Biaryls, particularly those containing one or more heterocyclic components, are ubiquitous among pharmaceutically active compounds, natural products, and agrochemicals. Palladium-catalyzed cross-coupling reactions have been extensively studied and practiced in both academic[1] and industrial[2] settings for their preparation. Although various palladium-catalyzed cross-coupling methods to form sp2-sp2 carbon-carbon bonds have been developed, the Negishi coupling is one of the most frequently utilized. The utility of Negishi couplings is partially due to the fact that organozinc reagents, despite considerable basicity, are compatible with a large number of sensitive functional groups.[3] Equally important is that a wide array of highly functionalized organozinc reagents can be readily accessed.[4] Additionally, the recent development of solid salt-stabilized organozinc reagents, which are much less sensitive towards air and moisture,[5,6] has also rendered the Negishi coupling a more practical and user-friendly technique for synthetic organic chemists.
Numerous studies have been devoted to the development of more general and efficient catalyst systems for Negishi cross-coupling reactions. In 2001, Fu described the first general protocol to effect the Negishi cross-coupling of aryl chlorides using Pd[P(tBu3)]2 (2 mol %) as the precatalyst in THF/NMP at 100 °C.[7] In 2004, our group reported a highly active catalyst based on dialkylbiarylphosphine L1 (RuPhos), which permitted the efficient generation of a wide range of sterically hindered tri- and tetra-ortho substituted biaryls in THF at 70 °C with low catalyst loadings.[8] In a series of publications, Knochel demonstrated the utility of biarylphosphine L2 (SPhos) as the supporting ligand for palladium-catalyzed Negishi coupling.[3,9] More recently, the Organ group has also developed a well-tailored Pd-PEPPSI-IPent precatalyst capable of combining a variety of extremely sterically hindered substrates to afford tetra-ortho substituted biaryls under exceptionally mild conditions with excellent yields.[10,11]
Despite these advances, significant challenges still remain. Notably, while simple aryl halides and arylzinc reagents are easily transformed, couplings involving heteroarylzinc reagents and heteroaryl halides are often less successful. This difficulty is due partly to the altered electronic properties of heterocyclic compounds, as well as to the presence of heteroatoms capable of binding to the transition-metal center and leading to catalyst deactivation and decomposition.[12] In particular, the transformation of five-membered heterocycles bearing more than one heteroatoms, such as pyrazoles and imidazoles, has proven to be challenging.[13,14] Thus, the development of a catalyst system capable of facilitating the coupling of a diverse range of heteroaryl and functionalized substrates under mild conditions is still highly desirable. Herein, we report a general catalyst system based on a palladacycle precatalyst ligated by dialkylbiarylphosphine ligand L3 (XPhos) for the palladium-catalyzed Negishi cross-couplings at ambient temperature or with low catalyst loadings. With this system myriad heteroaryl coupling partners, many of which were previously unsuccessful substrates,[8] can now be effectively coupled. In addition, we report the success of this system for the Negishi coupling of polyfluoroarylzinc reagents.
