Abstract
Background
Differences in genetic influences on disordered eating are present across puberty in girls. Heritability is 0% before puberty, but over 50% during and after puberty. Emerging data suggest that these developmental differences may be due to pubertal increases in ovarian hormones. However, a critical piece of evidence is lacking, namely, knowledge of genetic influences on disordered eating across puberty in boys. Boys do not experience increases in ovarian hormones during puberty. Thus, if pubertal increases in genetic effects are present in boys, then factors in addition to ovarian hormones may drive increases in heritability in girls. The current study was the first to examine this possibility in a sample of 1,006 male and female twins from the Michigan State University Twin Registry.
Methods
Disordered eating was assessed with the Minnesota Eating Behaviors Survey. Pubertal development was assessed with the Pubertal Development Scale.
Results
No significant differences in genetic influences on disordered eating were observed in males across any developmental stage. Heritability was 51% in boys during pre-puberty, puberty, and young adulthood. By contrast, in girls, genetic factors accounted for 0% of the variance in pre-puberty, but 51% of the variance during puberty and beyond. Sex differences in genetic effects were only significant during pre-puberty, as the best-fitting models constrained heritability to be equal across all males, pubertal females, and young adult females.
Conclusions
Results highlight sex-specific effects of puberty on genetic risk for disordered eating and provide indirect evidence of a role for ovarian hormones and/or other female-specific factors.
Keywords: eating disorders, males, genetic, twins, puberty, sex differences
Several twin studies document differences in genetic influences on disordered eating before and after puberty in girls (Culbert et al., 2009, Klump et al., 2003, Klump et al., 2007, Silberg and Bulik, 2005). Before puberty, genetic influences account for ~0% of the variability in disordered eating, whereas genetic factors account for over 50% during and after puberty (Culbert et al., 2009, Klump et al., 2003, Klump et al., 2007). Emerging data suggest that these developmental differences may be due to increases in ovarian hormones during puberty in girls. In particular, estrogen appears to moderate genetic influences on disordered eating, as there are no genetic influences on disordered eating symptoms (e.g., weight preoccupation, body dissatisfaction, binge eating) in twins with low estradiol levels during puberty, but significant genetic effects in twins with high estradiol levels (Klump et al., 2010). Collectively, these findings have led our team (Klump and Culbert, 2007, Klump et al., 2010) and others (Young, 2010) to speculate that ovarian hormones may “activate” genetic risk for disordered eating in girls during puberty.
However, at least one additional piece of evidence is lacking – namely, are the same pubertal increases in genetic effects present in boys? Boys do not experience increases in ovarian hormones during puberty, as their pubertal development is driven by increases in testosterone. If pubertal increases in genetic effects are present in boys, then factors other than (or in addition to) ovarian hormones may drive increases in genetic effects in girls. Conversely, if pubertal increases in genetic effects are observed in girls only, then there would be additional, albeit indirect, evidence that sex-specific processes (e.g., ovarian hormones) differentially influence the magnitude of genetic risk across sex during puberty.
No study has examined genetic influences on disordered eating in boys prior to puberty. Disordered eating is ~50% heritable in males in late adolescence (Baker et al., 2009) and young adulthood (Keski-Rahkonen et al., 2005, Reichborn-Kjennerud et al., 2003, Reichborn-Kjennerud et al., 2004, Slof-Op't et al., 2008, Tholin et al., 2005), an estimate on par with that for adult females (Bulik et al., 2000, Suisman and Klump, 2011). Thus, at least in late adolescence and adulthood, the magnitude of genetic influences on disordered eating appears to be similar in males and females.
The next important step is to examine whether patterns of genetic effects are similar in males and females from pre-adolescence into adulthood. If ovarian hormones contribute to increases in genetic effects during puberty in girls, we would expect no effect of puberty on genetic risk in boys. Indeed, we would expect stable estimates of heritability across development since ovarian hormones do not play a major role in boys’ development.
The aim of our study was to examine these possibilities by investigating differences in the magnitude of genetic influences in male twins during pre-puberty, puberty, and young adulthood. We further compared patterns of genetic effects in males to a sample of female twins who previously exhibited pubertal increases in genetic effects (see Culbert et al., 2009). The comparison of our male twins to an archival female group allowed us to directly examine sex differences in effects – without the females, we would be confined to visual comparisons of findings in male versus female twins rather than a quantitative test of sex differences.
Methods
Participants
Participants were 1,006 male and female same-sex twins (372 male and 634 female twins; see Table 1) ages 10–15 and 18–28 years from the Michigan State University Twin Registry (MSUTR) (Klump and Burt, 2006). Although the male MSUTR twins have never been examined, the female twins were included in a previous report showing pubertal increases in genetic effects (Culbert et al., 2009, Slane et al., in press) where 71% of the total sample of pre-pubertal female twins, 61% of the pubertal twins, and 100% of the young adult female twins were included in analyses.
Table 1.
