Abstract
In gene therapy, tissue-specific promoters are useful tools to direct transgene expression and improve efficiency and safety. Macrophage-specific promoters (MSPs) have previously been published using different delivery systems. In this study, we evaluated five different MSP fused with green fluorescence protein (GFP) to delineate the one with highest specificity using lentiviral delivery. We compared three variants of the CD68 promoter (full length, the 343 base pair (bp) proximal part and the 150 bp proximal part) and two variants (in forward and reverse orientation) of a previously characterized synthetic promoter derived from elements of transcription factor genes. We transduced a number of cell lines and primary cells in vitro. In addition, hematopoietic stem cells were transduced with MSPs and transferred into lethally irradiated recipient mice. FACS analysis was performed to determine the GFP expression in different cell populations both in vitro and in vivo. We showed that MSPs can efficiently be used for lentiviral gene delivery and that the 150 bp proximal part of the CD68 promoter provides primarily macrophage-specific expression of GFP. We propose that this is the best currently available MSP to use for directing transgene expression to macrophage populations in vivo using lentiviral vectors.
Keywords: Macrophage, promoter, lentivirus
INTRODUCTION
In gene therapy, it is advantageous to use selective targeting strategies to control expression of therapeutic genes. Transgene expression driven by tissue-specific promoters may help reduce the effects of off-target expression and improve the efficiency and safety of lentiviral vectors for gene therapy. Lentivirus-derived vectors are efficient delivery vehicles for genes as they can target and permanently integrate into hematopoietic stem cells and non-dividing cells, e.g. macrophages, with high efficiency. Using gene therapy with a tissue specific promoter, macrophages (or other cell types) can be targeted to either overexpress or silence a specific protein. The use of tissue specific promoters can limit both genotoxicity (e.g. by limitation of enhancer activity in nontarget cells which leads to reduced activation of oncogenes near the vector integration site) and cytotoxicity (ectopic transgene expression in nontarget cells).1
Macrophages are key players in several common diseases e.g. infections,2 arthritis,3 atherosclerosis4 and cancer.5, 6 Macrophage-specific promoters (MSPs) can be used for gene delivery approaches as valuable tools to study the function of the macrophage per se , or to treat diseases in a macrophage-dependent fashion. Several different MSPs have earlier been published in different settings.7–16 Among these, previous studies indicate that the CD68 promoter7, 8 and a synthetic promoter (generated by randomly ligating myeloid-specific cis promoter elements for several different transcription factors and inserting them upstream of the p47phox basal promoter)16 result in the highest transgene expression levels and macrophage specificity. However, these promoters exist in different lengths and orientations and further characterization is required to assess which promoter is superior.
In this study we sought to find an MSP with high specificity resulting in high protein expression in macrophages and low leakage in non-target cells using lentiviral delivery. To achieve an appropriate comparison between promoters, we constructed lentiviral vectors containing promoters fused with GFP. We compared three variants of the CD68 promoter (full length, the 343 base pair (bp) proximal region and the 150 bp proximal region) and two variants of the synthetic promoter (in forward and reverse orientation) in vitro and in vivo . We show that MSPs can efficiently be used for lentiviral gene delivery and that the 150 bp proximal region of the CD68 promoter results in significant protein expression and is currently the best available promoter for macrophage specific expression of transgenes.
RESULTS
The constructs
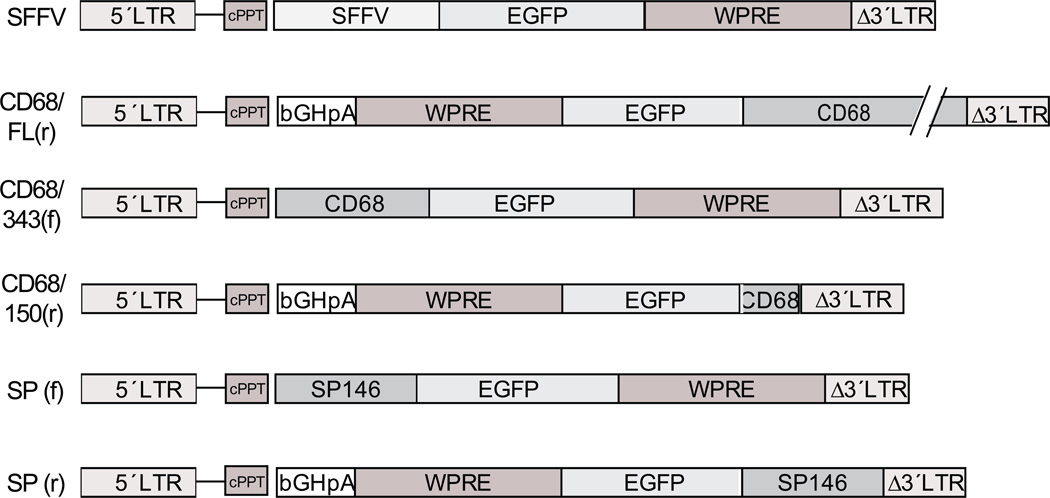
We generated lentiviral constructs with expression cassettes containing promoters, GFP and woodchuck post-transcriptional regulatory element (WPRE). The following promoters were used: (i) the spleen focus forming virus long terminal repeat promoter (SFFV), used as a positive control; (ii) full length human CD68 promoter in reverse orientation (CD68FL(r)); (iii) the proximal 343 bp of the CD68 promoter (CD68/343(f)); (iv) the proximal 150 bp of the CD68 promoter (CD68/150(r)); (v) the synthetic promoter in the forward orientation (SP(f)); and (vi) the synthetic promoter in the reverse orientation (SP(r)). All constructs are shown in Figure 1.
Figure 1. Lentiviral contructs used in this study.
LTR, long terminal repeat; SFFV, spleen focus forming virus; GFP, green fluorescent protein; WPRE, woodchuck post-transcriptional regulatory element; cPPT, central polypurine tract; bGHpA, bovine growth hormone poly adenylation signal; SP, synthetic promoter.
