Abstract
Human mobility plays an important role in the dissemination of malaria parasites between regions of variable transmission intensity. Asymptomatic individuals can unknowingly carry parasites to regions where mosquito vectors are available, for example, undermining control programs and contributing to transmission when they travel. Understanding how parasites are imported between regions in this way is therefore an important goal for elimination planning and the control of transmission, and would enable control programs to target the principal sources of malaria. Measuring human mobility has traditionally been difficult to do on a population scale, but the widespread adoption of mobile phones in low-income settings presents a unique opportunity to directly measure human movements that are relevant to the spread of malaria. Here, we discuss the opportunities for measuring human mobility using data from mobile phones, as well as some of the issues associated with combining mobility estimates with malaria infection risk maps to meaningfully estimate routes of parasite importation.
Introduction
One of the biggest challenges facing the African countries considering malaria elimination is the ongoing threat of imported infections between different regions within a country and across borders. In highly endemic regions almost everyone in the population has parasites and most have no symptoms. Asymptomatic individuals are therefore reservoirs of infection that can carry parasites when they travel and contribute to transmission in endemic regions, or renew transmission in areas that remain vulnerable to malaria following control. As transportation infrastructure across Africa improves, the role of importation of parasites carried by asymptomatic people becomes increasingly important, particularly in countries with spatially heterogeneous transmission settings. Tools for understanding human mobility are currently limited, but the near ubiquity of mobile phones in many malaria-endemic countries offers a new way to examine national population dynamics on an unprecedented scale.
Although many qualitative surveys have explored the impacts of travel and transportation on health, economics, and development in Africa [1], there is a huge deficit of quantitative data on individual mobility from these regions. The definitive data on the topic remain the observational analyses on population movements across the continent by Prothero between 1960 and 1995 [2, 3]. Since then, most studies have focused on migration and long distance human movements [4], with very few analyses of regular, short-distance journeys between different regions. Clearly, there is great need for a “theoretical conception of mobility” [5] grounded in quantitative data, not only in order to understand infectious disease transmission, but also for a better understanding of population dynamics in general.
Mobile phones, which have been rapidly adopted across the globe, offer a unique way to track millions of individuals over time and to understand the dynamics of malaria-endemic populations. The use of mobile phones as “human sensors” to measure human mobility patterns is a rapidly growing field [6, 7]. Recent work of this kind analyzing call data records (CDRs) from Europe and North America has focused on the development of statistical rules of movement that seem to apply across different spatial and temporal scales [7–9]. Much less is known about patterns of human movement in low-income countries, especially in Africa, although mobility has rapidly increased across the continent in recent years [10]. The types of journey made in low-income countries are different in their range and frequency and occur for different reasons than in the developed world. Migrant workers, seasonal pastoralists, rural-to-urban migrants, and refugees all play important roles in the transmission of infectious diseases ranging from malaria to cholera and HIV [2, 3, 5, 11].
Understanding how human movements contribute to the spread of disease requires the integration of mobility data with information about infection risk. The human movements that are relevant to the transmission of vector-borne infections like malaria will be different from those that are important for sexually transmitted infections like HIV, pathogens spread through the environment like cholera, or respiratory pathogens such as influenza. For example, most densely populated urban centers experience a high volume of human traffic, making cities critical for the spread of directly transmitted infections. The paucity of mosquito vectors in most cities makes these movements less important for malaria transmission, however. Mathematical models can be used to understand how human mobility impacts the spread of infection [12–18]. For example, studies of the spread of influenza have simulated mobility using census or airline data and applied gravity models to scale them to global travel patterns, using a dynamical model of the spread of infection within subpopulations [9–14].
Here, we focus on the application of mobile phone data to understanding human mobility in relation to malaria, although much of the discussion could apply to many infectious diseases. Others have reviewed the importance of understanding human mobility for reducing malaria transmission and containing drug resistance [19–22], and the data sources currently available [23, 24]. We discuss the opportunities that mobile phone data provide and the challenges associated with using call data records (CDRs) for understanding how malaria parasites are carried between regions. We first briefly describe the range of movements that can be measured using mobile phone data, and their relevance for transmission. We then discuss the spatio-temporal resolution of CDRs, issues related to sample bias and validation, and review studies that have used CDRs to estimate human mobility. Finally we focus on applying these estimates to malaria data in a meaningful way, and the key gaps in biological knowledge that limit the accuracy of our estimates.
1. The impact of mobility on malaria
The range and frequency of human movement patterns, coupled with the mode and dynamics of disease transmission, will determine the impact of human mobility on infectious disease epidemiology. Stoddard et al. [19] adapted Prothero’s landmark studies to identify the importance of these spatiotemporal scales for vector-borne diseases, and Le Menach et al [20] describe the importance of human and mosquito movements for malaria epidemiology. Local and regional movements between areas with different malaria risks have three main consequences for the transmission and epidemiology of the disease:
individuals from low malaria risk regions traveling to high risk regions are particularly susceptible to disease because they lack well-developed immune responses. For example, increased rates of disease have been observed among migrants from low risk regions when they moved to highly endemic areas of Ethiopia, Indonesia, and Brazil [3, 25]. If these individuals return home they may also transport malaria parasites back to their region of origin if it is receptive to transmission, and this has been shown to contribute significantly to local ongoing transmission in Zanzibar [20].