Descriptive Statistics and Twin Correlations.
| Males | Females | |||||
|---|---|---|---|---|---|---|
| Variables | Pre-Pubertal | Pubertal | Young Adult | Pre-Pubertal | Pubertal | Young Adult |
| Sample Sizes: | ||||||
| MZ | 80 | 30 | 104 | 68 | 88 | 192 |
| DZ | 72 | 24 | 62 | 64 | 90 | 132 |
| Total | 152 | 54 | 166 | 132 | 178 | 324 |
| Age: | ||||||
| Range | 10–15 years | 13–15 years | 18–28 years | 10–13 years | 10–15 years | 18–28 years |
| Mean (SD) | 12.07 (1.27) | 14.42 (0.84) | 20.99 (2.41) | 11.47 (0.89) | 13.71 (1.14) | 20.68 (2.45) |
| MEBS Total Score: | ||||||
| Range (max score = 30) | 0–18 | 0–16 | 0–19 | 0–19 | 0–22 | 0–29 |
| Mean (SD) | 4.57 (4.13) | 3.79 (3.92) | 3.23 (3.40) | 5.20 (4.62) | 6.40 (5.48) | 8.00 (6.16) |
| Alphas | .83 | .84 | .82 | .86 | .88 | .90 |
| r with EDE-Q total score | .77*** | .96*** | .84*** | .86*** | .78*** | .89*** |
| r with BMI | .24*** | .34*** | .32*** | .53*** | .24*** | .37*** |
| r with depression symptoms | .45*** | .40*** | .32*** | .54*** | .49*** | .50*** |
| Twin Correlationsa: | ||||||
| MZ Correlation | .46*** | .64** | .46*** | .27* | .71*** | .59*** |
| 95% Confidence Interval | 0.26–0.63 | 0.32–0.82 | 0.29–0.60 | 0.03–0.48 | 0.57–0.81 | 0.49–0.68 |
| N for concordant pairs | 72 | 24 | 104 | 66 | 66 | 192 |
| DZ Correlation | .13 | .03 | .07 | .22† | .42** | .19* |
| 95% Confidence Interval | −0.12–0.36 | −0.44–0.49 | −0.18–0.31 | −0.04–0.45 | 0.18–0.61 | 0.02–0.35 |
| N for concordant pairs | 64 | 18 | 62 | 58 | 56 | 132 |
| Test of Equality (z) | 2.01* | 2.15* | 2.58** | 0.37 | 2.36* | 4.10*** |
| Effect Size (q) | .37 | .73 | .43 | .05 | .44 | .49 |
Note: MEBS = Minnesota Eating Behaviors Survey; r = correlation; EDE-Q = Eating Disorder Examination Questionnaire; BMI = body mass index. Sample sizes indicate the number of twins. Assessments of depressive symptoms and anxiety levels were tailored to the twins’ developmental level such that depressive symptoms were assessed with the Children’s Depression Inventory (Kovacs, 1985) in pre-pubertal and pubertal twins, and the Beck Depression Inventory-II (Beck et al., 1996) in young adult twins. The “Test of Equality (z)” tests for differences between the MZ and DZ twin correlations. Effect size (q) represents indexes the magnitude of the difference between MZ and DZ twin correlations.
Twin correlations were calculated with twins who were concordant for pubertal status (i.e., pre-pubertal versus pubertal) only. Sample sizes are noted in the row “N for concordant pairs”.
p < .10,
p<.05,
p < .01,
p < .001.
The correlation is greater than zero.
Twins were originally recruited using a variety of methods (e.g., birth records, advertisements), although the registry is now population-based via birth record recruitment. MSUTR twins appear to be demographically representative of the recruitment region (Culbert et al., 2008).
Zygosity Determination
Zygosity was determined using physical similarity questionnaires completed by the twins or their mothers (for adolescents) that have demonstrated over 95% accuracy (Lykken et al., 1990, Peeters et al., 1998). Indeterminable zygosity scores were resolved by MSUTR investigators (KLK or SAB) who examined item endorsements and photographs of the twins.
Measures
Disordered Eating
The 30-item total score from the Minnesota Eating Behavior Survey1 (MEBS) (von Ranson et al., 2005) was used to assess disordered eating in the areas of body dissatisfaction (i.e., dissatisfaction with body size and/or shape), weight preoccupation (i.e., preoccupation with dieting and the pursuit of thinness), binge eating (i.e., episodes of overeating and thinking about binge eating), and compensatory behavior (i.e., self-induced vomiting, laxative use). The MEBS assesses these disordered eating symptoms using a true/false item format where items endorsed as “true” are summed to create the total score. Higher total scores represent more pathological attitudes and behavior.
We focused on the MEBS total score because it has been the focus of previous developmental twin studies (Culbert et al., 2009, Klump et al., 2000, 2003, Klump et al., 2007) and studies of male and female twins (Slane et al., in press, von Ranson et al., 2005). For example, the MEBS total score exhibits factor congruence and excellent internal consistency (α = .84–.89) in males and females (Marderosian et al., in preparation, von Ranson et al., 2005). Convergent validity with the Eating Disorder Examination Questionnaire total score (Fairburn and Beglin, 1994) in early and late adolescence is also excellent (r’s = .84–.89) (Marderosian et al., in preparation, von Ranson et al., 2005). In the current study, across both sex and developmental stage, the total score exhibited excellent internal consistency (all α’s ≥ .82; see Table 1), strong convergent validity with EDE-Q total scores (r’s = .77–.96), and expected correlations with external correlates such as body mass index (BMI) and depressive symptoms (see Table 1).