Expression of GFP in cell line and primary macrophages
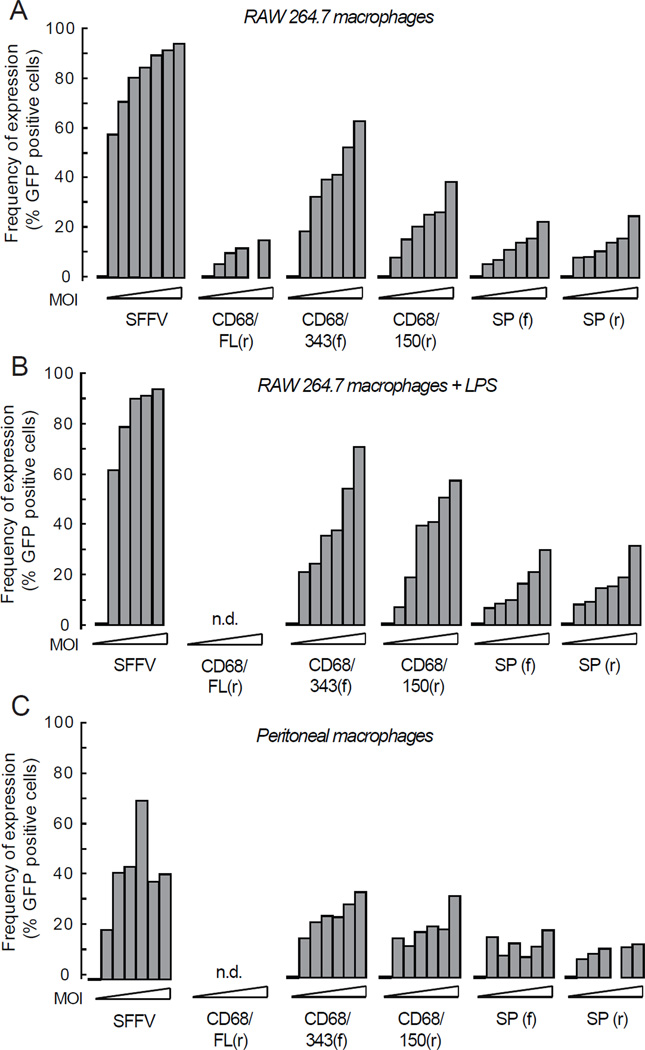
In order to determine if lentiviral particles encoding GFP driven by MSPs could transduce macrophages and lead to protein expression, we transduced various cell lines and primary cells. FACS analysis was performed to determine the GFP expression in different cell populations and typical gating is shown in Figure 2. To compare the MSP driven GFP expression in macrophages, a mouse macrophage cell line (RAW 264.7) was initially used. We found that all MSPs except CD68FL(r) were functional and lead to GFP expression in a concentration-dependent manner, both regarding number of cells (Figure 3a and Table S1) and mean fluorescence intensity (MFI) (Figure S1), although to a lower extent compared with SFFV. CD68FL(r) was excluded from further experiments because of low GFP expression levels. Activation of macrophages by addition of LPS after transduction only marginally increased the number of GFP-positive cells (Figure 3b). To verify GFP expression in primary cells, we also transduced peritoneal macrophages with lentiviral particles encoding GFP driven by the MSPs. All the MSPs lead to significant expression of GFP in primary macrophages (Figure 3c, Figure S1 and Table S1). Taken together, these data show that CD68/343(f) and CD68/150(r) resulted in the highest expression in cell line and primary macrophages. Because SP(f) and SP(r) expressed GFP to the same extent, we excluded SP(r) from further experiments.
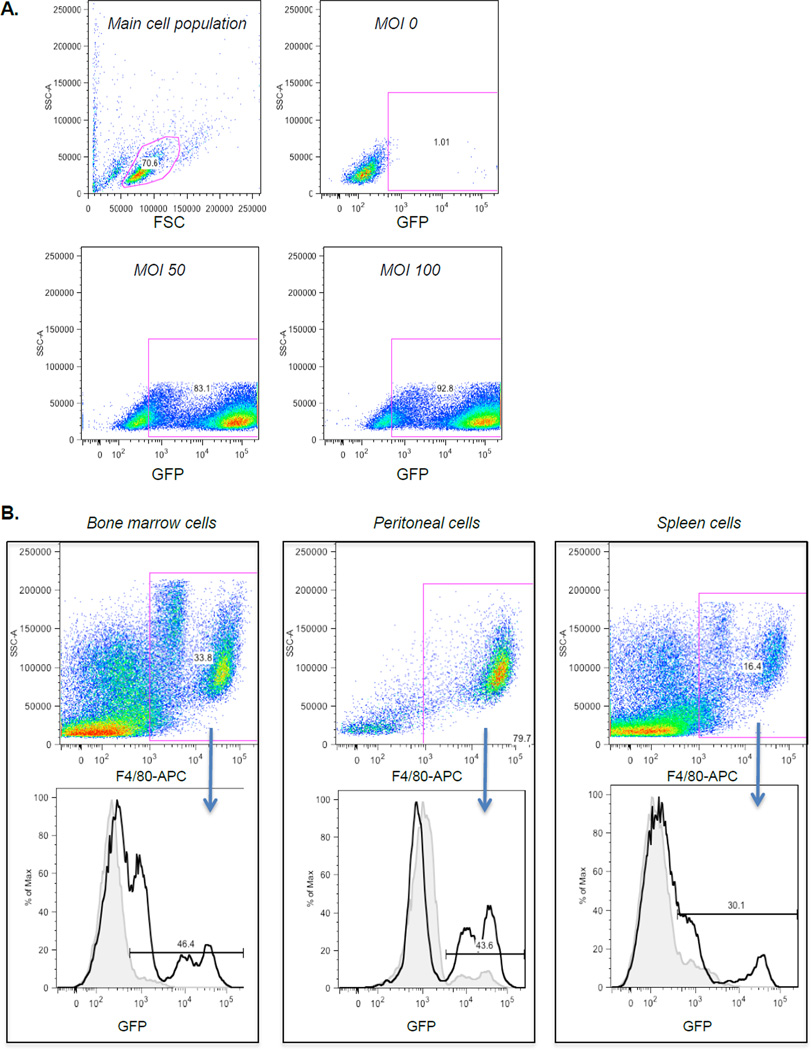
Figure 2. FACS gating for detection of GFP positive cells in vitro and ex vivo.