Individuals from high risk regions traveling to low risk regions may carry parasites with them, potentially sparking outbreaks and renewed or maintained malaria transmission.
The movement of people between different endemic regions can bring together populations of parasites that would otherwise remain genetically distinct. This can not only lead to the introduction of novel strains of the parasite into communities that do not have pre-existing immunity to them, but also spread drug resistance mutations. The emergence of drug resistance holds particular worry for malariaendemic countries [21]; in the past, drug resistance has been spread across Africa by humans, rather than arising spontaneously in multiple places, and currently only one drug regimen (artemisinin combination therapy) remains efficacious everywhere [26–28].
Traditional approaches to measuring human movements on these regional scales rely on survey data from national censuses or other household surveys, small scale GPS studies, or road network and traffic data [23, 29–31]. Mobile phones offer individual-level information on a scale previously impossible, providing a “big data” approach to understanding human mobility [6]. The scale and resolution of CDRs will continue to increase as mobile phone penetration reaches saturation globally; in Africa alone there were 280 million mobile phone subscribers in 2008 and this number is projected to rise to 735 million by the end of 2012 [32]. Importantly, the most rapid adoption of mobile phone technologies has occurred in low-income countries, which are arguably undergoing the greatest societal change and have the most to gain from insights into human mobility for infrastructure development and public health. Below we will discuss the nature of mobile phone data and the challenges and opportunities that CDRs pose for the estimation of human mobility.
2. Measuring mobility using mobile phones
Spatial and temporal estimates of individual location
Every time a mobile phone is used to make or receive a call, text, send money, or top up airtime, a digital data point is logged that registers the SIM card and the routing cell tower. These call data records (CDRs) are routinely stored by mobile phone operators and on occasion can be anonymized and shared with researchers as a retrospective data set, usually on a case-by-case basis. This ad hoc basis for the release of CDRs is problematic for the widespread use of mobile phone data, since data sets usually cannot be shared with the research community. Currently, accessing CDRs for research relies on a personal relationship with a particular operator so the data provided vary, but generally include a timestamp, hashed identifiers for the sender and the receiver, the duration of calls, and the cell tower identifier. The national focus of mobile phone operators means that CDRs cannot be used to analyze cross-border movements at present, and the importance of protecting the privacy of mobile phone subscribers means that collecting detailed information about each individual is also not currently possible.
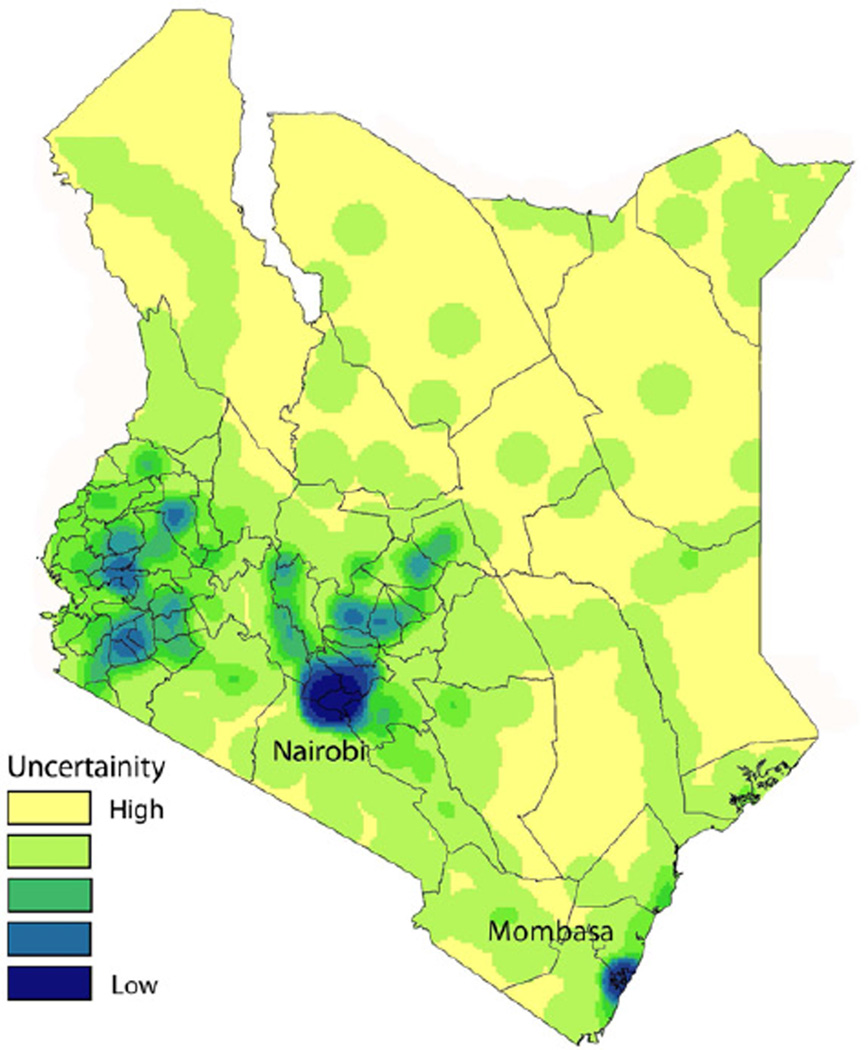
The service area for each cell tower generally falls between one and three kilometers [8, 9], providing an approximate location for the individual using the phone. In areas where cell towers are dense, such as in cities, the user’s location can be estimated down to the resolution of a city block, but this resolution drops off as cell tower density decreases. The spatial distribution of cell towers tends to reflect the distribution of people however, with cell tower density correlating strongly with population density. For many infectious diseases where transmission is correlated with population density, therefore, the spatial resolution of estimates is generally not problematic. Figure 1 shows the density of cell towers across Kenya, for example, and illustrates the level of uncertainty associated with mobility estimates in different parts of the country. The dry and sparsely populated northern regions of the country have far fewer cell towers, and as a result mobility estimates will be relatively coarse-grained.