Pubertal Development
The Pubertal Development Scale (PDS) (Petersen et al., 1988) was used to determine pubertal status in twins ages 10–15 years. Twins self-reported secondary sex characteristic (e.g., height spurts, body hair, breast development (girls), voice changes (boys)) development using a four-point scale: 1) development has not yet begun, 2) development has barely started, 3) development is definitely underway, and 4) development seems completed. Females rated menses as either present (coded 1) or absent (coded 4). Consistent with previous studies (Culbert et al., 2009, Klump et al., 2003), PDS items were summed and averaged, and this score was used to categorize twins as pre-pubertal (score < 2.5) or pubertal (score ≥ 2.5) (see Table 1 for sample sizes). The PDS demonstrated excellent reliability and validity in previous research (Petersen et al., 1988) and showed good internal consistency in our sample (α’s ≥ .80).
Twins ages 18 and older did not complete the PDS since the vast majority (>99%) of twins in this age range are post-pubertal (Herman-Giddens et al., 2001, Lee, 2001, Parent et al., 2003). These twins were instead categorized as the “young adult” group.
BMI
BMI (weight (in kilograms)/height (in meters)2) was calculated using height and weight measured with a digital scale and wall mounted ruler, respectively.
Statistical Analyses
MEBS total scores were prorated for 8 (1.63%) twins missing ≤10% of the items and were coded as missing for 5 (0.99%) twins missing ≥10% of the items. MEBS total scores were positively skewed, and thus, scores were rank-ordered and Blom transformed by sex. Age and BMI were regressed out of each twin’s score to ensure that differences between groups were not due to these variables.
Twin Correlations
Twin intraclass correlations were used as initial indicators of genetic effects on disordered eating in each group. Only pairs concordant on pubertal status (i.e., both pre-pubertal or pubertal) could be included in the twin correlations, although concordant and discordant twins were included in some biometric models (see below). Fortunately, the number of excluded pairs due to discordance on pubertal status was small (n = 14 male and 32 female pairs), and twins from concordant versus discordant pairs did not differ significantly on disordered eating (males, t (32.46) = −1.36, p = .18; females, t (86.42) = −0.85, p = .40).
Biometric Model-Fitting
Constraint Models
We first used constraint models to estimate the contribution of additive genetic (A; genetic influences that add across genes), shared environmental (C; environmental influences that are shared by twins and are a source of behavioral similarity), and nonshared environmental (E; environmental influences that are not shared by twins and are a source of behavioral dissimilarity, including measurement error) factors to disordered eating across sex and development. Previous studies have focused on these constraint models to examine developmental differences in girls; our use of the identical approach ensures comparability of our findings in boys to those published previously in girls.
Because of the potentially large number of models that could be fit across sex and developmental stage, we initially fit the fully unconstrained model that allows A, C, and E to vary across sex and development. We then examined model parameters and identified submodels that differentially constrained parameters across groups. This approach allowed us to test theoretically relevant submodels without unduly increasing the number of tests. In addition to these submodels, we also fit the fully constrained model that constrains parameters to be equal across sex and developmental stage, as this model is the most parsimonious and directly tests the null hypothesis (i.e., that there are no differences in A, C, and E across groups). Notably, like the twin correlations, co-twins must be concordant on pubertal status (i.e., both pre-pubertal or pubertal) to estimate group differences in these models.
Moderation Models
In addition to the constraint models, we also conducted moderation models (Purcell, 2002) to directly test sex and pubertal moderation of heritability for disordered eating. The advantages of these models are that discordant twins can be included in analyses, and the models provide a direct statistical test of the sex × puberty interaction on genetic influences on disordered eating. These models therefore provide a constructive replication of our initial results using an alternative statistical approach.
Because we were interested in whether sex and puberty moderate genetic risk for disordered eating, we used the two-moderator (sex × puberty) models (Purcell, 2002). These models estimate three sets of parameters that conjointly index whether genetic influences on disordered eating differ across sex, puberty, or sex and puberty (i.e., there is a sex × puberty interaction). The first set of parameters contain the “paths” or intercepts (i.e., a, c, e) and estimate the degree of genetic and environmental influence on disordered eating at the lowest level (i.e., codes of 0) of the moderators (i.e., males in the pubertal group; see coding information below). The second set of parameters are the sex moderators (i.e., βXS, βYS, βZS) which assess whether genetic and environmental influences are lower or higher in females relative to males. The third set of parameters are the puberty moderators (i.e., βXP, βYP, βZP) which assess whether there are increases or decreases in etiologic influences on disordered eating across puberty. The final set of parameters are the sex × puberty moderators (i.e., βXSxP, βYSxP, βZSxP) that test our primary hypothesis that sex differences in etiologic effects depend upon the level of pubertal development. Because we hypothesized that genetic effects would be lower in pre-pubertal females as compared to males and all other female groups, we coded the sex (Ms: females = 1, males = 0) and puberty (Mp: pre-puberty = 1, puberty and young adulthood = 0; see Results for the rationale for combining pubertal and young adult groups) moderator values so that the sex*puberty moderator would compare the magnitude of etiologic influences in pre-pubertal females versus all other groups (MS*MP: pre-pubertal females = 1, pre-pubertal males = 0, pubertal females = 0, pubertal males = 0).
