interleukins and matrixins. Adv Cardiol. 2008;45:44–64. doi: 10.1159/000115187. [DOI] [PubMed] [Google Scholar]
- 81.Mastej K, Adamiec R. Neutrophil surface expression of cd11b and cd62l in diabetic microangiopathy. Acta Diabetol. 2008;45:183–190. doi: 10.1007/s00592-008-0040-0. [DOI] [PubMed] [Google Scholar]
- 82.Stegenga ME, van der Crabben SN, Dessing MC, Pater JM, et al. Effect of acute hyperglycaemia and/or hyperinsulinaemia on proinflammatory gene expression, cytokine production and neutrophil function in humans. Diabet Med. 2008;25:157–164. doi: 10.1111/j.1464-5491.2007.02348.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Oncul O, Yildiz S, Gurer US, Yeniiz E, et al. Effect of the function of polymorphonuclear leukocytes and interleukin-1 beta on wound healing in patients with diabetic foot infections. J Infect. 2007;54:250–256. doi: 10.1016/j.jinf.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 84.Ochoa O, Torres FM, Shireman PK. Chemokines and diabetic wound healing. Vascular. 2007;15:350–355. doi: 10.2310/6670.2007.00056. [DOI] [PubMed] [Google Scholar]
- 85.Dinh T, Tecilazich F, Kafanas A, Doupis J, et al. Mechanisms involved in the development and healing of diabetic foot ulceration. Diabetes. 2012;61:2937–2947. doi: 10.2337/db12-0227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Caballero AE, Arora S, Saouaf R, Lim SC, et al. Microvascular and macrovascular reactivity is reduced in subjects at risk for type 2 diabetes. Diabetes. 1999;48:1856–1862. doi: 10.2337/diabetes.48.9.1856. [DOI] [PubMed] [Google Scholar]
- *87.Veves A, Akbari CM, Primavera J, Donaghue VM, et al. Endothelial dysfunction and the expression of endothelial nitric oxide synthetase in diabetic neuropathy, vascular disease, and foot ulceration. Diabetes. 1998;47:457–463. doi: 10.2337/diabetes.47.3.457. This study found a close association with peripheral neuropathy with skin microvascular changes in diabetes. [DOI] [PubMed] [Google Scholar]
- 88.Menzoian JO, LaMorte WW, Paniszyn CC, McBride KJS, et al. Symptomatology and anatomic patterns of peripheral vascular disease: Differing impact of smoking and diabetes. Ann Vasc Surg. 1989;3:224–228. doi: 10.1016/S0890-5096(07)60028-4. [DOI] [PubMed] [Google Scholar]
- 89.Arora S, Pomposelli F, LoGerfo FW, Veves A. Cutaneous microcirculation in the neuropathic diabetic foot improves significantly but not completely after successful lower extremity revascularization. J Vasc Surg. 2002;35:501–505. doi: 10.1067/mva.2002.121126. [DOI] [PubMed] [Google Scholar]
- 90.Boska M. Estimating the atp cost of force production in the human gastrocnemius/soleus muscle group using 31p mrs and 1h mri. NMR Biomed. 1991;4:173–181. doi: 10.1002/nbm.1940040404. [DOI] [PubMed] [Google Scholar]
- *91.Greenman RL, Panasyuk S, Wang X, Lyons TE, et al. Early changes in the skin microcirculation and muscle metabolism of the diabetic foot. Lancet. 2005;366:1711–1717. doi: 10.1016/S0140-6736(05)67696-9. This study identified microvascular changes in the skin of patients with diabetes compared to patients without diabetes. [DOI] [PubMed] [Google Scholar]
- 92.Greenman RL, Rakow-Penner R. Evaluation of the rf field uniformity of a double-tuned 31p/1h birdcage rf coil for spin-echo mri/mrs of the diabetic foot. J Magn Reson Imaging. 2005;22:427–432. doi: 10.1002/jmri.20372. [DOI] [PubMed] [Google Scholar]
- 93.Pomposelli FB, Kansal N, Hamdan AD, Belfield A, et al. A decade of experience with dorsalis pedis artery bypass: Analysis of outcome in more than 1000 cases. J Vasc Surg. 2003;37:307–315. doi: 10.1067/mva.2003.125. [DOI] [PubMed] [Google Scholar]
- 94.Greenman RL. Quantification of the 31p metabolite concentration in human skeletal muscle from rare image intensity. Magn Reson Med. 2004;52:1036–1042. doi: 10.1002/mrm.20258. [DOI] [PubMed] [Google Scholar]
- 95.Steed DL. Clinical evaluation of recombinant human platelet-derived growth factor for the treatment of lower extremity diabetic ulcers. Diabetic ulcer study group. J Vasc Surg. 1995;21:71–78. doi: 10.1016/s0741-5214(95)70245-8. discussion 79–81. [DOI] [PubMed] [Google Scholar]
- 96.Villela DL, Santos VL. Evidence on the use of platelet-rich plasma for diabetic ulcer: A systematic review. Growth factors. 2010;28:111–116. doi: 10.3109/08977190903468185. [DOI] [PubMed] [Google Scholar]
- 97.Saad Setta H, Elshahat A, Elsherbiny K, Massoud K, et al. Platelet-rich plasma versus platelet-poor plasma in the management of chronic diabetic foot ulcers: A comparative study. Int Wound J. 2011;8:307–312. doi: 10.1111/j.1742-481X.2011.00797.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Balingit PP, Armstrong DG, Reyzelman AM, Bolton L, et al. Norleu3-a(1–7) stimulation of diabetic foot ulcer healing: Results of a randomized, parallel-group, double-blind, placebo-controlled phase 2 clinical trial. Wound Repair Regen. 2012;20:482–490. doi: 10.1111/j.1524-475X.2012.00804.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *99.Bowen-Pope DF, Malpass TW, Foster DM, Ross R. Platelet-derived growth factor in vivo: Levels, activity, and rate of clearance. Blood. 1984;64:458–469. This study established the natural history of platelet-derived growth factor in vivo. [PubMed] [Google Scholar]
- 100.Lazarous DF, Shou M, Scheinowitz M, Hodge E, et al. Comparative effects of basic fibroblast growth factor and vascular endothelial growth factor on coronary collateral development and the arterial response to injury. Circulation. 1996;94:1074–1082. doi: 10.1161/01.cir.94.5.1074. [DOI] [PubMed] [Google Scholar]