Figure 1. The density of mobile phone towers across Kenya.
A spatial kernel density was estimated in Kenya using the location of all mobile phone towers owned by the leading mobile phone operator in the country. The density of mobile phone towers is an estimate of uncertainty of using CDR data to quantify travel. In Nairobi and Mombasa, the two largest cities in the country, the mobile phone tower density is high leading to more certainty of mobility estimate. In the rural areas of eastern and northern Kenya, the uncertainty is lower due to poor tower coverage. The density of towers is strongly correlated with population density (R2 = 0.688, p < 0.001).
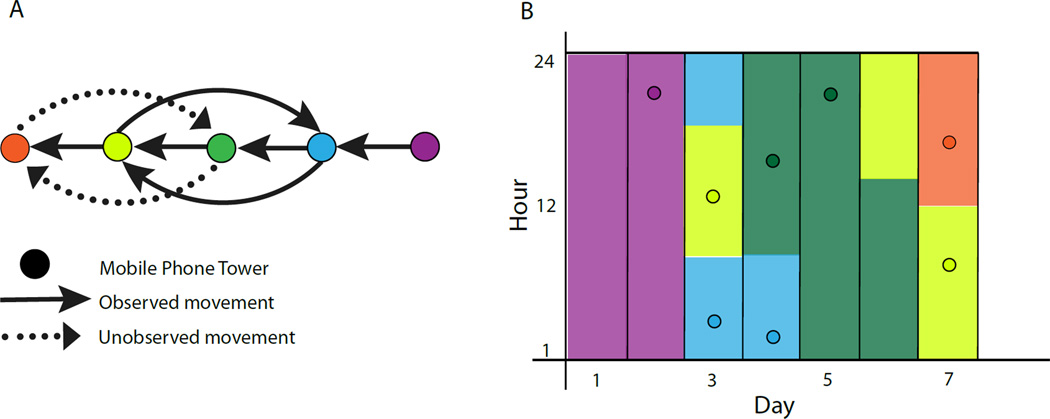
To understand how individual locations contribute to population-level dynamics, subscribers can be followed over time to generate longitudinal travel patterns (Figure 2). Here, assumptions must be made about where individuals are located at time points when they do not use their phone, and generally it is assumed that movement between two successive data points at tower A and tower B occurred half way between the time stamps. The temporal resolution of these estimates is dependent on how often the phone is used. Analyzed as a time series, CDRs can be interpolated in this way to provide estimates of each individual’s daily, weekly, and monthly movements, and these can be aggregate to provide populationlevel insights into mobility. A significant advantage of the scale of these data is the ability to discard individuals from the data set who do not use their phone often enough to generate a meaningful sample.
Figure 2. Approach to using CDRs to approximate location and movement for a subscriber.
A) Observed and unobserved movements between cell towers. Each circle represents a mobile phone tower with arrows indicating the day and direction of movement. Solid arrows indicate movement that can be measured using CDRs. Dotted arrows indicate movements that will not be measured. B) The estimated location and time of the subscriber for all 7 days. Points show the time of each call record (y axis) for each day of the week (x axis), with the color indicating the tower at which the call was made. Column colors represent the inferred location of the subscriber; it is assumed that the caller is at the mobile phone tower that routed their most recent call or text, and transitions between towers occur half way between two calls.
Sampling biases and phone sharing
Ideally, mobility estimates would be based on a random sample of the population and each registered SIM card would represent the movements of a single individual. However, individuals who own and use mobile phones are more likely to be male, affluent, and urban than individuals who do not [33, 34], so CDRs are unlikely to reflect a random sample of the population. Furthermore, it is common practice for individuals in low-income settings to use multiple SIM cards for different purposes in malaria-endemic regions, and phone sharing is extremely prevalent in low-income settings and among particular demographics like rural women [34]. These biases may not be homogeneously distributed. In Kenya, for example, income, education and gender disparities between mobile phone owners and non-owners are most pronounced in rural areas. In addition, the market for mobile phone operators is becoming increasingly fragmented [32], so even among mobile phone subscribers a single CDR data set will generally represent only a fraction of a country’s subscriber base. Although many of these issues are rapidly disappearing as mobile phones reach even the most rural, low income settings, sampling bias will continue to be an important limitation of mobile phone data, particularly with regard to the movement of children, who represent an important demographic for malaria and other infections that are highly prevalent in young age groups.
Defining the spatial distribution of human populations
There has been much interest in using CDRs to understand the statistical principles underlying human mobility based on the radius of gyration, an aggregated metric that combines the frequency and distance of travel patterns [8, 9]. For spatially explicit models of movement, however, these metrics are difficult to interpret and apply. In the context of infectious disease, and specifically malaria, quantifying the number and duration of trips between subpopulations of known malaria-endemicity provides a more meaningful way to analyze pathogen movements because it allows the integration of spatial and temporal information about infection risk [18, 20, 22].