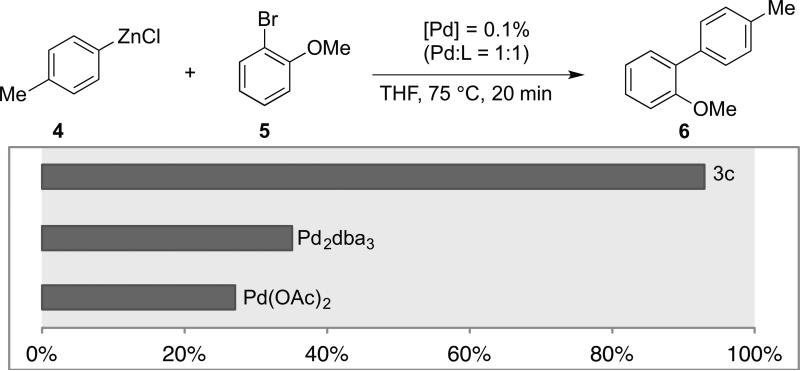
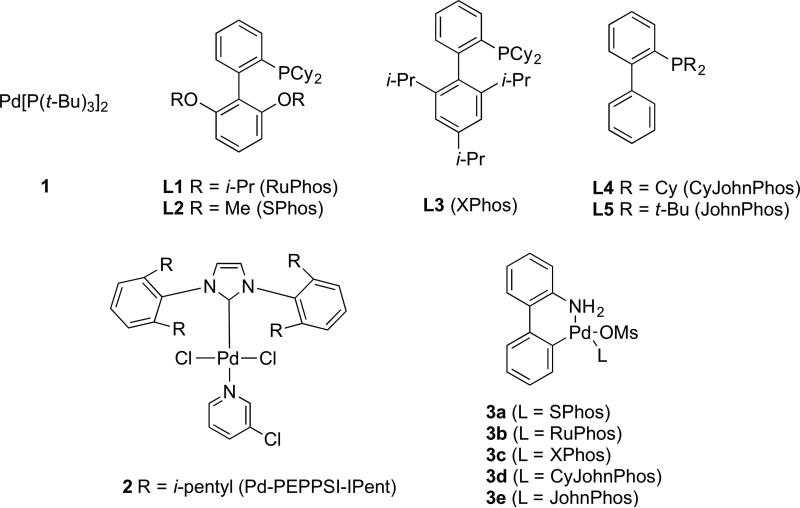
We recently reported the development of a new class of easily prepared, air- and moisture-stable aminobiphenyl-based palladacycle mesylate precatalysts capable of rapidly and quantitatively generating the catalytically active L1Pd(0) species under basic conditions at room temperature.[15-18] Given their intrinsic basicity, we reasoned that organozinc reagents could readily activate these precatalysts in situ, and furthermore that the efficient and rapid formation of L1Pd(0) facilitated by these precatalysts would allow for Negishi cross-couplings under mild conditions and, potentially, with low catalyst loadings. Thus, palladacycle precatalyst 3c was compared with other commonly used palladium sources in combination with L3 for the Negishi cross-coupling of p-tolylzinc chloride (4) and 2-bromoanisole (5) (Figure 1). Interestingly, the protocol employing palladacycle precatalyst 3c facilitated the coupling in 92% yield after just 20 minutes, whereas the use of other palladium sources such as Pd(OAc)2 and Pd2dba3 resulted in product yields lower than 40% in the same amount of time. These results clearly indicate that palladacycle precatalyst 3 generates the catalytically active L1Pd(0) species most efficiently.
Figure 1.
Comparison of precatalyst 3c with several other palladium sources. Conditions: p-tolylzinc chloride (0.65 mmol), 2-bromoanisole (0.5 mmol), Pd (0.1%, Pd:L = 1:1), L = L3, THF, 75 °C, 20 min; yields were determined by GC analysis of the crude reaction mixture.
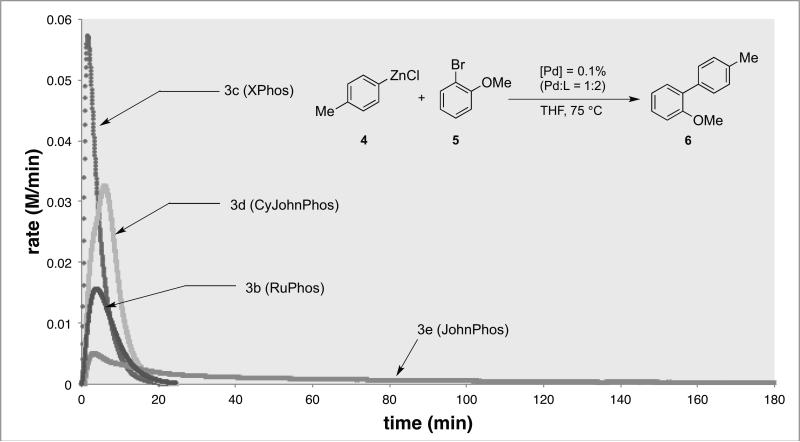
We next re-evaluated the ligand effects using palladacycle precatalysts of type 3.[8] Given the success of bulky monophosphinobiaryl ligands L3[17,19] and L5[20] in facilitating Suzuki-Miyaura cross-coupling with high reactivity, we were interested in carefully evaluating their activity for Negishi cross-couplings. Differences in reaction rates for catalyst systems derived from ligand L1, L3, L4 and L5 were determined by monitoring the reaction progress using calorimetric analysis.[21a] As depicted by Figure 2, reaction rates for all of the catalyst systems derived from L1, L4 and L5 are significantly lower than that observed when L3 was used as the supporting ligand. While catalyst systems employing L3 and L4 both facilitated full conversion of 2-bromoanisole after approximately 30 min, the initial rate of the catalyst generated from L4 was about 50% lower than the rate of the catalyst derived from L3. This result illustrates the influence of the size of the substituents on the non-phosphorus-containing ring of the dialkylbiarylphosphine ligand on catalyst activity, in accordance with our previous findings.