(a) Typical gating of RAW 264.7 macrophages transduced with SFFV of increasing MOI. (b) Typical gating of cell populations expressing GFP in bone marrow, peritoneal cells and spleen cells after bone marrow transplantation using CD68/150(r) transduced hematopoetic stem cells. The population of cells labeled with APC-F4/80 is shown in the upper panels. The percentage of GFP positive cells from animals transplanted with bone marrow transduced with CD68/150(r) (solid black line) was compared with background in non-transplanted animals (gray line) and are shown in the lower panels.
Figure 3. GFP expression in cell line and primary macrophages in vitro and ex vivo.
(a) RAW 264.7, (b) RAW 264.7 (stimulated 24 h after transduction with LPS (0.5 µg/ml)) and (c) macrophages from peritoneal lavage were transduced with MSPs of increasing MOI. At day 5 after transduction, the cells were harvested and GFP expression was analyzed by flow cytometry. Results show data pooled from two independent experiments. See statistical analyses in Supplementary Table S1.
Specificity of MSPs in vitro
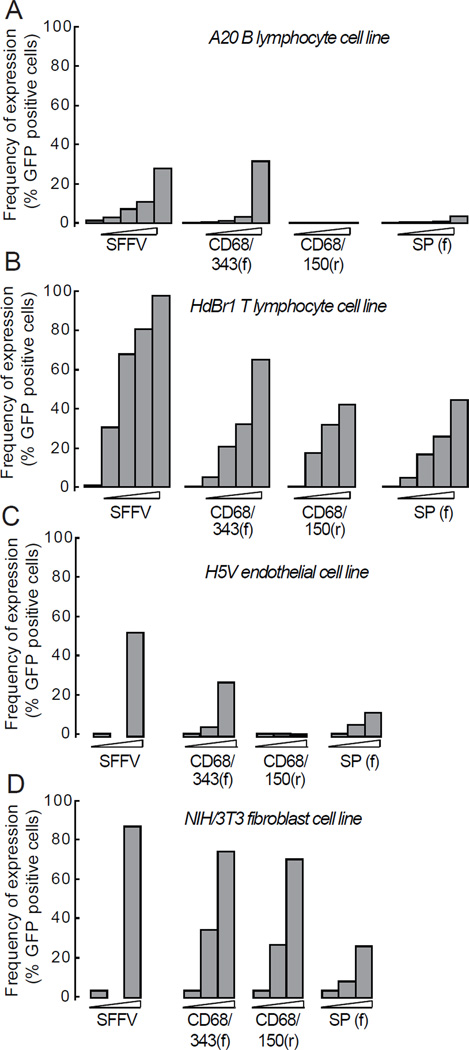
To study the specificity of MSPs in non-macrophage cells in vitro , we used a B cell line (A20), a T cell line (HDBR1), an endothelial cell line (H5V) and a fibroblast cell line (NIH/3T3). In B cells, transduction with CD68/343(f) resulted in significant expression of GFP (5% positive cells) (Figure 4a and Table S2). In T cells, (CD68/343(f) and CD68/150(r) resulted in 25% and 63% GFP positive cells, respectively) (Figure 4b) (Table S2). In endothelial cells, all MSPs except CD68/150(r) led to significant expression of GFP ((CD68/343(f) and SP(f) resulted in 10% and 6% GFP positive cells, respectively) (Figure 4c and Table S2). In fibroblasts (which express fairly high levels of CD6817), transduction with CD68/343(f) and CD68/150(r) resulted in significant expression of GFP (35% and 31%, respectively) (Figure 4d and Table S2). Thus, transduction of non-macrophage cell lines in vitro shows that all MSPs tested were to some extent promiscuous. However, CD68/150(r) and SP(f) showed a more specific expression pattern than CD68/343(f).
Figure 4. GFP expression in non-macrophage cell lines in vitro.
(a) NIH/3T3 fibroblasts, (b) H5V endothelial cells, (c) HDBR1 T cells, (d) A20 B cells were transduced with MSPs of increasing MOI. Fibroblasts and endothelial cells were harvested at day 5 and T and B cells at day 7 after transduction and GFP expression was analyzed by flow cytometry. Results show data pooled from two independent experiments. See statistical analyses in Supplementary Table S2.
Expression and specificity of MSPs in vivo