We fit a series of nested models to test the significance of each moderator. We first fit the “full” model which freely estimated all four sets of parameters (e.g., for additive genetic effects, this model included: a + βXSMS + βXP MP + βXSxP MSMP). In the remaining models, we tested the significance of each moderator by constraining the moderator coefficient (e.g., βXS) to 0 and comparing the fit of the reduced models to the full model.
Model Fit and Selection
All models were fit to the raw data using the maximum likelihood option in Mx (Neale, 1999). This option treats missing data as missing-at-random and is expected to produce less biased and more consistent estimates than other techniques (e.g., listwise deletion).
Comparisons of model fit were made by taking the difference in minus twice the log-likelihood (−2lnL) between the “full” and reduced models, which is chi-squared distributed under the null hypothesis implied by the reduced model. Large (statistically significant) differences led to a rejection of the nested model in favor of the full model, as this suggests that dropping the parameters resulted in a significantly worse fit. For the constraint models, the “full” model was the fully unconstrained model that freely estimated A, C and E across sex and pubertal stage. For the moderation models, the “full” model included all paths and moderators (i.e., sex, puberty, and sex × puberty). Akaike’s information criterion was also used to select the best fitting model, where models that minimize Akaike’s information criterion (AIC=χ2−2*Δdf) (Akaike, 1987) were preferred..
Following previous recommendations (Purcell, 2002), we report unstandardized (or absolute) parameter estimates. Unstandardized estimates are generally preferred as they more accurately depict absolute differences in genetic and environmental influences than standardized estimates which represent differences as proportions of the total variance. Nonetheless, we also report standardized estimates from the constraint models only, as these estimates allow for a direct comparison of our findings to previous developmental twin studies that focused on standardized estimates of genetic/environmental effects.
Results
Descriptive Statistics
Descriptive statistics are shown in Table 1. Pre-pubertal and pubertal male twins were significantly older than the female twins in the same pubertal groups (p’s < .001); these age differences likely reflect the earlier age of pubertal onset in girls versus boys (Lee, 2001). As shown previously (Culbert et al., submitted), there were no sex differences in mean levels of disordered eating in male and female twins before puberty (p = .24), but during and after puberty, female twins scored higher than male twins (all p’s < .001). Despite these mean-level differences, variability in MEBS scores was present in both male and female twins, as scores ranged from 0–19 in males and 0–29 in females, standard deviations were generally greater than 3.40, and there were no significant sex differences in variances (all p’s > .05). A number of male (n = 11; 3%) and female twins (n = 63; 10%) scored above the clinical cut-off on the MEBS (score = 15) (von Ranson et al., 2005), although the percentage was larger in females.
Twin Correlations
Twin correlations provided initial support for study hypotheses (see Table 1). There appeared to be genetic effects on disordered eating during and after puberty in male and female twins, as the MZ twin correlations were significantly greater than the DZ twin correlations in both sexes. By contrast, sex differences were present before puberty. The MZ twin correlation was significantly greater than the DZ twin correlation in pre-pubertal males, indicating the presence of genetic effects. However, in pre-pubertal females, the MZ and DZ twin correlations were very similar and not significantly different from each other, suggesting a lack of genetic effects. Overall, results indicated significant genetic influences on disordered eating in males regardless of developmental stage, whereas genes were only important in females during and after puberty.
Biometric Model Fitting
Constraint Models
Parameter estimates from the fully unconstrained model indicated substantial sex differences in the magnitude of genetic effects during pre-puberty, but seemingly no sex differences after puberty (see Table 2). Although our 95% confidence intervals for genetic estimates frequently included 0, the high degree of similarity between the twin correlations and the pattern of genetic effects suggest that small sample sizes contribute to broad confidence intervals rather than a lack of fit of the model to the observed data.
Table 2.
Parameter Estimates and Fit Statistics from Constraint Models.