[21b,c] Further, the benefit of using a ligand with cyclohexyl rather than tert-butyl substituents on the phosphorous atom of the monophosphinobiaryl ligand is highlighted by the 10-fold difference in reaction rate between catalysts based on L4 and L5. Interestingly, a catalyst derived from L1 furnished only 55% conversion at 0.1 mol% Pd loading, indicating that the catalyst based on L1 is less effective than that generated from L3 at low palladium loadings.[22] Taken together, these studies suggest that a catalyst system based on L3 exhibits the highest activity for Negishi couplings.
Figure 2.
Comparison of precatalysts with different dialkylbiarylphosphine ligands. Conditions: p-tolylzinc chloride (0.65 mmol), 2-bromoanisole (0.5 mmol), 3 (0.1%), L = L2-5 (0.1%), THF, 75 °C.
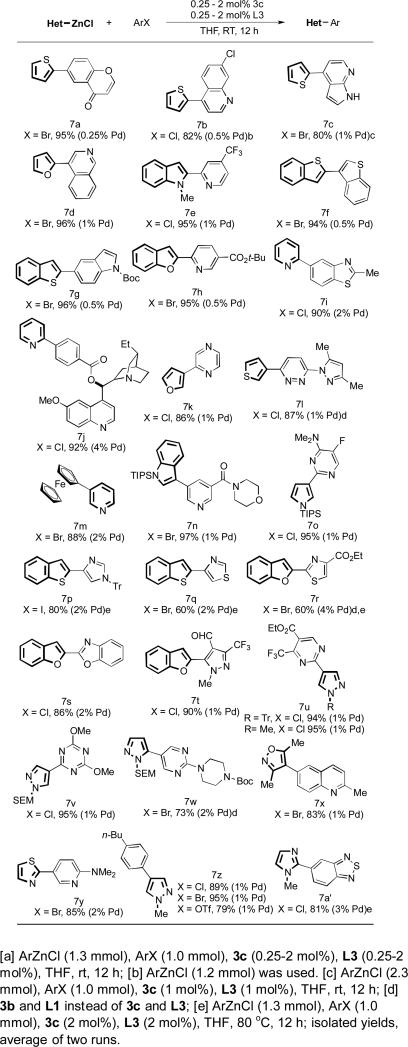
In light of the importance of heterocyclic compounds in medicinal chemistry and materials chemistry,[23] we focused on the Negishi cross-coupling of heteroarylzinc reagents with heterocyclic halides and pseudohalides (Table 1). We were interested in the Negishi coupling of five-membered 2-heteroaromatic zinc chlorides (i.e., 2-furyl, 2-thienyl, 2-benzofuranyl, 2-benzothiophenyl and 2-indolyl zinc chlorides) and 2-pyridylzinc chlorides, as the corresponding organoboron reagents are difficult substrates for Suzuki-Miyaura coupling due to the rapid protodeboronation,[17] and, in the case of 2-pyridylboronate,[24] the relatively slow rate of transmetallation.[25] We found that by using 0.25–4 mol% of 3c, these heteroaryl zinc reagents (7a–7j) could be efficiently coupled at room temperature to furnish heterobiaryls in excellent yield. 3-Furyl, 3-thienyl, 3-pyrroryl, 3-indolyl and 3-pyridylzinc chlorides were equally effective under the current protocol (7k–7o). Azole coupling partners were also evaluated with the current catalyst system. 4-Iodo-1-tritylimidazole (7p), 2- and 4-bromothiazoles (7q and 7r) proved to be more challenging substrates, requiring higher reaction temperatures to obtain appreciable amounts of coupled product. Benzo-fused azole (7s) and N-substituted pyrazole (7t) electrophiles were excellent substrates for this methodology and could be converted to the desired products in excellent yields at room temperature.[26] Azole containing nucleophilic coupling partners such as pyrazolyl (7u, 7v, 7w and 7z), 4-isoxazolyl (7x), 2-thiazolyl (7y) and 2-imidazolyl (7a’) zinc chlorides can also be smoothly cross-coupled using our protocol. Finally, different types of halides and pseudohalides can all be effectively coupled with organozinc reagents under our conditions.