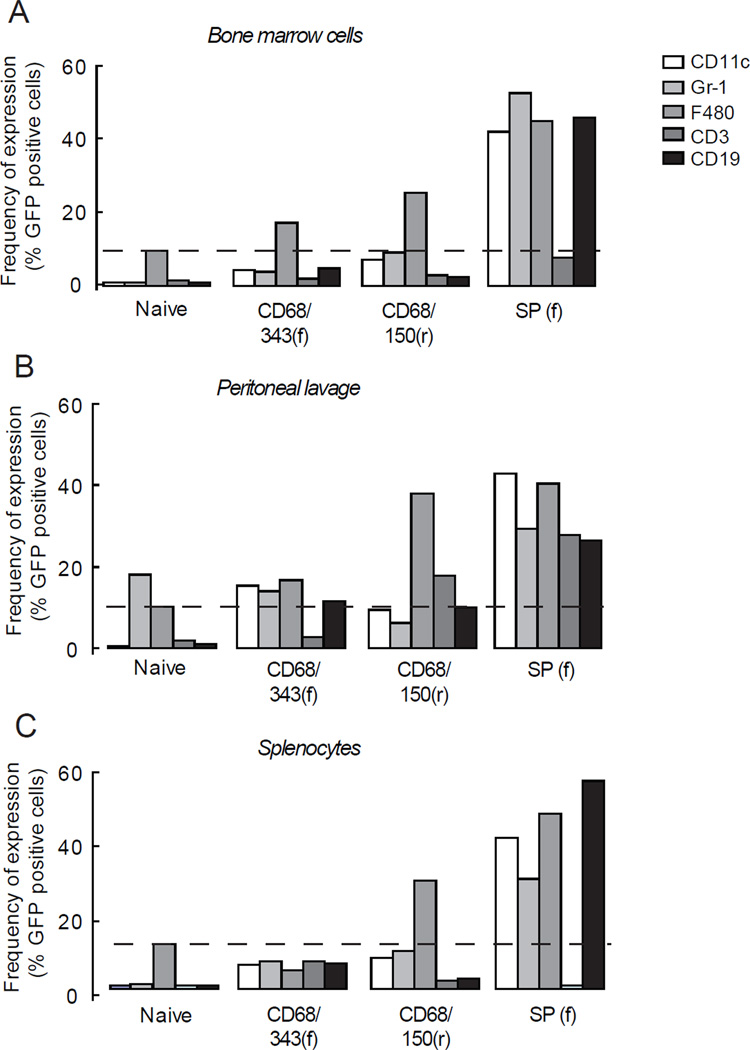
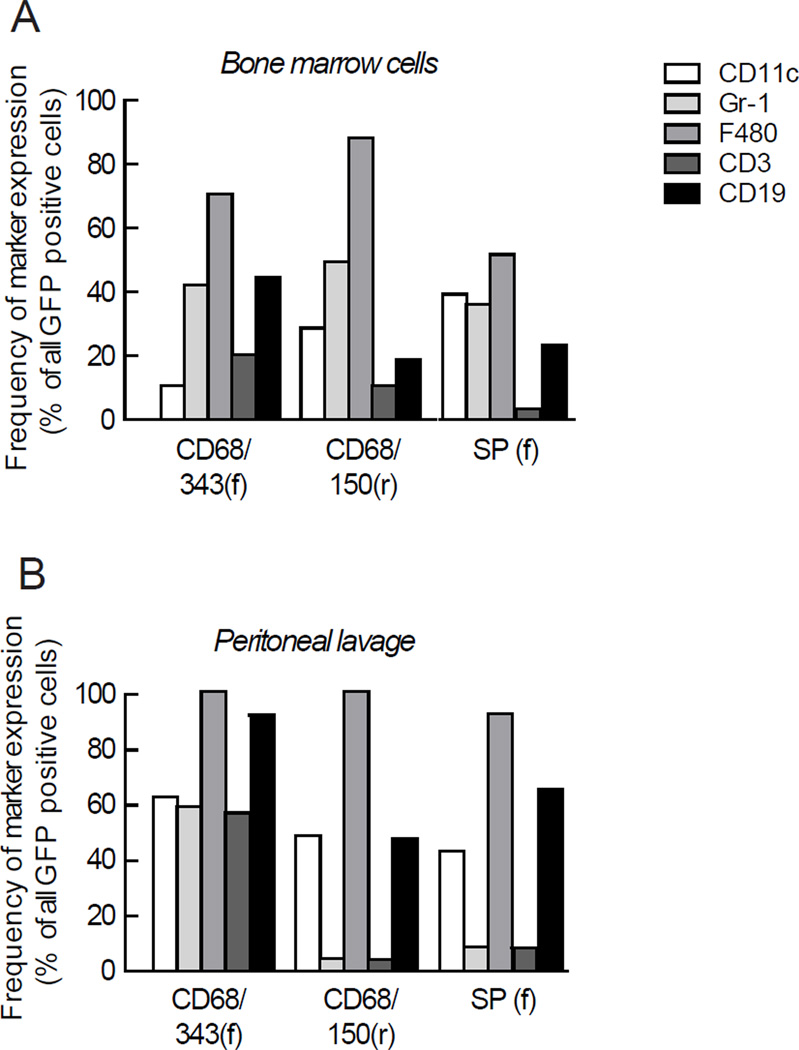
To study the expression and specificity of the MSPs in different cell populations in vivo, hematopoietic stem cells were transduced with CD68/343(f), CD68/150(r) or SP(f) and transplanted into C57Bl6 recipient mice. We found that all the selected MSPs were well integrated both in bone marrow and in peripheral cells 8 weeks after transplantation (Table 1). FACS analysis was performed to determine the GFP expression in different cell populations in bone marrow, peritoneal lavage and spleen (Figure 2). Using CD68/150(r), 25- 40% of the macrophages (F4/80+ cells) expressed GFP in the different organs (Figure 5a–c). When we combine data from bone marrow, peritoneal lavage and spleen, the average GFP positivity for macrophages was 31% ± 4, P <0.001. On the other hand, dendritic cells (DCs) (CD11c+), neutrophils (Gr-1+), T cells (CD3+) and B cells (CD19+) did not express significant levels of GFP (Table S3), suggesting a highly macrophage specific expression pattern. In contrast, SP(f) showed a more nonspecific pattern, as both macrophages (44 %, P<0.05) as well as DCs (42%, P<0.05), neutrophils (37%, P<0.05) and B cells (44%, P<0.05) expressed GFP (Figure 5a–c and Table S3). Transduction with CD68/343(f) showed that surprisingly few cells expressed GFP and in a nonspecific pattern (Figure 5 a–c). Analyses of MFI also showed that CD68/150(r) lead to macrophage-specific expression of GFP. In addition, when markers were analyzed in all GFP-expressing cells, CD68/150(r) resulted in 90–100% cells that were positive for the macrophage-marker F4/80, while expression of T-cell, B-cell, DC, and neutrophil markers were as low as 10–50% (Figure 6). Our results clearly demonstrate that CD68/150(r) provides primarily macrophage-specific expression of GFP and is an excellent MSP to use for directing transgene expression to macrophage populations in vivo using lentiviral vectors.
Table 1.
| At the day of transplantation | At 8 weeks after transplantation | ||
|---|---|---|---|
| Hematopoetic stem cells (copies/1000 cells) |
Bone marrow | Spleen | |
| (copies/1000 cells) | (copies/1000 cells) | ||
| Naïve | n.d. | 1.3±1 | 8.1±2 |
| CD68/343(f) | 48000 | 120±110 | 150±110 |
| CD68/150(r) | 21000 | 56±50 | 70±50 |
| SP(f) | 43000 | 950±730 | 790±360 |
Number of integrated viral copies in genomic DNA in transduced hematopoetic stem cells at the day of transplantation, and in whole bone marrow and spleen at 8 weeks after transplantation. Data at 8 weeks are shown as mean ± SEM. Not determined (n.d.)
Figure 5. Reconstitution of lethally irradiated mice with MSPs encoding GFP transduced hematopoetic stem cells.