| Estimates | Fully Unconstrained |
Constrain all except Pre-Pub Females |
Constrain A to 0 in Pre- Puberty |
Fully Constrained |
|---|---|---|---|---|
| Parameter Estimates | ||||
| Males: | ||||
| Pre-Pubertal Twins (N = 136) | ||||
| A | .46 (.00–.68) | .55 (.37–.63) | .00 (.00–.00) | .51 (.32–.59) |
| C | .00 (.00–.40) | .00 (.00–.15) | .29 (.06–.49) | .00 (.00–.17) |
| E | .54 (.32–.84) | .45 (.37–.54) | .71 (.51–.94) | .49 (.41–.57) |
| Pubertal Twins (N = 42) | ||||
| A | .51 (.00–.81) | -- | .56 (.33–.64) | -- |
| C | .08 (.00–.76) | -- | .00 (.00–.20) | -- |
| E | .41 (.18–.81) | -- | .44 (.36–.54) | -- |
| Young Adult Twins (N = 166) | ||||
| A | .44 (.00–.63) | -- | -- | -- |
| C | .00 (.00–.39) | -- | -- | |
| E | .56 (.37–.80) | -- | -- | -- |
| Females: | ||||
| Pre-Pubertal Twins (N = 124) | ||||
| A | .00 (.00–.48) | .00 (.00–.48) | .00 (.00–.00) | -- |
| C | .25 (.00–.47) | .25 (.00–.47) | .25 (.003–.47) | -- |
| E | .75 (.51–.99) | .75 (.51–.99) | .75 (.53–.99) | -- |
| Pubertal Twins (N = 122) | ||||
| A | .58 (.00–.83) | .55 (.37–.63) | .56 (.33–.64) | -- |
| C | .13 (.00–.61) | .00 (.00–.15) | .00 (.00–.20) | -- |
| E | .29 (.17–.50) | .45 (.37–.54) | .44 (.36–.54) | -- |
| Young Adult Twins (N = 324) | ||||
| A | .56 (.23–.67) | -- | -- | -- |
| C | .00 (.00–.28) | -- | -- | -- |
| E | .44 (.33–.59) | -- | -- | -- |
| Model Fit Statistics | ||||
| −2lnL (df) | 2414.23 (885) | 2422.60 (897) | 2424.82 (896) | 2431.76 (900) |
| χ2Δ (df) | -- | 8.37 (12) | 10.59 (110) | 17.53 (15) |
| p | -- | .76 | .48 | .29 |
| AICa | −32.77 | −48.40 | −44.18 | −45.24 |
Note. A = additive genetic effects; C = shared environmental effects; E = nonshared environmental effects; −2lnL = −2 times the log likelihood; χ2Δ = chi-square change; AIC = Akaike’s Information Criteria. Parameter estimates are standardized with 95% confidence intervals in parentheses. The “Fully Unconstrained” model allowed the A, C, and E estimates to vary across sex and developmental stage. The “Constrain all except Pre-Pub Females” constrained A, C, and E estimates to be equal across all male twins, and pubertal and adult female twins, but allowed estimates to vary in the pre-pubertal, female twin group. The “Constrain A to be 0 in Pre-Puberty” constrained the A estimates to be 0 in pre-puberty for male and female twins, and constrained A, C, and E to be equal in pubertal and young adult male and female twins. The “Fully Constrained” model constrained the A, C, and E estimates to be equal across sex and developmental stage. Dashed lines for constrained models indicate that parameter estimates were constrained to be equal to those for the preceding group. The best-fitting model is noted in bolded text.
All AICs were calculated by taking the difference in −2lnL values between a baseline, unrestricted model (i.e., a model that freely estimates variances, covariances, and means; −2lnL (df) = 2375.00 (849)) and all other models.
Based on these results, we fit two submodels the data. The first tested sex differences in genetic effects during puberty only by constraining A, C, and E to be equal in males and females across all groups except the pre-pubertal females. The second model additionally constrained genetic effects to be 0 in male and female twins during pre-puberty (while continuing to constrain A, C, and E across pubertal and adult groups). Together, these submodels provided a rigorous test of our hypothesis that genetic effects would be significant in male but not female twins before puberty.
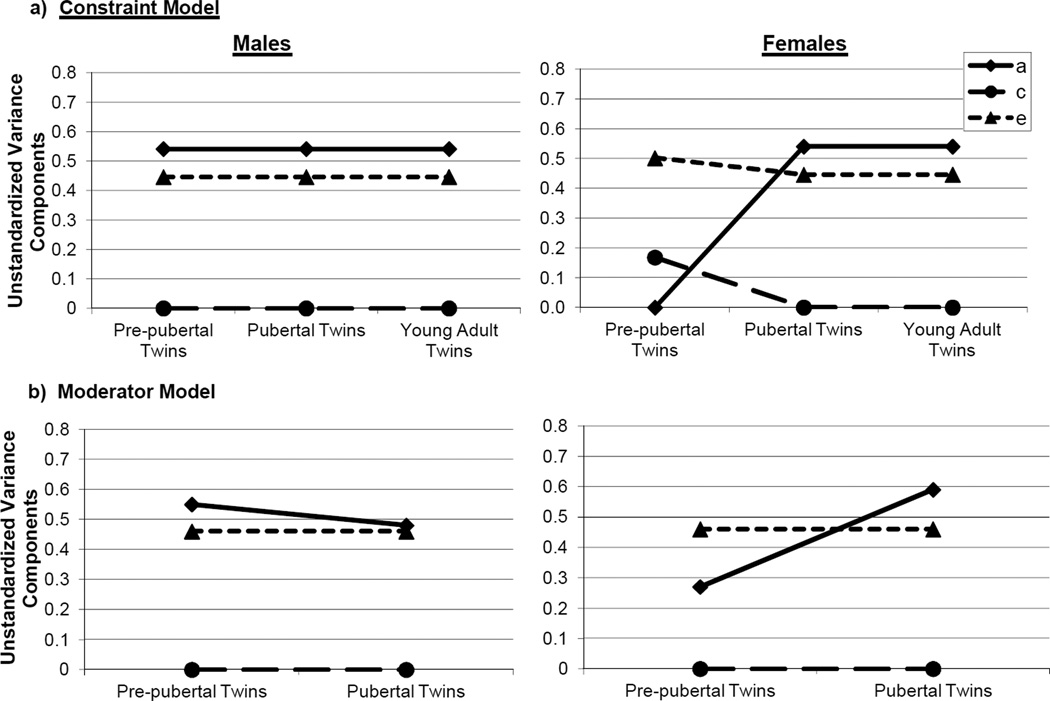
Although none of the submodels fit significantly worse than the full model (i.e., chi-square differences were non-significant; see Table 2), the model constraining A, C, and E to be equal across all groups except pre-pubertal females was chosen as best-fitting, based on its lower AIC value. Standardized (see Table 2) and unstandardized (see Figure 1a) parameter estimates from this model indicated that shifts in additive genetic and shared environmental influences primarily accounted for group differences. In the pre-pubertal female twins, genetic influences were estimated at 0%, with shared and nonshared environmental factors accounting for all of the variance. By contrast, in all of the male and other female groups, additive genetic (51%) and nonshared environmental (49%) influences predominated with no shared environmental influences.
