Abstract
Caspases are either apoptotic or inflammatory. The inflammatory Caspases-1 and -11 trigger pyroptosis, a form of programmed cell death. Whereas both can be detrimental in inflammatory disease, only Caspase-1 has an established protective role during infection. Herein, we report that Caspase-11 is required for innate immunity to cytosolic, but not vacuolar, bacteria. While Salmonella typhimurium and Legionella pneumophila normally reside in the vacuole, specific mutants (sifA and sdhA, respectively) that aberrantly enter the cytosol triggered Caspase-11, enhancing clearance of S. typhimurium sifA in vivo. This response did not require NLRP3, NLRC4, or ASC inflammasome pathways. Burkholderia species that naturally invade the cytosol also triggered Caspase-11, protecting mice from lethal challenge with B. thailandensis and B. pseudomallei. Thus, Caspase-11 is critical for surviving exposure to ubiquitous environmental pathogens.
Canonical inflammasomes, such as NLRP3, NLRC4, and AIM2, are cytosolic sensors that detect pathogens or danger signals and activate Caspase-1, leading to secretion of the proinflammatory cytokines interleukin (IL)-1β and IL-18, and pyroptosis, a form of programmed cell death (1). Pyrin domain-containing inflammasomes, including NLRP3, signal through the ASC adaptor protein to recruit Caspase-1 (Fig. S1). Many diverse agonists cause cytosolic perturbations that are detected through NLRP3; however the underlying mechanisms remain obscure (2). In contrast, the CARD domain-containing inflammasome NLRC4 can signal directly to Caspase-1 resulting in pyroptosis, as well as indirectly through ASC to promote IL-1β and IL-18 secretion (Fig. S1) (1, 3). NLRC4 detects bacterial flagellin and type III secretion system (T3SS) rod or needle components within the macrophage cytosol (4–6). Together, NLRC4 and the ASC dependent inflammasomes account for all known canonical Caspase-1 activation pathways.
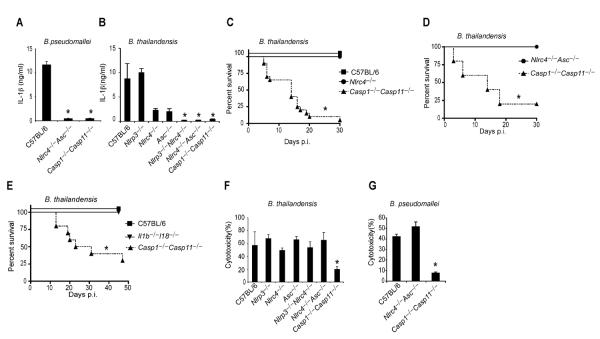
Burkholderia pseudomallei is a Gram-negative bacterium endemic to Southeast Asia that causes mellioidosis and is a potential biologic weapon (7). B. pseudomallei uses a T3SS to escape the phagosome and replicate in the cytosol. NLRC4 and NLRP3 both detect B. pseudomallei, promoting IL-1β secretion from murine bone marrow-derived macrophages (BMM) ((8) and Fig. 1A). Despite encoding many of the same virulence factors as B. pseudomallei, including T3SS and T6SS, the closely related B. thailandensis is far less virulent (9). We therefore hypothesized that NLPR3 and NLRC4 also detect B. thailandensis, and indeed, NLRP3 and NLRC4 accounted for all IL-1β secretion in response to B. thailandensis (Fig. 1B). We next determined whether inflammasome activation is critical to survival following B. thailandensis challenge using Caspase-1 deficient mice. Kayagaki et al. recently showed that all existing Caspase-1 deficient mice also lack Caspase-11 due to the backcrossing of a mutant Casp11 allele from 129 into C57BL/6 mice (10). Inflammasome detection was critical for resistance to B. thailandensis, as Casp1−/−Casp11−/− animals succumbed to the infection (Fig. 1C, S2A). In contrast, wild type C57BL/6 mice survived high dose intraperitoneal or intranasal challenge (Fig. 1C, S2A). Surprisingly, Nlrc4−/−Asc−/− mice that are deficient in all known canonical inflammasomes were also resistant (Fig. 1D, S2B). This indicated that an unknown signaling pathway provides protection via either Caspase-1 or -11 (see pathway schematic Fig. S1). Resistance to B. thailandensis was at least partially independent of IL-1β and IL-18, depending on the route of infection (Fig. 1E, S2C), suggesting that both cytokines and pyroptosis can contribute to protection. We therefore examined pyroptosis in vitro, and found that cytotoxicity in response B. thailandensis was impaired in Casp1−/−Casp11−/− BMM (Fig. 1F). Consistent with our in vivo data, pyroptosis in vitro did not require Nlrc4 or Asc (Fig. 1F). B. pseudomallei similarly triggered pyroptosis in Nlrc4−/−Asc−/− macrophages (Fig. 1G). These results indicate that a pyroptosis-inducing pathway distinct from all known canonical inflammasomes detects B. thailandensis and protects against lethal infection.