Defining the distribution of human subpopulations is often difficult in low-income settings [35–37]. Settlement maps have been used [18, 38–41] that combine the Landsat Enhanced Thematic Mapper (ETM), Radarsat imagery, and derived texture layers to generate estimates of population densities at a 30 m spatial resolution. The population maps based on some of these techniques have been developed for African countries and are freely available online (http://www.afripop.org/). In the absence of information about human settlements, cell towers could theoretically be aggregated as a proxy, however there is no clear methodology for clustering contiguous towers into meaningful subpopulations. On a larger geographic area, information is regularly available on political boundaries such as providence, country, or district boundaries that can be used to aggregate mobile phone towers to smaller set of geographic areas.
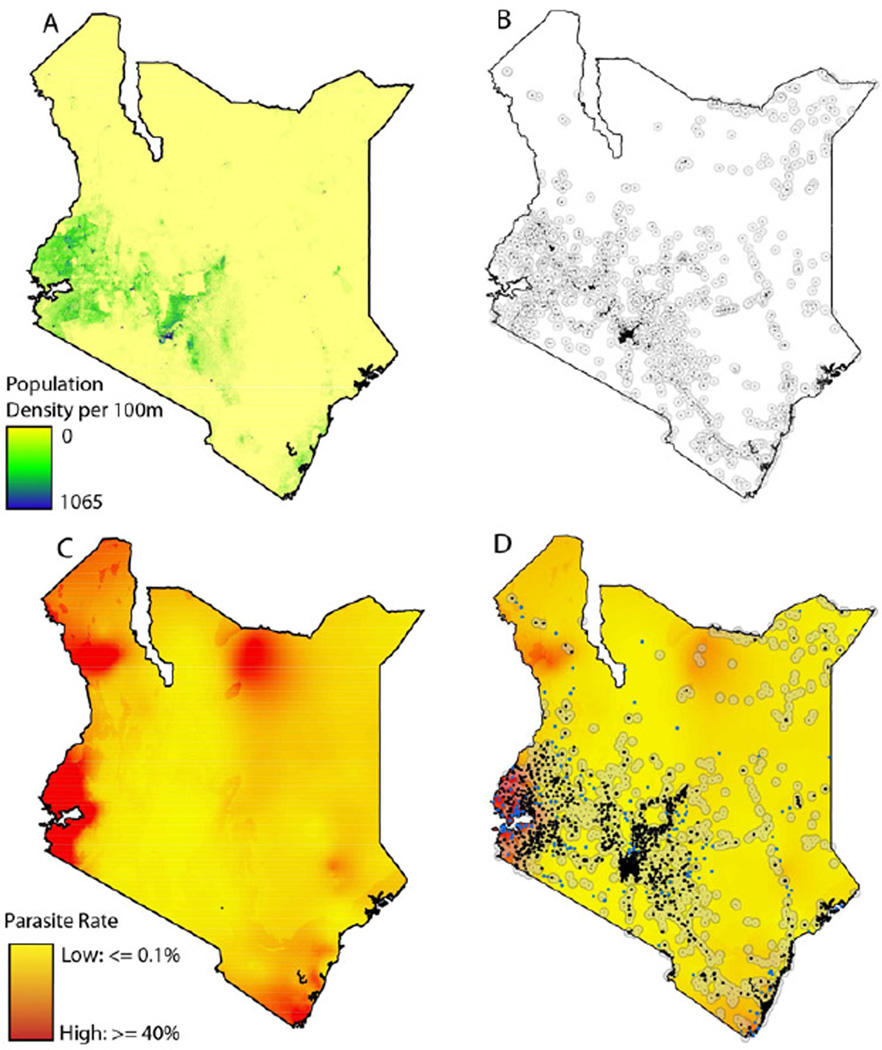
Together, the demographics of a mobile phone subscriber base, the spatial distribution of cell towers, and data on the distribution of human populations will all determine the extent to which CDRs can help generate a meaningful picture of the human dynamics that underlie the spread of disease (Figure 3 shows the different types of data available for Kenya, for example). Once these elements are combined, they form the foundation upon which details of spatial infection risk and disease dynamics can be overlaid to predict routes of pathogen importation. We next discuss approaches to this process in the context of malaria.
Figure 3. Data layers for defining resolution of mobility estimates.
A) The population density of Kenya with data from Afripop. B) The location of settlements in Kenya. A 10km buffer is drawn around each settlement to define a catchment area. C) The parasite rate in Kenya with data from MAP. D) The location of mobile phone tower in Kenya overlaid on a settlement and parasite rate maps.