Single cells from (a) bone marrow, (b) peritoneal lavage and (c) spleen were stained stained for DCs (CD11c), neutrophils (Gr-1), macrophages (F4/80), T cells (CD3) and B cells (CD19) and analyzed by FACS 8 weeks after transplantation. The dashed line represents the level of background in F4/80 stained cells. Data are presented as mean of two representative observations. See statistical analyses in Supplementary Table S3.
Figure 6. Breakdown of cell lineages of GFP expressing cells in bone marrow and spleen of individual reconstituted mice.
Single cells from (a) bone marrow, (b) peritoneal lavage and (c) spleen were stained for DCs (CD11c), neutrophils (Gr-1), macrophages (F4/80), T cells (CD3) and B cells (CD19) and analyzed by FACS 8 weeks after transplantation.
DISCUSSION
In this study, we used a lentiviral vector system to compare different MSPs with the aim of finding the promoter with the highest macrophage specificity that lead to stable high protein expression. Our data show that the 150 bp proximal part of the CD68 promoter is superior to the other promoters tested and results in the highest macrophage specificity and significant protein expression.
Previous studies using MSPs have shown that the CD68 promoter7, 8 and a synthetic promoter generated by fusing transcription factors to the p47phox minipromoter16 result in high transgene expression levels and macrophage specificity. However, these promoters have never been compared and moreover they exist in different lengths and orientations. Further evaluation of these promoters is consequently required to assess which promoter is superior. We therefore compared three different CD68 promoters of different length and orientation (CD68FL(r), CD68/343(f) and CD68/150(r)) and two constructs of a macrophage-specific synthetic promoter of different orientation (SP(f) and SP(r)). The rationale for using different orientations is that the full length CD68 contains the 83bp CD68 IVS1, which on the one hand mediates enhancer activity when inserted downstream of the promoter,18 but on the other hand introns contained within the insert may be removed during vector replication.19 Indeed, previous attempts to use the CD68 full length promoter in forward orientation show that it does not result in any GFP expression at all (I Gjertsson, unpublished observation) and the full length CD68 promoter is therefore used in reverse orientation (CD68FL(r)). However, CD68/343(f) has previously been shown to be functional in forward orientation.20
Construction of CD68/150(r) (which lacks the intron), was cloned in the same vector backbone as CD68FL(r), thus this construct is also in reverse orientation. The constructs of the synthetic promoter are tested in forward SP(f) orientation as well as reverse (SP(r)) orientation.
Our data show that all tested MSPs except CD68FL(r), are functional in vitro and in vivo . Surprisingly, the shortest version of the CD68 promoter, CD68/150(r) gives the highest macrophage specificity in vitro as well as in vivo . This promoter has been characterized previously,8 but has to our knowledge not been used for any functional studies. Previous characterization of the CD68 promoter has shown that sequences between −150 and +2 of the CD68 gene give maximal promoter activity,8 most likely due to the fact that multiple transcription factors bind to this sequence. Ets transcription factors (e.g. PU.1) are the most important regulatory element for macrophage specificity.21, 22 At -110 bp of the CD68 promoter, there is an Ets cis-element that binds PU.1, which is crucial for promoter activity.8 Furthermore, at -145 of the CD68 promoter there is an AP-1 transcription factor cis-element, which has been shown to act synergistically together with PU.1 to activate the mouse CD68 promoter. These previous notions together with our results suggest that the proximal -150 bp of the CD68 promoter is enough for maximal promoter activity specifically in macrophages and it is possible that inclusion of larger parts of the promoter sequence actually decreases specificity.
The synthetic promoter has been suggested to be highly specific for macrophages,16 but in vivo characterization of promoter specificity in other types of bone marrow derived cells has so far been limited. Our results suggest that the synthetic promoter is specific for all cells of hematopoietic lineage.
Our data show that the macrophage-specificity for CD68/150(r) as well as CD68/343(f) gives higher specificity in vivo than in vitro. One reason for this discrepancy may be that the analyzed cells in vivo are fully differentiated cells and are immunologically defined. Cell lines are not defined in the same way and do not display the cell-specific expression patterns as cells do in vivo.
Lentiviral vectors have become popular delivery vehicles for gene therapy. In particular, the capacity of lentiviral vectors to integrate into non-dividing cells and provide long-term, and stable gene expression in vivo is an attractive attribute for gene therapy approaches. As a consequence, clinical trials for treatment of human immunodeficiency virus (HIV) (reviewed in23) and β-thalassaemia24 have been performed using lentiviral vector delivery systems with general viral promoters driving ubiquitous expression in all cell types. However, it is advantageous to use selective targeting strategy to control expression of therapeutic genes. Transgene expression driven by tissue-specific promoters may help reduce the effects of offtarget expression and improve the efficiency and safety of lentiviral vectors for gene therapy. Gene therapy with a macrophage-specific promoter could for instance be an option in some lysosomal storage diseases such as Morbus Gaucher, where the genetic defect is confined to macrophages25.
In conclusion, we found that CD68/150(r) is the currently available MSP with highest macrophage-specific activity and that this promoter is exceedingly suitable for lentiviral delivery in vitro and in vivo.