Fig. 1. Burkholderia detection and protection conferred by Casp1/11 is independent of all known canonical inflammasomes.
Lipopolysaccharide (LPS) primed BMMs were infected with B. pseudomallei (A, G) or B. thailandensis (B, F) for 4h. (A, B) IL-1β secretion was determined by ELISA or (F, G) cytotoxicity was determined by LDH release assay. (C, D, E) Survival curves of wild type C57BL/6 or the indicated knockout mice infected 2×107 cfu i.p. with B. thailandensis. Data are representative of at least 3 (A, B, F, G) or 2 (D, E) experiments. (C) Data are pooled from 3 experiments. For number of mice in each panel see Table S2. Statistically significant differences with respect to controls are indicated (Student's T-test or log rank test for survival; * = p ≤ 0.05, n.s. = p > 0.05).
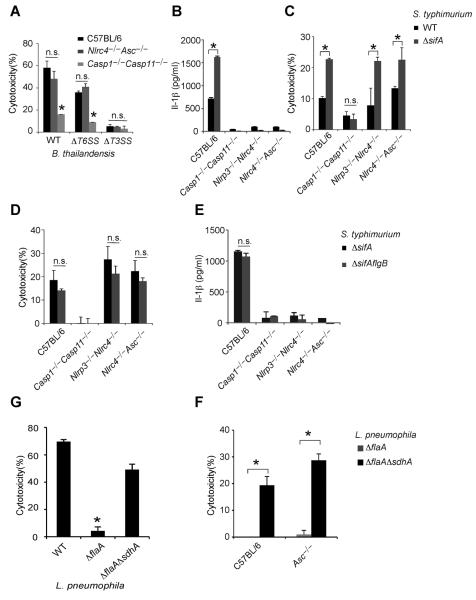
Inflammasomes discriminate pathogens from non-pathogens by detecting contamination or perturbation of the cytosolic compartment (11). The B. thailandensis T3SS facilitates bacterial access to the cytosol, and was required for induction of pyroptosis, whereas the virulence-associated T6SS was dispensable (Fig. 2A). We therefore hypothesized that macrophages detect vacuolar lysis or release of bacteria into the cytosol.
Fig. 2. Diverse cytosolic bacteria activate pyroptosis independent of NLRC4, NLRP3 and ASC.
(A) LPS-primed BMMs were infected for 4h with either B. thailandensis or the indicated mutants and cytotoxicity was determined. BMMs were infected for 8h with (B) S. typhimurium or S. typhimurium ΔsifA, (D) S. typhimurium ΔsifA or S. typhimurium ΔsifA flgB (D) and cytotoxicity was determined. LPS-primed BMM were infected for 8h with S. typhimurium or S. typhimurium ΔsifA (C), S. typhimurium ΔsifA or S. typhimurium ΔsifA flgB (E) and IL-1β secretion was determined. (F, G) Cytotoxicity in wild type or Asc−/− BMMs infected for 4h with L. pneumophila, L. pneumophila ΔflaA or L. pneumophila ΔsdhA ΔflaA. Cytotoxicity was determined by LDH release and IL-1β secretion by ELISA. Data are representative of at least 3 experiments. Statistically significant differences with respect to controls are indicated (Student's T-test; * = p ≤ 0.05, n.s. = p > 0.05).