3. Applying human mobility data to understand malaria transmission
Combining mobile phone data with malaria models
The congruence of the spatial resolution of CDRs, human population distributions, and infection risk data sources will determine the resolution of estimates of parasite dynamics. One advantage of this approach for vector-borne infections like malaria is the ability to define meaningful spatial infection risks, since assumptions do not have to be made about face-to-face interactions between people. By definition, the spatial scale of models based on CDRs will be greater than the scale of the pronounced local heterogeneities in transmission that occur due to household structure and mosquito breeding sites [42, 43]. The nature of CDRs therefore prescribes a regional spatial scale for modeling transmission, based on the assumption that it is possible to define a meaningful average infection risk at the scale of settlements. Major mapping projects like the Malaria Atlas Project (MAP) have produced high resolution (1-km2) maps of the prevalence of malaria infections among children age 2 to 10 years for most of the malariaendemic world [44]. Although currently lacking seasonal variations in these prevalence estimates, these provide a way to estimate transmission intensity within a given settlement and assign the probability that an individual from a particular location is infected, as well as the risk of an individual becoming infected when traveling to particular regions. Below we focus on the issues associated with calculating these values.
Mathematical models of malaria transmission have been used to design control policies for over a century, but there are still some major knowledge gaps that are particularly pertinent to understanding the impact of human mobility on the spread of disease (Figure 4). Open questions relating to malaria transmission and human mobility relate broadly to two main issues: i) measuring transmission intensity and ii) parameterizing the within-host dynamics of infection. Note that we are not dealing here with the many uncertainties associated with mosquito ecology and behavior because of the relatively coarse spatial resolution of CDRs.
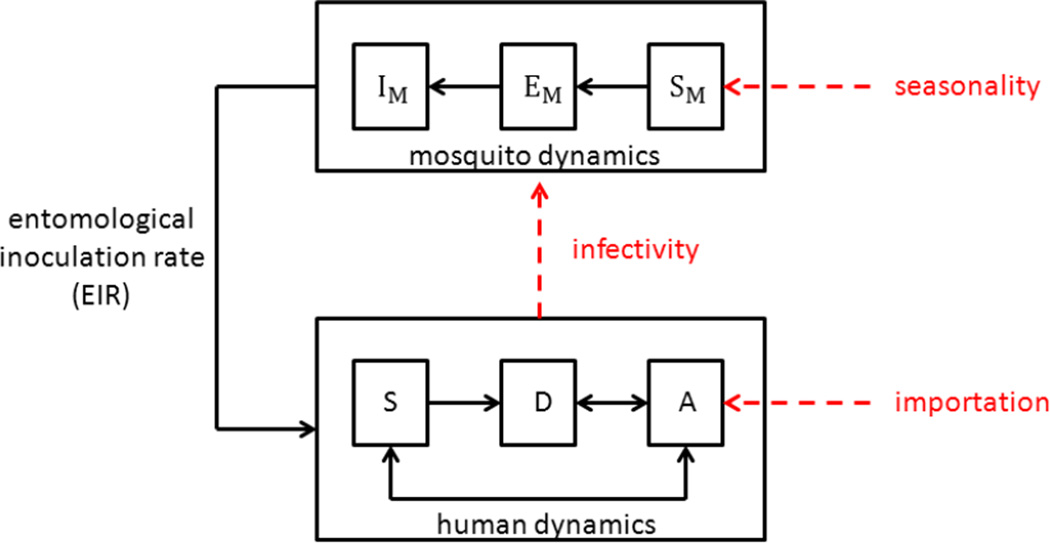
Figure 4. Knowledge gaps in malaria transmission models.
Important factors that are expected to have significant effects on malaria transmission but are currently poorly quantified include: importation of asymptomatic infections, human infectivity to mosquitoes and the effects of seasonality on mosquito abundance. The specific model assumptions made about these factors will impact the modeled entomological inoculation rate (EIR) and ultimately effect model predictions. Basic models for the human dynamics of malaria transmission include susceptible (S), diseased (D) and asymptomatic (A) individuals and for mosquito dynamics include susceptible (S_M), exposed (E_M) and infectious (I_M) mosquitoes.
Modeling the impact of mobility in areas of variable transmission intensity
First, measures of transmission intensity that underlie model estimates are uncertain and often poorly correlated with each other [45–47]. Although the prevalence of infection in human populations is the most common measure of transmission intensity, and the one available from MAP databases, there are a number of issues associated with using these measures to estimate parasite importation. Parasite prevalence - used to infer the probability that an individual is infected when they travel - is often obtained in clinical settings from children with symptomatic malaria, and may not provide an accurate estimate of the parasite prevalence in asymptomatic adults, who are most likely to contribute to parasite importation. Furthermore, to calculate the probability that travelers will acquire infections when they visit an endemic region it is necessary to convert prevalence into a per night probability of being infected, or a nightly entomological inoculation rate (EIR). The complexities associated with malaria transmission mean that malaria prevalence in mosquitoes may not correspond to malaria prevalence in people so this conversion relies on many simplifying assumptions [45, 47]. Even in areas where EIR estimates are available, most estimates are monthly or even annual and rarely reflect seasonal fluctuations in mosquito biting rates.
Uncertain estimates of transmission intensity also make it difficult to estimate the impact of imported infections in different regions. Infectious disease models generally rely on the basic reproductive rate (R0) to determine the potential for transmission following the importation of a pathogen. For malaria, however, there are pronounced heterogeneities in transmission on local scales due to variations in the distributions of hosts (both human and mosquito), mosquito larval habitats and host attractiveness to mosquitoes. Since the spatial and temporal scales used to estimate the quantities that determine R0 rarely capture these inherent heterogeneities, estimates of R0 usually represent the transmission potential of a “smoothed” setting [48, 49]. This measure of average transmission may not adequately predict the impact of imported infections that will also be heterogeneously distributed. Furthermore, since infection prevalence in a population will reflect transmission in the presence of the control efforts in place at the time, the impact of imported infections is more accurately described by a basic reproductive rate under control RC. Thus, in many places malaria prevalence reflects a combination of the underlying potential for transmission and the historical and current control programs [50]. These intrinsic uncertainties make it difficult to estimate the impact of imported infections or place confidence intervals on estimates, and previously we have taken a more transparent approach by simply quantifying the expected standing population of infected individuals within particular settlements.