Figure 1.
Unstandardized Estimates of Additive Genetic (a), Shared Environmental (c), and Nonshared Environmental (e) Effects from the Best-Fitting: a) Constraint Model; and b) Moderator Model.
Moderation Models
Because constraint models showed no differences in genetic/environmental influences across puberty and young adulthood, we combined the pubertal and young adult samples into one group within each sex for the moderation models. This reduced the number of puberty moderator levels from 3 (pre-puberty, puberty, young adulthood) to 2 (pre-puberty, puberty (0)), thereby increasing statistical power. Despite their many advantages, moderation models require large samples and fewer moderator levels to detect significant effects, particularly when examining multiple moderators (Purcell, 2002).
As discussed previously, we were particularly interested in whether the sex × puberty moderator of A could be dropped from the model, since this coefficient directly tests whether genetic influences on disordered eating vary by sex and pubertal development. This moderator appeared to be statistically significant; although the chi-square change comparing the “Drop A moderators” to the full model didn’t quite reach significance (p = .17; see Table 3), the sex × puberty moderator of A (βXSxD = –.30) was statistically significant in the model (i.e., 95% CI = −.58, −.02). These findings strongly suggest that the degree of genetic influence on disordered eating varies by sex during pre-puberty or puberty only. Confirming these impressions, the best fitting model (as indicated by the lowest AIC and a non-significant chi-square change) dropped all C and E moderators, but did not drop the A moderators. Unstandardized estimates from this model (see Figure 1b) showed increased genetic effects from pre-puberty to puberty in girls, but minimal changes in boys. These findings replicate constraint model results and strongly suggest sex differences in genetic effects during pre-puberty only.
Table 3.
Model Fit Comparisons for the Moderation Models.
| Model | −2lnL (df) | χ2Δ (df) | p | AIC |
|---|---|---|---|---|
| Full Model | 2706.76 (984) | -- | -- | -- |
| Drop Sex Moderator | 2711.06 (990) | 4.3 (6) | .64 | −7.70 |
| Drop Puberty Moderator | 2713.90 (990) | 7.14 (6) | .30 | −4.86 |
| Drop Sex × Puberty Moderator | 2710.13 (987) | 3.37 (3) | .33 | −2.63 |
| Drop Moderators on A, C or E: | ||||
| Drop all moderators on A | 2711.25 (987) | 4.99 (3) | .17 | −1.01 |
| Drop all moderators on C | 2706.76 (987) | 0 (3) | 1.00 | −6.00 |
| Drop all moderators on E | 2707.39 (987) | 0.63 (3) | .89 | −5.37 |
| Drop all moderators on C and E | 2710.39 (990) | 0.63 (6) | .99 | −11.37 |
| Drop all Moderators on A, C and E | 2713.99 (993) | 7.23 (9) | .61 | −10.77 |
Note. χ2Δ = chi-square change; Full Model = model with paths and all three moderators. Each nested model is compared to the full model when calculating the χ2Δ and degrees of freedom. The best-fitting model is noted by bolded text; unstandardized estimates (with 95% confidence intervals in parentheses) from this model are as follows: a = .69 (.54, .84), c = .00 (−.33, .33), e = .68 (.63, .74), βXS = .08 (−.09, .34), βYS = 0, βZS = 0, βXD = .05 (−.16, .27), βYD = 0, βZD = 0, βXSxD = −.30 (−.58, −.02), βYSxD = 0, βZSxD = 0,
We were somewhat puzzled by the lack of significant “c” moderation. Notably, large samples are needed to detect “c” moderation which is notoriously difficult to identify using the classical twin study (i.e., c effects; see Martin et al., 1978) and was of small magnitude in our study (i.e., 25% in pre-pubertal versus 0% in pubertal/young adult females; see Table 2). Importantly, the larger “a” estimates for pre-pubertal females in the moderation versus constraint models likely reflect the presence of undetected “c” effects, as these effects would be included in the “a” estimates within moderation models.