In order to establish their intracellular vacuolar growth niche, Salmonella typhimurium and Legionella pneumophila use T3SS and T4SS, respectively, to translocate effector proteins that work in concert to maintain the stability of these altered bacteria-containing vacuoles (12–14). Loss of the S. typhimurium SifA or L. pneumophila SdhA effectors causes rupture of the vacuole and release of bacteria into the cytosol (15–17). S. typhimurium uses two distinct T3SS encoded by the Salmonella pathogenicity island 1 (SPI1) and SPI2; these two T3SS translocate distinct batteries of effectors, such as SifA by SPI2 (18). While S. typhimurium-expressing SPI1 and flagellin are readily detected by NLRC4 (19, 20), bacteria grown under conditions that mimic the vacuolar environment express SPI2 and repress flagellin, minimizing canonical inflammasome detection (1, 11, 21). Infection of BMMs with S. typhimurium that lacked sifA, however, significantly increased IL-1β secretion and pyroptosis (Fig. 2B–C). IL-1β secretion was dependent on canonical inflammasomes (Fig. 2B), whereas pyroptosis was still observed in Nlrc4−/−Asc−/− and Nlrp3−/−Nlrc4−/− macrophages (Fig. 2C). Furthermore, the NLRC4 inflammasome agonist flagellin was not required for these responses (Fig. 2D, 2E). Thus, macrophages detect S. typhimurium when it aberrantly enters the cytosol, activating pyroptosis independent of all known canonical inflammasomes.
L. pneumophila also translocates flagellin through its T4SS. Thus, L. pneumophila mutants lacking flagellin (ΔflaA) evaded NLRC4 detection (Fig. 2F) (2). In contrast, L. pneumophila ΔflaA ΔsdhA mutants induce Caspase-1 activation (16, 17), IL-1β secretion (17), and pyroptosis (Fig. 2F; (17)). The AIM2-ASC canonical inflammasome has been implicated in L. pneumophila ΔflaA ΔsdhA-induced IL-1β secretion, likely by detecting DNA released from bacteria lysing in the cytosol; however, the role of AIM2-ASC in pyroptosis was not examined (17). Analogous to S. typhimurium ΔsifA, L. pneumophila ΔflaA ΔsdhA induced pyroptosis in the absence of flagellin and ASC (Fig. 2G), ruling out all canonical inflammasomes in triggering pyroptosis under these infection conditions. These data demonstrate that diverse bacteria are detected in the cytosol.
Because IL-1β secretion required the canonical inflammasomes whereas pyroptosis did not, we hypothesized that cell death is triggered by a distinct mechanism mediated by Caspase-11. Like Caspase-1, Caspase-11 is an inflammatory caspase that can directly trigger pyroptosis (Fig. S1). Caspase-11 can also promote IL-1β secretion dependent upon NLRP3, ASC, and Caspase-1 (10, 22–24). Because Caspase-1 is activated by recruitment to an oligomerized platform known as the inflammasome, Kayagaki et al. hypothesized that a similar oligomeric structure would activate Caspase-11, which they termed the non-canonical inflammasome (10). Although the cholera toxin B subunit and many different Gram-negative bacteria can trigger Caspase-11 activation in vitro (10, 22–24), the nature of the physiologic stimulus that activates Caspase-11 during infection remains uncertain.