Understanding parasite dynamics within the host
A second problem directly related to understanding imported malaria is that the dynamics of malaria infections within individuals are poorly understood. Calculating the probability that an infected individual will generate secondary infections when they travel to a receptive region requires assumptions about how long individual infections last, how infectious asymptomatic infections are, and how infectiousness correlates with immunity [51–56]. These basic parameters of infection are still not well understood, and since asymptomatic malaria-infected adults are the least well-studied group of transmitters, but the most important for importation between regions, these issues are likely to be critical for the accurate quantification of imported infections. There are many reasons that these biological aspects of malaria transmission remain unresolved: the malaria parasite exhibits vast genetic diversity and as a result human immunity requires repeated infections to develop and is probably never sterilizing. Parasite diversity also makes it difficult to distinguish recrudescent parasites from new infections, making it difficult to estimate the duration of infection. Furthermore, the transmission stages of the parasite have remained relatively under-studied because they are not associated with disease and occur at very low densities in the blood when parasite densities are low, making them particularly difficult to detect in asymptomatic individuals. In general, simplifying these uncertainties by assuming a fixed duration of infection (and infectiousness) is likely to over-estimate the true number of imported infections.
Another important reason to assess the impact of mobility on malaria transmission is to understand the spread of parasite drug resistance [21, 57, 58]. Since movement between different foci of transmission are likely to bring unrelated populations of parasites together, mobility facilitates the spread of resistance mutations through recombination between parasite genotypes. Indeed, it is thought that human movements have been responsible for the spread of mutations conferring resistance to choloroquine and sulphadoxine-pyrimehtamine from Southeast Asia to Africa [58]. Estimates of mobility from CDRs can give insights into the rate at which mutations are expected to spread, but will depend upon rates of recombination and the relationship between transmission intensity and multiplicity of infection. These issues remain very poorly understood. However, as sequencing technology improves and rapid genetic analysis of populations of parasites from field samples becomes feasible, our understanding of these parameters will improve our understanding of parasite population genetics. Systematic collection of parasite genetic data would also provide a different to assessing human mobility, since parasite genetic differentiation will reflect the connectivity between populations, and could provide important validation of models based on CDRs.
Conclusions
The potential for using CDRs to provide unprecedented insights into population dynamics in malariaendemic countries and inform policy is exciting. Using simple assumptions about how human populations and malaria risk are distributed, it is possible to pinpoint specific settlements with high risks of imported malaria and generate maps that show how the parasite may be traveling around a country within human carriers [18]. Here we have focused on some of the technical considerations for conducting these analyses and many of the problems associated with accurate estimation of malaria importation. As the biology of malaria transmission becomes clearer, however, many of the uncertain parameters associated with quantifying imported infections will become easier as malaria models improve. Although the privacy of mobile phone subscribers must be carefully protected, mobile phones offer a promising tool not only for understanding the dynamics of populations more completely than ever before, but also for engaging with them.
Acknowledgements
A.P.W. was supported by the National Science Foundation Graduate Research Fellowship program (#0750271). R.W.S. is supported by the Wellcome Trust as Principal Research Fellow (# 079080). A.W. is E.H and C.O.B were supported by Award Number U54GM088558 from the National Institute Of General Medical Sciences. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute Of General Medical Sciences or the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Statement
The authors declare no competing interests.