Table 1.
Cross-Coupling of Heteroarylzinc Reagents and Heteroaryl Halidesa.
|
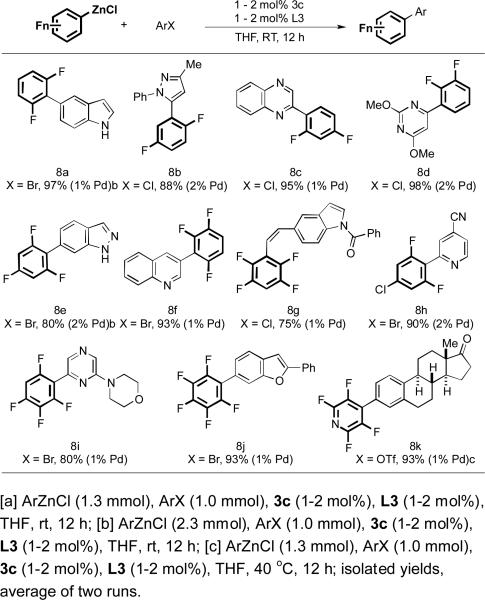
To further demonstrate the utility of our methodology, we sought to extend the scope of our catalyst system to reactions of polyfluorophenylzinc reagents (Table 2). We were particularly intrigued by the coupling of fluorinated arylzinc reagents because methods for the preparation of polyfluorinated biaryls remain underdeveloped. Reactions of corresponding polyfluorophenyl boronic acids constitute a challenging family of nucleophiles for Suzuki-Miyaura coupling due to rapid protodeboronation.[17] For example, the half-life time of 2,3,6-trifluorophenylboronic acid under our most recently developed conditions for Suzuki-Miyaura coupling is only 2 min,[17] rendering the Suzuki-Miyaura coupling of this boronic acid a formidable task. We therefore reasoned that Negishi coupling could serve as an important alternative to achieve this type of transformation.[27] Subjecting various types of polyfluorophenylzinc reagents to the current protocol furnished coupling products in uniformly good yields (8a-8j). Moreover, by increasing the reaction temperature to 40 °C, perfluoro-4-pyridylzinc chloride reacted well with an aryl triflate derived from estrone (8k).[28,29]
Table 2.
Cross-Coupling of Polyfluoroarylzinc Reagents and Heteroaryl Halides at Room Temperaturea.