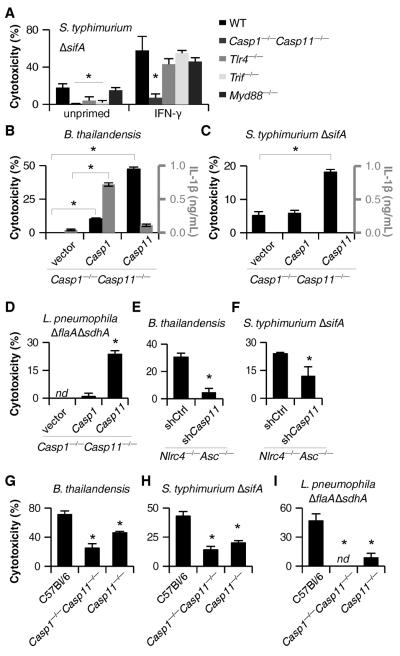
Caspase-11 activation requires priming through a Toll like receptor 4 (TLR4)-TRIF-STAT1 pathway (10, 22–24). Consistent with this, Tlr4−/− and Trif−/− macrophages did not undergo pyroptosis after S. typhimurium ΔsifA infection, whereas cell death was observed in macrophages deficient in the other TLR4 adaptor, Myd88 (Fig. 3A). This dependence could be overcome by priming the macrophages with interferon (IFN)-γ (Fig. 3A), which signals through STAT1. Interestingly, IFN-γ or LPS priming significantly increased the sensitivity of macrophages to S. typhimurium ΔsifA (Fig. 3A, S3A). These priming effects correlated with increased Caspase-11 expression (Fig. S3B–C), but could also be mediated by enhancing aberrant vacuolar rupture. We used retroviruses to complement Casp1−/−Casp11−/− macrophages with either Casp1 or Casp11 in order to determine which was involved. Caspase-11 alone promoted pyroptosis without IL-1β secretion after B. thailandensis infection, whereas Caspase-1 enabled both responses (Fig. 3B). This is consistent with B. thailandensis detection through NLRC4 and/or NLRP3 activating Caspase-1 (8) and an additional pathway activating Caspase-11. In contrast, the responses to S. typhimurium ΔsifA or L. pneumophila ΔflaA ΔsdhA acted through Caspase-11, and not Caspase-1 (Fig. 3C–D). We further confirmed that Caspase-11 was responsible for the cell death observed in Nlrc4−/−Asc−/− macrophages using short hairpin (sh)RNAmir (Fig. 3E–F, S3E). Finally, Casp11−/− BMM revealed that Caspase-11 was required for pyroptosis after B. thailandensis, S. typhimurium ΔsifA, and L. pneumophila ΔflaA ΔsdhA (Fig. 3G–I). Although a previous report suggested that NLRC4 signals through Caspase-11 to alter phagosomal trafficking (25), we saw no evidence that NLRC4 contributes to Caspase-11 dependent cell death (Fig. 1F, 2D, S4). Pyroptosis initiated by Caspase-11 was morphologically similar to pyroptosis triggered by Caspase-1 (Fig. S5A–B). Therefore, macrophages activate Caspase-11 in response to cytosolic B. thailandensis, S. typhimurium, or L. pneumophila (Fig. S1).
Fig. 3. Caspase-11 mediates pyroptosis after infection by cytosolic bacteria. (A–I).
Macrophage cytotoxicity and IL-1β secretion were determined after infection with S. typhimurium ΔsifA (8h), L. pneumophila ΔflaA ΔsdhA (4h), or B. thailandensis (4h). (A) C57BL/6, Casp1−/−Casp11−/−, Tlr4−/−, Trif−/−, and Myd88−/− BMM infected with S. typhimurium ΔsifA with or without IFN-γ priming prior to infection. (B–C) Retroviral transduction was used to complement Casp1 or Casp11 in Casp1−/−Casp11−/− iBMM. Macrophages were primed with LPS (B) or IFN-γ (C) and responses to B. thailandensis (B) or S. typhimurium ΔsifA (C) infection were examined. (D) Control or complemented Casp1−/−Casp11−/− BMM infected with L. pneumophila ΔflaA ΔsdhA. (E–F) Retroviral transduction was used to introduce control or Casp11-targeting shRNAmir into Nlrc4−/−Asc−/− iBMM. Macrophages were primed overnight with LPS (E) or IFN-γ (F) and then infected as indicated. (G–I) C57BL/6, Casp1−/−Casp11−/−, and Casp11−/− BMM infected with B. thailandensis (G), S. typhimurium ΔsifA (H), or L. pneumophila ΔflaA ΔsdhA (I). Data are representative of at least 3 (A–C, E, G, H) or 2 (D, F, I) independent experiments. Statistically significant differences with respect to controls are indicated (Student's T-test; * = p ≤ 0.05). nd, none detected.