MATERIALS AND METHODS
Generation of lentiviral constructs
The following constructs were used in this study and are summarized in Figure 1. The SFFV promoter construct (previously referred to as pHR'SINcPPT-SEW) that is used as a positive control has been described previously.26 To generate the construct containing the full length human CD68 promoter (2940 bp) in reverse orientation (the CD68FL(r)), its expression cassette was put together stepwise in the cloning plasmid pTZ18R (Pharmacia) using standard cloning procedures. Briefly, pTZ18R was digested with HindIII / EcoRI and a multiple cloning site (MCS) linker, SalI-AscI-HindIII-XbaI-PacI-PmeIClaI- XhoI-NheI-MfeI, was inserted with overhang that destroys the original HindIII / EcoRI sites. A 3bp HindIII / XbaI fragment containing the CD68 promoter was excised from pcDNA3 7 and inserted into the corresponding sites in the pTZ18R MCS. The WPRE27 was excised from SFFV using ClaI and inserted into the corresponding site in the MCS. The bovine growth hormone poly adenylation signal (bGHpA) was amplified by a polymerase chain reaction (PCR) from pcDNA3.1 (Invitrogen, Carlsbad, CA, USA) to generate a 248 bp fragment with suitable sites (Forward primer: 5′-GTA TGT CGA CAC TGT GCC TTC TAG TTG G-3′, Reverse primer: 5′-GTA TTC TAG AAG CCA TAG AGC CCA C-3′). The PCR product was subcloned to pCR2.1 TOPO (Invitrogen, Carlsbad, CA, USA) and verified by sequencing. The bGHpA fragment was then excised with SalI / XbaI and inserted into the XhoI / NheI sites in the MCS. The EGFP was excised from pEGFP1 (Clontech, Mountain View, CA, USA) with BamHI / NotI and inserted into the corresponding sites in another pTZ18R plasmid with its MCS exchanged to PacI-SalI-BamHI-SnaBI-NotI-PmeI. The EGFP was then excised with PacI / PmeI and inserted into the corresponding sites in the MCS in the first pTZ18R plasmid. Finally, the whole expression cassette was excised from pTZ18R with MfeI / SalI and inserted, in reverse orientation, into SFFV using the EcoRI / XhoI site and hence exchanging the SFFV-GFP-WPRE expression cassette. Generation of a promoter consisting of the proximal 343 bp of the CD68 promoter (CD68/343(f)) has been described previously 20. To generate a promoter consisting of the proximal 150 bp of the CD68 promoter (CD68/150(r)), the 150 bp CD68 proximal promoter was PCR amplified from pBSCD68 generating a 187 bp fragment with suitable sites (Forward primer: 5′-GAT AGG CGC GCC AAG CTT CTG AGG CCC CTG AGT CAG-3′, Reverse primer: 5′-GAT AGT CGA CTT AAT TAA TGG CTG AAC CGC CTC AC-3′). The PCR product was subcloned to pCR2.1 TOPO and verified by sequencing. The promoter fragment was then excised with AscI / SalI and inserted into the corresponding sites in the CD68FL(r) exchanging the 3097 bp CD68 promoter fragment. To generate the synthetic promoter (SP) in the forward orientation (SP(f)), a 1720 bp EcoRI / SacII fragment containing the SP, GFP and part of the WPRE, was excised from SP-GFP 16 and inserted into the corresponding sites of SFFV, exchanging the SFFV-GFP-WPRE expression cassette. To generate the SP in the reverse orientation (SP(r)), the CD68FL(r) was digested with AscI / SalI, removing the full length CD68 promoter, and a MCS linker, AscI-EcoRI-XmaI-SalI, was inserted. The SP promoter was then excised from SP-GFP 16 with EcoRI / AgeI and inserted into the EcoRI / XmaI sites in the MCS linker.
Production of lentiviral particles
Vesicular stomatitis virus-G (VSV-G) pseudotyped lentivirus was produced by transient transfection of 293FT cells with three plasmids: one of the self inactivating transfer vector plasmids (SFFV, CD68FL(r), CD68/343(f), CD68/150(r), SP(f), SP(r)); the multi-deleted packaging plasmid pCMVΔR8.74; and the VSV-G envelope pMD.G2 using calcium phosphate co-precipitation (ProFection Mammalian Transfection System Promega Biotech AB, Stockholm, Sweden). Because this method did not lead to a sufficiently high titer of lentiviral particles encoding CD68/150(r), this particular transfer vector plasmid was co-precipitated with pCMVΔR8.74 and pMD.G2 using polyethylenimine (Sigma-Aldrich, St Louis, MO, USA). At 72 h post transfection, the medium was harvested and concentrated by ultracentrifugation at 90,000 g. The pellets were resuspended in PBS containing 2% FCS and stored at −80°C. The concentration of lentiviral particles was determined by transducing a defined number of HeLa cells with serially diluted lentiviral particles and measuring the expression of GFP using Facs Calibur (BD Biosciences, San Jose, CA, USA) and analyzed by FlowJo Software (Tree Star Inc., Ashland, OR, USA).
Transduction of peritoneal macrophages and cell lines
Peritoneal macrophages were isolated by flushing the peritoneum of wild-type C57Bl6/J mice with PBS. Cells were collected and cultured for 2 h at 37°C in serum-free RPMI 1640 media supplemented with sodiumpyruvate (2mM), non-essential amino acids, sodium bicarbonate (1.5 g/l), penicillin (100 U/ml) and streptomycin (100mg/l). The cells were washed three times with PBS and cultured at 37°C in RPMI 1640 media containing 10% FCS supplemented as described above. The following mouse cell lines were used: the B cell line A20, the T cell line HDBR1, the macrophage cell line RAW 267.4, the endothelial cell line H5V and the fibroblast cell line NIH/3T3. All cells were cultured in a humified incubator with 5% CO2 at 37°C. Cells were transduced at the different multiplicity of infections (MOIs) indicated. The cells were harvested after 5 days (peritoneal macrophages, RAW 267.4, NIH/3T3 and H5V) or 7 days (A20 and HDBR1) and the GFP expression was analyzed by flow cytometry.
Fluorescence activated cell sorting (FACS) analyses
To detect GFP expression in different cell populations, a single cell preparation of 1×106 cells from cell culture, spleen, bone marrow and peritoneal cells were placed in 96-well plates and pelleted (3 min, 300g, 4°C). To avoid nonspecific binding via Fc-receptor interactions, cells were incubated with Fc-block (2.4G2, BD Bioscience) for 10 min at room temperature. Antibodies used were anti-CD19 (ID3), anti-CD11c (HL3), anti-Gr1 (RB68C), anti-CD3 (145-2C11) purchased from (BD Bioscience) and anti-F4/80 (BM8) purchased from (eBioscience, San Diego, CA, USA). All antibodies were diluted in FACS-buffer (PBS containing, 1% FCS and 0.5 mM EDTA). The antibodies were directly conjugated with fluorescein isothiocyanate (FITC), phycoerythin PE, allophycocyanin (APC) and APC-H7. Cells were stained as previously described and gating of cells was performed using fluorochrome minus one settings 28 and detected by FACSCanto II™ (BD Biosciences, San Jose, CA,USA). Analyses with respect to the number of cells and mean flourescence intensity (geometric mean) were performed using FlowJo Software, Tree Star Inc. (Ashland, OR, USA). Typical gating is shown in Figure 2.