|
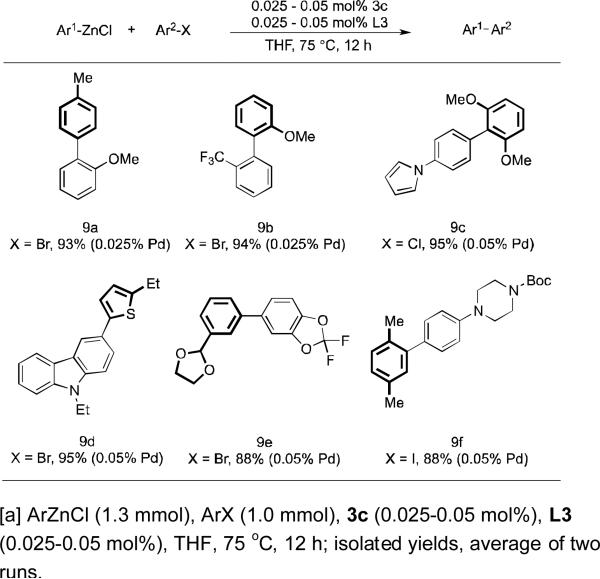
Encouraged by the high level of reactivity of our catalyst system, we set out to examine the scope of this system at extremely low levels of catalyst loading (Table 3). We were able to lower the catalyst loading to 0.025-0.05 mol% (turnover number = 2,000-4,000) while tolerating a variety of functional groups, including an acetal (9e), a tertiary amine (9f), an amide (9f) as well as heterocycles (9c and 9d). These results clearly demonstrate the ability of our catalyst to operate at low concentrations of catalyst for a range of functionalized substrates. However, we note that with the current catalyst system, substrates bearing an ortho coordinating substituent such as an ester or a ketone usually require catalyst loadings higher than 0.1 mol% to achieve full conversion.
Table 3.
Cross-Coupling of Arylzinc Chlorides and Aryl Halides at Low Catalyst Loadingsa.
|
In summary, through the use of our recently developed palladacycle precatalysts, we have identified a highly active catalyst system based on L3 for the Negishi cross-coupling of heteroarylzinc reagents and polyfluoroaryl zinc reagents under mild reaction conditions. Our method is effective with a broad scope of heteroaryl halides, pseudohalides and other types of challenging substrates, delivering a wide range of heterobiaryls that represent structural motifs frequently found in biologically active compounds.
Supplementary Material
Scheme 1.
Precatalysts and Ligands Employed for Negishi Cross-Coupling Reactions.
Acknowledgments
We thank the National Institutes of Health for financial support of this work (Grant GM46059). We thank Robert Todd (Aldrich) for a helpful discussion and a gift of ZnCl2·THF complex. We are grateful to Dr. Laurent Pellegatti (Massachusetts Institute of Technology) for insightful discussions and Dr. Meredeth A. McGowan (Massachusetts Institute of Technology) for help with the preparation of this manuscript. We acknowledge Nicholas C. Bruno (Massachusetts Institute of Technology) for providing the precatalyst 3 used in this study. MIT has patents on some of the ligands and precatalysts used in this work from which S.L.B. receives royalty payments.
References
- 1.a de Meijere A, Diederich F. Metal-Catalyzed Cross-Coupling Reactions. 2nd ed. Wiley-VCH; Weiheim: 2004. [Google Scholar]; b Negishi E.-i. Handbook of Organopalladium Chemistry for Organic Synthesis. Wiley-Interscience; New York: 2002. [Google Scholar]
- 2.a Magano J, Donetz JR. Chem. Rev. 2011;111:2177–2250. doi: 10.1021/cr100346g. [DOI] [PubMed] [Google Scholar]; b Corbet JP, Mignani G. Chem. Rev. 2006;106:2651–2710. doi: 10.1021/cr0505268. [DOI] [PubMed] [Google Scholar]