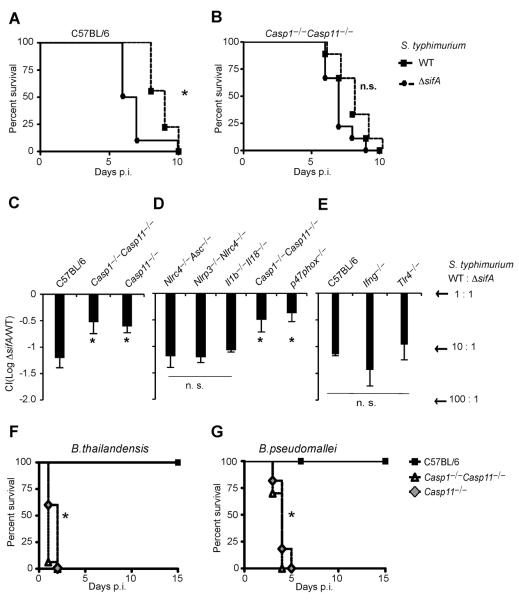
S. typhimurium ΔsifA is attenuated (15), attributed to the role of SifA in coordinating intracellular trafficking of the Salmonella-containing vacuole. We hypothesized that this attenuation was actually due to innate immune detection though Caspase-11. Indeed, S. typhimurium ΔsifA was mildly attenuated in C57BL/6 mice as expected, but this was not replicated in Casp1−/−Casp11−/− mice (Fig. 4A–B). We next determined the relative clearance of S. typhimurium ΔsifA during co-infection with wild type S. typhimurium, a more quantitative measure of virulence than lethal challenge. 16-fold fewer S. typhimurium ΔsifA were recovered from C57BL/6 mice 48h post infection. However, only a 4-fold reduction was seen in Casp11−/− mice (Fig. 4C), indicating that Caspase-11 clears S. typhimurium ΔsifA in vivo; in contrast, wild type S. typhimurium effectively evades Caspase-11 (23) by remaining within the vacuole. The remaining S. typhimurium ΔsifA attenuation likely reflects the role of sifA as a virulence factor promoting intracellular replication. Moreover, all known canonical inflammasomes were dispensable for S. typhimurium ΔsifA clearance, as were IL-1β and IL-18 (Fig. 4D), implicating pyroptosis as the mechanism of clearance. Clearance of bacteria after pyroptosis is mediated by neutrophils through generation of reactive oxygen (21). Consistent with this, NADPH oxidase deficient p47phox−/− mice were also defective for clearance of S. typhimurium ΔsifA (Fig. 4D). Interestingly, TLR4 and IFN-γ were not required (Fig. 4E), suggesting that there is redundant priming of Caspase-11 pathways in vivo. Therefore, Caspase-11 protects mice from S. typhimurium ΔsifA, and because IL-1β and IL-18 are not required, pyroptosis is likely to be the mechanism of bacterial clearance in this case.
Fig. 4. Caspase-11 protects against cytosolic bacteria in vivo.
(A, B) S. typhimurium or S. typhimurium ΔsifA were injected i.p. into C57BL/6 (1000 cfu) or Casp1−/−Casp11−/− mice (250 cfu) and survival was monitored. (C–E) The indicated mice were infected with 5×104 cfu of both wild type S. typhimurium and S. typhimurium ΔsifA marked with ampicillin or kanamycin resistance, respectively. Bacterial loads from 3–4 mice per genotype were determined 48h later and competitive index calculated (CI = log (S. typhimurium ΔsifA/ S. typhimurium)). A CI of −1 corresponds to 10 cfu of S. typhimurium for every 1 cfu of S. typhimurium ΔsifA. (F–G) C57BL/6, Casp1−/−Casp11−/−, or Casp11−/− mice were infected with (F) 2×107 cfu mouse passaged B. thailandensis i.p. or (G) 100 cfu B. pseudomallei i.n. (A, B, F, G) Data are pooled from two independent experiments. (C–E) Representative of 2 experiments. For number of mice in each panel see Table S2. Statistically significant differences with respect to controls are indicated (Student's T-test or log rank test for survival; * = p ≤ 0.05, n.s. = p > 0.05).
We next examined the susceptibility of Casp11−/− mice to the naturally cytosolic pathogens B. thailandensis and B. pseudomallei. While C57BL/6 mice are resistant to B. thailandensis infection, Casp11−/− mice succumbed (Fig. 4F). Likewise, Casp11−/− succumbed to B. pseudomallei infection, whereas C57BL/6 mice survived (Fig. 4G). Since Nlrc4−/− mice are also susceptible to B. pseudomallei infection (8), we conclude that both Caspase-1 and Caspase-11 play critical roles in limiting B. pseudomallei infection.