Discussion
This is the first study to examine the influence of puberty on genetic risk for disordered eating in boys and girls. Findings were significant in highlighting the presence of significant sex differences in puberty’s effects. Moderate heritabilities (~50%) were observed for males regardless of developmental stage, and these heritabilities were equal to those in females from puberty onward. Results were in sharp contrast to those of females, where genetic factors accounted for 0% of the variance during pre-puberty, but over 50% during puberty and young adulthood. Our findings were consistent across two analytic approaches confirming that sex differences in genetic influences are present during pre-puberty only.
Overall, findings highlight important sex differences in the role of puberty in genetic risk for disordered eating. In girls, these data add to a growing literature suggesting that puberty may activate genetic risk in girls via sex-specific processes. Previous theories have focused on ovarian hormones (Klump and Culbert, 2007, Klump et al., 2010, Klump et al., 2007), primarily because these hormones drive pubertal development in girls and regulate gene transcription within neuronal systems disrupted in eating disorders (e.g., serotonin) (Ostlund et al., 2003). New data directly implicating estradiol in increases in genetic effects during puberty in girls (Klump et al., 2010) further suggest that ovarian hormones likely contribute. Although our findings cannot confirm a role for ovarian hormones, they provide additional evidence that female-specific pubertal processes (like hormones) may be important. Indeed, the sex-differentiated pattern of genetic risk suggests that hormones that increase during puberty in girls but not boys (i.e., ovarian hormones) may activate genetic risk for girls.
Importantly, our findings do not rule out the possibility that psychosocial factors also contribute. Girls are exposed to more sociocultural (e.g., pressures for thinness) and peer (e.g., affiliation with weight-focused peers) factors that increase risk for eating disorders during puberty (Cafri et al., 2005, Thompson and Stice, 2001). Increased exposure to these risk factors may activate genetic risk and increase the heritability of disordered eating in girls versus boys. These gene × environment interactions may then contribute to enhanced phenotypic risk for girls during the pubertal period and beyond. Notably, while these mechanisms could help explain sex differences in puberty’s effects on genetic risk, they do not easily explain the steep increase in genetic effects (from 0 to 51%) in girls. Many girls experience peer pressure and pressures for thinness prior to puberty, pressures that would presumably activate some portion of genetic risk prior to the pubertal period. Multiple studies show an absence of genetic effects on disordered eating prior to puberty in girls (Culbert et al., 2009, Klump et al., 2003, Klump et al., 2007), suggesting that psychosocial risk factors alone cannot account for puberty’s effects. Indeed, it may be that some combination of hormonal activation and heightened psychosocial risk contribute to gene-environment interactions and increased heritability in girls during puberty. This possibility should be examined in future studies using hormone and psychosocial assessments in male and female twins.
It also will be important to understand factors underlying the stability of genetic risk for disordered eating in boys across development. Disordered eating was influenced by genetic influences in boys in pre-puberty, puberty, and young adulthood, with no differences in the magnitude of these effects across development. The genetic factors contributing to this stability currently remain unknown. One interesting candidate is testosterone exposure. Emerging research suggests that early testosterone exposure has major organizational effects on the brain (Gagnidze et al., 2010) and may protect against the development of disordered eating (Culbert et al., 2008, Klump et al., 2006, Smith et al., 2010). Given the genomic effects of testosterone (Gagnidze et al., 2010), its presence early in life in boys (but not girls) (Gagnidze et al., 2010), and its enduring effects on the brain and behavior (Breedlove, 1994, Collaer and Hines, 1995, Ryan and Vandenbergh, 2002, Wade, 1972), early testosterone might organize genetic risk for disordered eating in boys and contributes to stability in genetic risk across adolescence and into adulthood.
Although these hypotheses are speculative, they dovetail nicely with phenotypic patterns of disordered eating risk across sex. Stable, yet relatively low levels, of phenotypic risk have been observed in boys across adolescence. For example, a recent study showed that boys’ disordered eating did not increase from pre- to post-puberty, but instead remained relatively constant at low levels of risk (Culbert et al., submitted). This pattern was categorically different from that observed in girls, where relatively low levels of disordered eating before puberty were replaced with significantly higher levels during puberty and beyond (Culbert et al., submitted). Collectively then, our data and those of others (Culbert et al., 2008, Culbert et al., submitted, Klump et al., 2006, Ryan and Vandenbergh, 2002, Smith et al., 2010, Wade, 1972) suggest that events early in life in boys (e.g., prenatal testosterone exposure), and events later in life in girls (i.e., ovarian hormone activation during puberty) may differentially affect genetic risk for disordered eating and result in sex-differentiated patterns of phenotypic risk across development (i.e., stable and low levels of phenotypic risk in boys; low risk followed by increasing levels of risk during puberty in girls). These possibilities should be examined in future research using human (e.g., opposite-sex twins; Culbert et al., 2008) and animal (e.g., binge eating proneness model; Boggiano et al., 2007) models.