- 3.a Manolikakes G, Hernandez CM, Schade MA, Metzger A, Knochel P. J. Org. Chem. 2008;73:8422–8436. doi: 10.1021/jo8015852. [DOI] [PubMed] [Google Scholar]; b Manolikakes G, Schade MA, Hernandez CM, Mayer H, Knochel P. Org. Lett. 2008;10:2765–2768. doi: 10.1021/ol8009013. [DOI] [PubMed] [Google Scholar]; c Manolikakes G, Dong Z, Mayr H, Li J, Knochel P. Chem. Eur. J. 2009;15:1324–1328. doi: 10.1002/chem.200802349. [DOI] [PubMed] [Google Scholar]
- 4.a Knochel P, Jones P. Organozinc Reagents, A Practical Approach. Oxford University Press; New York: 1999. [Google Scholar]; b Knochel P, Singer RD. Chem. Rev. 1993;93:2117–2188. [Google Scholar]; c Hagg B, Mosrin M, Ila H, Malakhov V, Knochel P. Angew. Chem. Int. Ed. 2011;50:9794–9824. doi: 10.1002/anie.201101960. [DOI] [PubMed] [Google Scholar]
- 5.Bernhardt S, Manolikakes G, Kunz T, Knochel P. Angew. Chem. Int. Ed. 2011;50:9205–9209. doi: 10.1002/anie.201104291. [DOI] [PubMed] [Google Scholar]
- 6.Stathakis CI, Bernhardt S, Quint V, Knochel P. Angew. Chem. Int. Ed. 2012;51:9428–9432. doi: 10.1002/anie.201204526. [DOI] [PubMed] [Google Scholar]
- 7.Dai C, Fu GC. J. Am. Chem. Soc. 2001;123:2719–2724. doi: 10.1021/ja003954y. [DOI] [PubMed] [Google Scholar]
- 8.Milne JE, Buchwald SL. J. Am. Chem. Soc. 2004;126:13028–13032. doi: 10.1021/ja0474493. [DOI] [PubMed] [Google Scholar]
- 9.Melzig L, Metzger A, Knochel P. Chem. Eur. J. 2011;17:2948–2956. doi: 10.1002/chem.201002850. [DOI] [PubMed] [Google Scholar]
- 10.Çalimsiz S, Sayah M, Mallik D, Organ MG. Angew. Chem. Int. Ed. 2010;49:2014–2017. doi: 10.1002/anie.200906811. [DOI] [PubMed] [Google Scholar]
- 11.For recent reviews, see: Valente C, Belowich ME, Hadei N, Organ MG. Eur. J. Org. Chem. 2010;23:4343–4354.Valente C, Çalimsiz S, Hoi KH, Mallik D, Sayah M, Organ MG. Angew. Chem. Int. Ed. 2012;51:3314–3332. doi: 10.1002/anie.201106131.
- 12.Shen Q, Shekhar S, Stambuli JP, Hartwig JF. Angew. Chem. Int. Ed. 2005;44:1371–1375. doi: 10.1002/anie.200462629. [DOI] [PubMed] [Google Scholar]
- 13.Su M, Buchwald SL. Angew. Chem. Int. Ed. 2012;51:4710–4713. doi: 10.1002/anie.201201244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.For a recent review, see: Schnurch M, Flasik R, Khan AF, Spina M, Mihovilovic MD, Stanetty P. Eur. J. Org. Chem. 2006:3283–3307.
- 15.Bruno NC, Tudge MT, Buchwald SL. Chem. Sci. 2012 doi: 10.1039/C2SC20903A. accepted manuscript, DOI: 10.1039/C2SC20903A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Biscoe MR, Fors BP, Buchwald SL. J. Am. Chem. Soc. 2008;130:6686–6687. doi: 10.1021/ja801137k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kinzel T, Zhang Y, Buchwald SL. J. Am. Chem. Soc. 2010;132:14073–14075. doi: 10.1021/ja1073799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.For a recent review on the development of preformed palladium precatalysts, see: Li H, Seechurn CCCJ, Colacot TJ. ACS Catal. 2012;2:1147–1164.