Collectively, these data demonstrate, for the first time, that Caspase-11 protects animals from lethal infection by bacteria that have the ability to invade the cytosol. This could be critical for defense against ubiquitous environmental bacteria such as B. thailandensis that encode virulence factors, but have not evolved to evade Caspase-11 detection. It will be interesting to determine whether Caspase-11 is activated in response to the process of vacuolar rupture or the presence of bacteria within the cytosol. Caspase-11 also responds to vacuolar bacteria under delayed kinetics, but such responses have not been shown to provide protection from infection in vivo (10, 22–24). LPS-induced septic shock is mediated by Caspase-11 (10), suggesting that Caspase-11 can be activated by other mechanisms besides cytosol-localized bacteria. Thus, we propose that Caspase-11 provides protection against pathogens, but is dysregulated during overwhelming infection, contributing to septic shock and mortality. It will be interesting to determine if Caspase-11 triggers eicosanoid secretion as is seen for Caspase-1, and whether these mediators contribute to septic shock (26). The identity of the hypothesized non-canonical inflammasome(s) that activate Caspase-11 and the precise nature of the activating signal will shed more light on the mechanisms by which Caspase-11 can both promote innate immunity and exacerbate immunopathology. These insights may lead to novel therapies to treat infection and sepsis.
Supplementary Material
Acknowledgments
The authors thank V. Dixit sharing mice (under a materials transfer agreement) and M. Heise for sharing mice, and S. Miller, J. Mougous, and H. Schweizer for sharing bacterial strains. We also thank D. Rodriguez and L. Zhou for managing mouse colonies. The data presented in this manuscript are tabulated in the main paper and in the supplementary materials. This work was supported by NIH grants AI097518 (EAM) and AI057141 (EAM and AA), AI065359 (PAC), AI075039 (REV), AI080749 (REV), and AI063302 (REV), Investigator Awards from the Burroughs Wellcome Fund and Cancer Research Institute (REV), and an NSF graduate fellowship (MFF).
References and Notes
- 1.Miao EA, Rajan JV, Aderem A. Caspase-1-induced pyroptotic cell death. Immunol Rev. 2011;243:206–214. doi: 10.1111/j.1600-065X.2011.01044.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Franchi L, Muñoz-Planillo R, Núñez G. Sensing and reacting to microbes through the inflammasomes. Nature Immunology. 2012;13:325–332. doi: 10.1038/ni.2231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Broz P, Von Moltke J, Jones JW, Vance RE, Monack DM. Differential requirement for Caspase-1 autoproteolysis in pathogen-induced cell death and cytokine processing. Cell Host Microbe. 2010;8:471–483. doi: 10.1016/j.chom.2010.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Miao EA, et al. From the Cover: Innate immune detection of the type III secretion apparatus through the NLRC4 inflammasome. Proceedings of the National Academy of Sciences. 2010;107:3076–3080. doi: 10.1073/pnas.0913087107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhao Y, et al. The NLRC4 inflammasome receptors for bacterial flagellin and type III secretion apparatus. Nature. 2011;477:596–600. doi: 10.1038/nature10510. [DOI] [PubMed] [Google Scholar]
- 6.Kofoed EM, Vance RE. Innate immune recognition of bacterial ligands by NAIPs determines inflammasome specificity. Nature. 2011;477:592–595. doi: 10.1038/nature10394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wiersinga WJ, Currie BJ, Peacock SJ. Melioidosis. N Engl J Med. 2012;367:1035–1044. doi: 10.1056/NEJMra1204699. [DOI] [PubMed] [Google Scholar]
- 8.Ceballos-Olvera I, Sahoo M, Miller MA, Barrio LD, Re F, Philpott DJ, editors. Inflammasome-dependent Pyroptosis and IL-18 Protect against Burkholderia pseudomallei Lung Infection while IL-1β Is Deleterious. PLoS Pathog. 2011;7:e1002452. doi: 10.1371/journal.ppat.1002452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wiersinga WJ, Van Der Poll T, White NJ, Day NP, Peacock SJ. Melioidosis: insights into the pathogenicity of Burkholderia pseudomallei. Nat Rev Micro. 2006;4:272–282. doi: 10.1038/nrmicro1385. [DOI] [PubMed] [Google Scholar]