References
- 1.Porter G. Living in a Walking World: Rural Mobility and Social Equity Issues in subSaharan Africa. World Development. 2002;30(2):285–300. [Google Scholar]
- 2.Prothero RM. Population mobility and trypanosomiasis in Africa. Bull World Health Organ. 1963;28(5-6):615–626. [PMC free article] [PubMed] [Google Scholar]
- 3.Prothero RM. Disease and mobility: a neglected factor in epidemiology. Int J Epidemiol. 1977;6(3):259–267. doi: 10.1093/ije/6.3.259. [DOI] [PubMed] [Google Scholar]
- 4.Anderson J. Space-time budgets and activity studies in urban geography and planning. Environment and Planning. 1971;3(4):353–368. [Google Scholar]
- 5.Deane KD, Parkhurst JO, Johnston D. Linking migration, mobility and HIV. Trop Med Int Health. 2010;15(12):1458–1463. doi: 10.1111/j.1365-3156.2010.02647.x. [DOI] [PubMed] [Google Scholar]
- 6.Salathe M, et al. Digital epidemiology. PLoS Comput Biol. 2012;8(7):e1002616. doi: 10.1371/journal.pcbi.1002616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Simini F, et al. A universal model for mobility and migration patterns. Nature. 2012;484(7392):96–100. doi: 10.1038/nature10856. [DOI] [PubMed] [Google Scholar]
- 8.Song C, et al. Limits of predictability in human mobility. Science. 2010;327(5968):1018–1021. doi: 10.1126/science.1177170. [DOI] [PubMed] [Google Scholar]
- 9.Gonzalez MC, Hidalgo CA, Barabasi AL. Understanding individual human mobility patterns. Nature. 2008;453(7196):779–782. doi: 10.1038/nature06958. [DOI] [PubMed] [Google Scholar]
- 10.van Dijk H, Foeken D, van Til K. In: Population mobility in Africa: an overview, in Mobile Africa: Changing Patterns of Movement in Africa and Beyond. van Dijk H, Foeken D, van Til K, editors. Brill Academic Publishing; 2001. [Google Scholar]
- 11.Mari L, et al. Modelling cholera epidemics: the role of waterways, human mobility and sanitation. J R Soc Interface. 2011 doi: 10.1098/rsif.2011.0304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hollingsworth TD, Ferguson NM, Anderson RM. Will travel restrictions control the international spread of pandemic influenza? Nat Med. 2006;12(5):497–499. doi: 10.1038/nm0506-497. [DOI] [PubMed] [Google Scholar]
- 13.Ferguson NM, et al. Strategies for containing an emerging influenza pandemic in Southeast Asia. Nature. 2005;437(7056):209–214. doi: 10.1038/nature04017. [DOI] [PubMed] [Google Scholar]
- 14.Longini IM, Jr, et al. Containing pandemic influenza at the source. Science. 2005;309(5737):1083–1087. doi: 10.1126/science.1115717. [DOI] [PubMed] [Google Scholar]
- 15.Longini IM, Jr, et al. Containing a large bioterrorist smallpox attack: a computer simulation approach. Int J Infect Dis. 2007;11(2):98–108. doi: 10.1016/j.ijid.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 16.Balcan D, et al. Multiscale mobility networks and the spatial spreading of infectious diseases. Proc Natl Acad Sci U S A. 2009;106(51):21484–21489. doi: 10.1073/pnas.0906910106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Balcan D, et al. Modeling the spatial spread of infectious diseases: the GLobal Epidemic and Mobility computational model. J Comput Sci. 2010;1(3):132–145. doi: 10.1016/j.jocs.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wesolowski A, et al. Quantifying the impact of human mobility on malaria. Science. 2012;338(6104):267–270. doi: 10.1126/science.1223467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stoddard ST, et al. The role of human movement in the transmission of vector-borne pathogens. PLoS Negl Trop Dis. 2009;3(7):e481. doi: 10.1371/journal.pntd.0000481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Le Menach A, Tatem AJ, Cohen JM, Hay SI, Randell H, Patil A, Smith DL. Travel risk, malaria importation and malaria transmission in Zanzibar. Scientific Reports. 2011;1:1–7. doi: 10.1038/srep00093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lynch C, Roper C. The transit phase of migration: circulation of malaria and its multidrug-resistant forms in Africa. PLoS Med. 2011;8(5):e1001040. doi: 10.1371/journal.pmed.1001040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tatem AJ, et al. The use of mobile phone data for the estimation of the travel patterns and imported Plasmodium falciparum rates among Zanzibar residents. Malar J. 2009;8:287. doi: 10.1186/1475-2875-8-287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pindolia DK, et al. Human movement data for malaria control and elimination strategic planning. Malar J. 2012;11(1):205. doi: 10.1186/1475-2875-11-205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tatem AJ, et al. Mapping populations at risk: improving spatial demographic data for infectious disease modeling and metric derivation. Popul Health Metr. 2012;10(1):8. doi: 10.1186/1478-7954-10-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sawyer D. Economic and social consequences of malaria in new colonization projects in Brazil. Soc Sci Med. 1993;37(9):1131–1136. doi: 10.1016/0277-9536(93)90252-y. [DOI] [PubMed] [Google Scholar]
- 26.Naidoo I, Roper C. Drug resistance maps to guide intermittent preventive treatment of malaria in African infants. Parasitology. 2011;138(12):1469–1479. doi: 10.1017/S0031182011000746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pearce RJ, et al. Multiple origins and regional dispersal of resistant dhps in African Plasmodium falciparum malaria. PLoS Med. 2009;6(4):e1000055. doi: 10.1371/journal.pmed.1000055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Plowe CV, et al. World Antimalarial Resistance Network (WARN) III: molecular markers for drug resistant malaria. Malar J. 2007;6:121. doi: 10.1186/1475-2875-6-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Paz-Soldan VA, et al. Assessing and maximizing the acceptability of global positioning system device use for studying the role of human movement in dengue virus transmission in Iquitos, Peru. Am J Trop Med Hyg. 2010;82(4):723–730. doi: 10.4269/ajtmh.2010.09-0496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vazquez-Prokopec GM, et al. Usefulness of commercially available GPS data-loggers for tracking human movement and exposure to dengue virus. Int J Health Geogr. 2009;8:68. doi: 10.1186/1476-072X-8-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Elgethun K, et al. Comparison of global positioning system (GPS) tracking and parent-report diaries to characterize children's time-location patterns. J Expo Sci Environ Epidemiol. 2007;17(2):196–206. doi: 10.1038/sj.jes.7500496. [DOI] [PubMed] [Google Scholar]