- 19.a Billingsley KL, Anderson KW, Buchwald SL. Angew. Chem. Int. Ed. 2006;45:3484–3488. doi: 10.1002/anie.200600493. [DOI] [PubMed] [Google Scholar]; b Billingsley KL, Buchwald SL. J. Am. Chem. Soc. 2007;129:3358–3366. doi: 10.1021/ja068577p. [DOI] [PubMed] [Google Scholar]; c Oberli MA, Buchwald SL. Org. Lett. 2012;14:4606–4609. doi: 10.1021/ol302063g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.a Wolfe JP, Buchwald SL. Angew. Chem. Int. Ed. 1999;38:2413–2416. doi: 10.1002/(sici)1521-3773(19990816)38:16<2413::aid-anie2413>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]; b Wolfe JP, Singer RA, Yang BH, Buchwald SL. J. Am. Chem. Soc. 1999;121:9550–9561. [Google Scholar]
- 21.a Blackmond DG. Angew. Chem. Int. Ed. 2005;44:4302–4320. doi: 10.1002/anie.200462544. [DOI] [PubMed] [Google Scholar]; b Strieter ER, Blackmond DG, Buchwald SL. J. Am. Chem. Soc. 2003;125:13978–13980. doi: 10.1021/ja037932y. [DOI] [PubMed] [Google Scholar]; c Strieter ER, Blackmond DG, Buchwald SL. Angew. Chem. Int. Ed. 2006;45:925–928. doi: 10.1002/anie.200502927. [DOI] [PubMed] [Google Scholar]
- 22.The use of an SPhos-based palladacycle gave similar results as observed with RuPhos.
- 23.a Joule JA, Mills K. Heterocyclic Chemistry. 5th edition Wiley; Chichester: 2010. [Google Scholar]; b Leurs R, Bakker RA, Timmerman H, de Esch IJP. Nat. Rev. Drug Discovery. 2005;4:107–120. doi: 10.1038/nrd1631. [DOI] [PubMed] [Google Scholar]
- 24.For Suzuki-Miyaura coupling of 2-pyridyl boronates, see: Billingsley KL, Buchwald SL. Angew. Chem. Int. Ed. 2008;47:4695–4698. doi: 10.1002/anie.200801465.Yang DX, Colletti SL, Wu K, Song M, Li GY, Shen HC. Org. Lett. 2009;11:381–384. doi: 10.1021/ol802642g. For improved palladium-catalyzed processes that occur in high yield, see: Deng JZ, Paone DV, Ginnetti AT, Kurihara H, Dreher SD, Weissman SA, Stauffer SR, Burgey CS. Org. Lett. 2009;11:345–347. doi: 10.1021/ol802556f.Knapp DM, Gillis EP, Burke MD. J. Am. Chem. Soc. 2009;131:6961–6963. doi: 10.1021/ja901416p.Dick GR, Woerly EM, Burke MD. Angew. Chem. Int. Ed. 2012;51:2667–2672. doi: 10.1002/anie.201108608.
- 25.a Molander GA, Biolatto B. J. Org. Chem. 2003;68:4302–4314. doi: 10.1021/jo0342368. [DOI] [PubMed] [Google Scholar]; b Ishiyama T, Isahida K, Miyaura N. Tetrahedron. 2001;57:9813–9816. [Google Scholar]
- 26.Our attempts to couple 5-bromoimidazoles, 3-bromoimidazo[1,2-a]pyridine, 3-bromoimidazo[1,2-a]pyrazine and 6-chloroimidazo[2,1-b]thiazole were unsuccessful.
- 27.Abarbri M, Dehmel F, Knochel P. Tetrahedron Lett. 1999;40:7449–7453. [Google Scholar]
- 28.At an early stage of this study, freshly prepared solutions of ZnCl2 in THF were utilized in our procedure. In an effort to develop a method of enhanced operational simplicity, we evaluated the effectiveness of commercially available ZnCl2 solutions, and the 2-MeTHF solution of ZnCl2 (commercially available from Aldrich and Acros) was identified as the optimal choice to furnish consistent results, completely excluding the use of a glovebox. 2-MeTHF solution of ZnCl2 was used for the syntheses of 7l, 7m, 7n, 7p, 7q, 7r, 7w and 7a’ (Table 2) as well as 8k (Table 3).
- 29.For alternative strategies to prepare polyfluorinated biaryls, see: Lafrance M, Rowley CN, Woo TK, Fagnou K. J. Am. Chem. Soc. 2006;128:8754–8756. doi: 10.1021/ja062509l.Do H-Q, Daugulis O. J. Am. Chem. Soc. 2008;130:1128–1129. doi: 10.1021/ja077862l.Nakamura Y, Yoshikai N, Ilies L, Nakamura E. Org. Lett. 2012;14:3316–3319. doi: 10.1021/ol301195x.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.