- 10.Kayagaki N, et al. Non-canonical inflammasome activation targets caspase-11. Nature. 2011;479:117–121. doi: 10.1038/nature10558. [DOI] [PubMed] [Google Scholar]
- 11.Miao EA, Rajan JV. Salmonella and Caspase-1: A complex Interplay of Detection and Evasion. Front Microbiol. 2011;2:85. doi: 10.3389/fmicb.2011.00085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schroeder N, Mota LJ, Méresse S. Salmonella-induced tubular networks. Trends Microbiol. 2011;19:268–277. doi: 10.1016/j.tim.2011.01.006. [DOI] [PubMed] [Google Scholar]
- 13.Shoma S, et al. Critical involvement of pneumolysin in production of interleukin-1alpha and caspase-1-dependent cytokines in infection with Streptococcus pneumoniae in vitro: a novel function of pneumolysin in caspase-1 activation. Infect Immun. 2008;76:1547–1557. doi: 10.1128/IAI.01269-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ge J, Shao F. Manipulation of host vesicular trafficking and innate immune defence by Legionella Dot/Icm effectors. Cell Microbiol. 2011;13:1870–1880. doi: 10.1111/j.1462-5822.2011.01710.x. [DOI] [PubMed] [Google Scholar]
- 15.Beuzón CR, et al. Salmonella maintains the integrity of its intracellular vacuole through the action of SifA. EMBO J. 2000;19:3235–3249. doi: 10.1093/emboj/19.13.3235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Creasey EA, Isberg RR. The protein SdhA maintains the integrity of the Legionella-containing vacuole. Proceedings of the National Academy of Sciences. 2012;109:3481–3486. doi: 10.1073/pnas.1121286109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ge J, Gong Y-N, Xu Y, Shao F. Preventing bacterial DNA release and absent in melanoma 2 inflammasome activation by a Legionella effector functioning in membrane trafficking. Proceedings of the National Academy of Sciences. 2012 doi: 10.1073/pnas.1117490109. doi:10.1073/pnas.1117490109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van der Heijden J, Finlay BB. Type III effector-mediated processes in Salmonellainfection. Future Microbiology. 2012;7:685–703. doi: 10.2217/fmb.12.49. [DOI] [PubMed] [Google Scholar]
- 19.Franchi L, et al. Cytosolic flagellin requires Ipaf for activation of caspase-1 and interleukin 1β in salmonella-infected macrophages. Nature Immunology. 2006;7:576–582. doi: 10.1038/ni1346. [DOI] [PubMed] [Google Scholar]
- 20.Miao EA, et al. Cytoplasmic flagellin activates caspase-1 and secretion of interleukin 1β via Ipaf. Nature Immunology. 2006;7:569–575. doi: 10.1038/ni1344. [DOI] [PubMed] [Google Scholar]
- 21.Miao EA, et al. Caspase-1-induced pyroptosis is an innate immune effector mechanism against intracellular bacteria. Nature Immunology. 2010;11:1136–1142. doi: 10.1038/ni.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gurung P, et al. TRIF-mediated caspase-11 production integrates TLR4- and Nlrp3 inflammasome-mediated host defense against enteropathogens. Journal of Biological Chemistry. 2012 doi: 10.1074/jbc.M112.401406. doi:10.1074/jbc.M112.401406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Broz P, et al. Caspase-11 increases susceptibility to Salmonella infection in the absence of caspase-1. Nature. 2012 doi: 10.1038/nature11419. doi:10.1038/nature11419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rathinam VAK, et al. TRIF Licenses Caspase-11-Dependent NLRP3 Inflammasome Activation by Gram-Negative Bacteria. Cell. 2012;150:606–619. doi: 10.1016/j.cell.2012.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Akhter A, et al. Caspase-11 Promotes the Fusion of Phagosomes Harboring Pathogenic Bacteria with Lysosomes by Modulating Actin Polymerization. Immunity. 2012 doi: 10.1016/j.immuni.2012.05.001. doi:10.1016/j.immuni.2012.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Von Moltke J, et al. Rapid induction of inflammatory lipid mediators by the inflammasome in vivo. Nature. 2012 doi: 10.1038/nature11351. doi:10.1038/nature11351. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.