- 32.GSMA. Africa Mobile Observatory. 2011. [Google Scholar]
- 33.Blumenstock JE, Eagle N. Mobile divides: gender, socioeconomic status, and mobile phone use in Rwanda; 4th International Conference on Information and Communication Technologies and Development; 2010. [Google Scholar]
- 34.Wesolowski A, et al. Heterogeneous mobile phone ownership and usage patterns in Kenya. PLoS One. 2012;7(4):e35319. doi: 10.1371/journal.pone.0035319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tatem AJ, et al. High resolution population maps for low income nations: combining land cover and census in East Africa. PLoS One. 2007;2(12):e1298. doi: 10.1371/journal.pone.0001298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tatem AJ, Riley S. Effect of poor census data on population maps. Science. 2007;318(5847):43. doi: 10.1126/science.318.5847.43a. author reply 43. [DOI] [PubMed] [Google Scholar]
- 37.Hay SI, et al. The accuracy of human population maps for public health application. Trop Med Int Health. 2005;10(10):1073–1086. doi: 10.1111/j.1365-3156.2005.01487.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Linard C, Tatem AJ. Large-scale spatial population databases in infectious disease research. Int J Health Geogr. 2012;11:7. doi: 10.1186/1476-072X-11-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Linard C, et al. Population distribution, settlement patterns and accessibility across Africa in 2010. PLoS One. 2012;7(2):e31743. doi: 10.1371/journal.pone.0031743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tatem A, Linard C. Population mapping of poor countries. Nature. 2011;474(7349):36. [Google Scholar]
- 41.Tatem AJ, et al. The effects of spatial population dataset choice on estimates of population at risk of disease. Popul Health Metr. 2011;9:4. doi: 10.1186/1478-7954-9-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bejon P, et al. Stable and unstable malaria hotspots in longitudinal cohort studies in Kenya. PLoS Med. 2010;7(7):e1000304. doi: 10.1371/journal.pmed.1000304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bousema T, et al. Hitting hotspots: spatial targeting of malaria for control and elimination. PLoS Med. 2012;9(1):e1001165. doi: 10.1371/journal.pmed.1001165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hay SI, et al. A world malaria map: Plasmodium falciparum endemicity in 2007. PLoS Med. 2009;6(3):e1000048. doi: 10.1371/journal.pmed.1000048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Smith DL, et al. A quantitative analysis of transmission efficiency versus intensity for malaria. Nat Commun. 2010;1:108. doi: 10.1038/ncomms1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Corran P, et al. Serology: a robust indicator of malaria transmission intensity? Trends Parasitol. 2007;23(12):575–582. doi: 10.1016/j.pt.2007.08.023. [DOI] [PubMed] [Google Scholar]
- 47.Smith T, et al. Relationships between the outcome of Plasmodium falciparum infection and the intensity of transmission in Africa. Am J Trop Med Hyg. 2004;71(2 Suppl):80–86. [PubMed] [Google Scholar]
- 48.Smith DL, et al. Revisiting the basic reproductive number for malaria and its implications for malaria control. PLoS Biol. 2007;5(3):e42. doi: 10.1371/journal.pbio.0050042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Smith T, Schapira A. Reproduction numbers in malaria and their implications. Trends Parasitol. 2012;28(1):3–8. doi: 10.1016/j.pt.2011.09.002. [DOI] [PubMed] [Google Scholar]
- 50.Gething PW, et al. A new world malaria map: Plasmodium falciparum endemicity in 2010. Malar J. 2011;10:378. doi: 10.1186/1475-2875-10-378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Owusu-Agyei S, et al. Does radical cure of asymptomatic Plasmodium falciparum place adults in endemic areas at increased risk of recurrent symptomatic malaria? Trop Med Int Health. 2002;7(7):599–603. doi: 10.1046/j.1365-3156.2002.00902.x. [DOI] [PubMed] [Google Scholar]
- 52.Mueller I, et al. Force of infection is key to understanding the epidemiology of Plasmodium falciparum malaria in Papua New Guinean children. Proc Natl Acad Sci U S A. 2012;109(25):10030–10035. doi: 10.1073/pnas.1200841109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Killeen GF, Ross A, Smith T. Infectiousness of malaria-endemic human populations to vectors. Am J Trop Med Hyg. 2006;75(2 Suppl):38–45. doi: 10.4269/ajtmh.2006.75.2_suppl.0750038. [DOI] [PubMed] [Google Scholar]
- 54.Li Y, Ruan S, Xiao D. The within-host dynamics of malaria infection with immune response. Math Biosci Eng. 2011;8(4):999–1018. doi: 10.3934/mbe.2011.8.999. [DOI] [PubMed] [Google Scholar]
- 55.Metcalf CJ, et al. Partitioning regulatory mechanisms of within-host malaria dynamics using the effective propagation number. Science. 2011;333(6045):984–988. doi: 10.1126/science.1204588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.de Roode JC, et al. Dynamics of multiple infection and within-host competition in genetically diverse malaria infections. Am Nat. 2005;166(5):531–542. doi: 10.1086/491659. [DOI] [PubMed] [Google Scholar]
- 57.Anderson T. Mapping the spread of malaria drug resistance. PLoS Med. 2009;6(4):e1000054. doi: 10.1371/journal.pmed.1000054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wongsrichanalai C, et al. Epidemiology of drug-resistant malaria. Lancet Infect Dis. 2002;2(4):209–218. doi: 10.1016/s1473-3099(02)00239-6. [DOI] [PubMed] [Google Scholar]