Transduction of hematopoietic stem cells
Bone marrow was harvested from femur from 3 months old C57Bl6/J donor mice, and hematopoietic stem cells were isolated with negative selection using EasySep® Mouse Hematopoietic Progenitor Cell Enrichment Kit (Stemcell Technologies, Manchester, United Kingdom). After isolation, hematopoietic stem cells were resuspended in StemSpan with 1% penicillin/streptomycin and the following cytokines (100 ng/ml mSCF, 100 ng/ml Flt-3L, 100 ng/ml IL-11, 20 ng/ml IL-3) and cultured in 12 well plates at a concentration of 1 × 106 cells/ml. The cells were transduced with lentiviral constructs CD68/343(f), CD68/150(r) and SP(f) at MOI 30 and incubated at 37°C overnight. The next morning, cells were washed with PBS twice, counted and resuspended at a concentration of 2 × 105 cells / 100 µl.
Bone marrow transplantation
C57Bl/6 were obtained from Charles Rivers and housed in a pathogen-free barrier facility (12-hr light/12-hr dark cycle) and fed rodent chow. All animal studies were approved by the local Animal Ethics Committee. One week prior to and two weeks after the transplantation 8-week-old C57Bl6/J recipient mice were given acidified water supplemented with neomycin (100 mg/l) and polymyxin B sulphate (10 mg/l). The recipient mice were irradiated with 9 Gy and reconstituted with transduced stem cells (2 × 105) by an intravenous injection. Mice were sacrificed after 8 weeks and tissues and cells were harvested for analyses.
Assessment of in vivo transgene integration by PCR
To detect vector integration in different cell populations, DNA was prepared from bone marrow and spleen using the QIAamp DNA mini kit (QIAGEN, Hilden, Germany) according to the manufacturer’s instructions and the WPRE was amplified with forward primer 5′-GGC ACT GAC AAT TCC GTG GT-3’, reverse primer 5’- AGG GAC GTA GCA GAA GGA CG-3’ and the probe 5′FAM-ACG TCC TTT CCA TGG CTG CTC GC-TAMRA 29. The WPRE copy number was normalized to exon 5 of the mouse titin gene, which was amplified with forward primer 5’-AAA ACG AGC AGT GAC GTG AGC-3’, reverse primer 5’- TTC AGT CAT GCT GCT AGC GC-3’ and the probe 5′FAM-TGC ACG GAA GCG TCT CGT CTC AGT C –TAMRA 3’. The real-time PCR was performed in a mixture containing 2 µl DNA, 1× Taqman Universal Mastermix (Applied Biosystems, Foster City, CA), 300nM each of forward and reverse primers and 200 nM FAM-labeled probe in a 20 µl reaction. The analysis was performed using the 7500 Real Time PCR System (Applied Biosystems, Foster City, CA). All primers were obtained from Sigma-Aldrich and probes from Applied Biosystems.
Statistical analyses
GFP expression was compared between constructs using a linear regression model with MOI as continuous variable and construct as fixed variable. The model included construct as a fixed term and MOI as an interaction term (MOI*construct), i.e. a regression model with different slopes and intercepts for each construct. For nonlinear responses, MOI was log transformed before analysis. Expression levels are presented as normalized expression calculated at an average MOI and is presented as mean ± SEM. Mean expression was compared to zero expression using t-test and was adjusted for multiple tests within each experiment using Bonferroni correction and a P-value less than 0.05 was considered significant. All statistical analyses were performed using IBM® SPSS® Statistics 19.
Supplementary Material
ACKNOWLEDGEMENTS
We thank Louise Henningsson, Kristina Skålén, Elin Stenfeldt and Maria Heyden (Gothenburg University) for expert technical assistance, Yan Ru Su and John Blakemore (Vanderbilt University) for stimulating discussions and technical expertise and Rosie Perkins (Gothenburg University) for editing the manuscript. This work was supported by the Swedish Research Council, VINNOVA Foundation, IngaBritt and Arne Lundgren Foundation, the Swedish Foundation for Strategic Research, the Sahlgrenska University Hospital ALF research grants, NIH grants HL105375, HL57986, HL106845 and HL65709.
Footnotes
CONFLICT OF INTEREST
None.
REFERENCES
- 1.Toscano MG, Romero Z, Munoz P, Cobo M, Benabdellah K, Martin F. Physiological and tissue-specific vectors for treatment of inherited diseases. Gene Ther. 2010 doi: 10.1038/gt.2010.138. [DOI] [PubMed] [Google Scholar]
- 2.Janeway C, Travers P, Walport M, Shlomchik M. Immunobiology, the immune system in health and disease. Garland Publishing; 2001. [Google Scholar]
- 3.Gierut A, Perlman H, Pope RM. Innate immunity and rheumatoid arthritis. Rheum Dis Clin North Am. 2010;36:271–296. doi: 10.1016/j.rdc.2010.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yan ZQ, Hansson GK. Innate immunity, macrophage activation, and atherosclerosis. Immunological reviews. 2007;219:187–203. doi: 10.1111/j.1600-065X.2007.00554.x. [DOI] [PubMed] [Google Scholar]
- 5.DeNardo DG, Johansson M, Coussens LM. Immune cells as mediators of solid tumor metastasis. Cancer Metastasis Rev. 2008;27:11–18. doi: 10.1007/s10555-007-9100-0. [DOI] [PubMed] [Google Scholar]
- 6.Johansson M, Denardo DG, Coussens LM. Polarized immune responses differentially regulate cancer development. Immunological reviews. 2008;222:145–154. doi: 10.1111/j.1600-065X.2008.00600.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gough PJ, Raines EW. Gene therapy of Apolipoprotein E-deficient mice using a novel macrophage-specific retroviral vector. Blood. 2003;101:485–491. doi: 10.1182/blood-2002-07-2131. [DOI] [PubMed] [Google Scholar]
- 8.O'Reilly D, Quinn CM, El-Shanawany T, Gordon S, Greaves DR. Multiple ets factors and interferon regulatory factor-4 modulate CD68 expression in a cell type-specific manner. J Biol Chem. 2003;278:21909–21919. doi: 10.1074/jbc.M212150200. [DOI] [PubMed] [Google Scholar]
- 9.Su YR, Ishiguro H, Major AS, Dove DE, Zhang W, Hasty AH, Babaev VR, Linton MF, Fazio S. Macrophage Apolipoprotein A1 expression protects against atherosclerosis in ApoE-deficient mice and up-regulates ABC transporters. Mol Ther. 2003;8:576–583. doi: 10.1016/s1525-0016(03)00214-4. [DOI] [PubMed] [Google Scholar]
- 10.Li AC, Guidez FR, Collier JG, Glass CK. The macrosialin promoter directs high levels of transcriptional activity in macrophages dependent on combinatorial interactions between pu.1 and c-jun. J Biol Chem. 1998;273:5389–5399. doi: 10.1074/jbc.273.9.5389. [DOI] [PubMed] [Google Scholar]
- 11.Horvai A, Palinski W, Wu H, Moulton KS, Kalla K, Glass CK. Scavenger receptor a gene regulatory elements target gene expression to macrophages and to foam cells of atherosclerotic lesions. Proc Natl Acad Sci U S A. 1995;92:5391–5395. doi: 10.1073/pnas.92.12.5391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dziennis S, Van Etten RA, Pahl HL, Morris DL, Rothstein TL, et al. The CD11b promoter directs high-level expression of reporter genes in macrophages in transgenic mice. Blood. 1995;85:319–329. [PubMed] [Google Scholar]
- 13.Hahn CN, del Pilar Martin M, Zhou XY, Mann LW, d'Azzo A. Correction of murine galactosialidosis by bone marrow-derived macrophages overexpressing human protective protein/cathepsin a under control of the colony-stimulating factor-1 receptor promoter. Proc Natl Acad Sci U S A. 1998;95:14880–14885. doi: 10.1073/pnas.95.25.14880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Whitman SC, Rateri DL, Szilvassy SJ, Cornicelli JA, Daugherty A. Macrophage-specific expression of class A scavenger receptors in LDL receptor(-/-) mice decreases atherosclerosis and changes spleen morphology. J Lipid Res. 2002;43:1201–1208. [PubMed] [Google Scholar]
- 15.Bellosta S, Mahley RW, Sanan DA, Murata J, Newland DL, Taylor JM, et al. Macrophage-specific expression of human Apolipoprotein E reduces atherosclerosis in hypercholesterolemic Apolipoprotein E-null mice. J Clin Invest. 1995;96:2170–2179. doi: 10.1172/JCI118271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.He W, Qiang M, Ma W, Valente AJ, Quinones MP, Wang W, et al. Development of a synthetic promoter for macrophage gene therapy. Hum Gene Ther. 2006;17:949–959. doi: 10.1089/hum.2006.17.949. [DOI] [PubMed] [Google Scholar]
- 17.Gottfried E, Kunz-Schughart LA, Weber A, Rehli M, Peuker A, Muller A, et al. Expression of CD68 in non-myeloid cell types. Scand J Immunol. 2008;67:453–463. doi: 10.1111/j.1365-3083.2008.02091.x. [DOI] [PubMed] [Google Scholar]
- 18.Greaves DR, Quinn CM, Seldin MF, Gordon S. Functional comparison of the murine macrosialin and human CD68 promoters in macrophage and nonmacrophage cell lines. Genomics. 1998;54:165–168. doi: 10.1006/geno.1998.5546. [DOI] [PubMed] [Google Scholar]
- 19.Coffin J, Hughes SH, Varmus HE. Retroviruses. Cold Spring Harbor Laboratory Press; 1997. [PubMed] [Google Scholar]
- 20.Su YR, Blakemore JL, Zhang Y, Linton MF, Fazio S. Lentiviral transduction of ApoA1 into hematopoietic progenitor cells and macrophages: Applications to cell therapy of atherosclerosis. Arteriosclerosis, thrombosis, and vascular biology. 2008;28:1439–1446. doi: 10.1161/ATVBAHA.107.160093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Feng R, Desbordes SC, Xie H, Tillo ES, Pixley F, Stanley ER, Graf T. Pu.1 and c/ebpalpha/beta convert fibroblasts into macrophage-like cells. Proc Natl Acad Sci U S A. 2008;105:6057–6062. doi: 10.1073/pnas.0711961105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tenen DG, Hromas R, Licht JD, Zhang DE. Transcription factors, normal myeloid development, and leukemia. Blood. 1997;90:489–519. [PubMed] [Google Scholar]
- 23.Lemiale F, Korokhov N. Lentiviral vectors for hiv disease prevention and treatment. Vaccine. 2009;27:3443–3449. doi: 10.1016/j.vaccine.2009.01.055. [DOI] [PubMed] [Google Scholar]
- 24.Cavazzana-Calvo M, Payen E, Negre O, Wang G, Hehir K, Fusil F, et al. Transfusion independence and hmga2 activation after gene therapy of human beta-thalassaemia. Nature. 2010;467:318–322. doi: 10.1038/nature09328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Beck M. Therapy for lysosomal storage disorders. IUBMB Life. 2010;62:33–40. doi: 10.1002/iub.284. [DOI] [PubMed] [Google Scholar]
- 26.Demaison C, Parsley K, Brouns G, Scherr M, Battmer K, Kinnon C, et al. High-level transduction and gene expression in hematopoietic repopulating cells using a human immunodeficiency [correction of imunodeficiency] virus type 1-based lentiviral vector containing an internal spleen focus forming virus promoter. Hum Gene Ther. 2002;13:803–813. doi: 10.1089/10430340252898984. [DOI] [PubMed] [Google Scholar]
- 27.Zufferey R, Donello JE, Trono D, Hope TJ. Woodchuck hepatitis virus posttranscriptional regulatory element enhances expression of transgenes delivered by retroviral vectors. J Virol. 1999;73:2886–2892. doi: 10.1128/jvi.73.4.2886-2892.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Perfetto SP, Chattopadhyay PK, Roederer M. Seventeen-colour flow cytometry: Unravelling the immune system. Nat Rev Immunol. 2004;4:648–655. doi: 10.1038/nri1416. [DOI] [PubMed] [Google Scholar]
- 29.Charrier S, Stockholm D, Seye K, Opolon P, Taveau M, Gross DA, et al. A lentiviral vector encoding the human wiskott-aldrich syndrome protein corrects immune and cytoskeletal defects in wasp knockout mice. Gene Ther. 2005;12:597–606. doi: 10.1038/sj.gt.3302440. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.