Finally, future studies should identify the factors contributing to sex similarities in genetic effects during and after puberty. It appears that once genetic influences are activated in girls, a similar proportion (i.e., ~50%) of variance in disordered eating is accounted for by genes in both sexes. It is important to note that our findings speak to similarities in the magnitude of genetic influences, but they do not tell us whether the type of genetic risk factors (e.g., hormones, psychosocial factors) are the same across sex. Indeed, studies of opposite-sex adult twins have obtained genetic correlations of ~.50, indicating that a substantial proportion of the genes contributing to disordered eating in females are not the same as the genetic factors in males (Baker et al., 2009, Reichborn-Kjennerud et al., 2003). Thus, in late adolescence and adulthood, there are sex specific genetic risk factors for disordered eating that should be a focus of future research.
Limitations of our study should be noted. Sample sizes were relatively small, particularly in the pubertal male and female groups where confidence intervals for genetic estimates were broad and sometimes included 0. Small samples also likely decreased our ability to detect changes in shared environmental effects in the moderation models. However, our results for genetic effects were consistent across two separate model-fitting techniques. Moreover, model-fitting results matched impressions from the twin correlations, and sample sizes in the most critical groups (i.e., pre-pubertal twins) were larger and adequately sized. A lack of power therefore is unlikely to have unduly influenced our model fit comparisons or primary study findings (i.e., genetic effects differ across sex during pre-puberty only). Nonetheless, our point estimates of genetic effects should be interpreted with some caution, and future research should replicate our results using larger samples of twins.
The PDS has been criticized for assessing self-perceptions of development (Dorn et al., 2006) that may not reflect objective pubertal changes (Coleman and Coleman, 2002). Additional research with other measures of puberty (e.g., physical exams) is needed to confirm that sex differences follow objective changes in pubertal stage. High correlations between PDS scores and physician ratings (Petersen et al., 1988) suggest that this is likely to be the case. Moreover, estrogen moderation of genetic influences on disordered eating in girls (Klump et al., 2010) further implicates pubertal biological processes in changes in genetic effects.
Acknowledgements
This research was supported by grants from the National Institute of Mental Health (NIMH) (1R01 MH092377-01) awarded to Drs. Klump, Burt, and Sisk, the NIMH awarded to Drs. Nigg, Klump, and Sisk (1R21 MH070542-01), the NIMH (1F31-MH084470) awarded to Ms. Culbert, and the Michigan State University Intramural Grants Program awarded to Dr. Klump. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Mental Health, or the National Institutes of Health.
Footnotes
The Minnesota Eating Behavior Survey (previously known as the Minnesota Eating Disorder Inventory) was adapted and reproduced by special permission of Psychological Assessment Resources, Inc., 16204 North Florida Avenue, Lutz, Florida 33549, from the Eating Disorder Inventory (collectively, EDI and EDI-2) by Garner, Olmstead, Polivy, Copyright 1983 by Psychological Assessment Resources, Inc. Further reproduction of the MEBS is prohibited without prior permission from Psychological Assessment Resources, Inc.
References
- Akaike H. Factor analysis and aic. Psychometrika. 1987;52:317–332. [Google Scholar]
- Baker JH, Maes HM, Lissner L, Aggen SH, Lichtenstein P, Kendler K. Genetic risk factors for disordered eating in adolescent males and females. Journal of Abnormal Psychology. 2009;118:576–586. doi: 10.1037/a0016314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck AT, Steer RA, Brown GK. Manual for the Beck Depression Inventory-II. San Antonio, TX: Psychological Corporation; 1996. [Google Scholar]
- Boggiano MM, Artiga AI, Pritchett CE, Chandler-Laney PC, Smith ML, Eldridge AJ. High intake of palatable food predicts binge-eating independent of susceptibility to obesity: an animal model of lean vs. obese binge-eating and obesity with and without binge-eating. International Journal of Obesity (London) 2007;31:1357–1367. doi: 10.1038/sj.ijo.0803614. [DOI] [PubMed] [Google Scholar]
- Breedlove SM. Sexual differentiation of the human nervous system. Annual Review of Psychology. 1994;45:389–418. doi: 10.1146/annurev.ps.45.020194.002133. [DOI] [PubMed] [Google Scholar]
- Bulik CM, Sullivan PF, Wade TD, Kendler KS. Twin studies of eating disorders: A review. International Journal of Eating Disorders. 2000;17:251–261. doi: 10.1002/(sici)1098-108x(200001)27:1<1::aid-eat1>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- Cafri G, Yamamiya Y, Brannick M, Thompson JK. The influence of sociocultural factors on body image: A meta-analysis. Clinical Psychology: Science and Practice. 2005;12:421–433. [Google Scholar]
- Coleman L, Coleman J. The measurement of puberty: A review. Journal of Adolescence. 2002;25:535–550. doi: 10.1006/jado.2002.0494. [DOI] [PubMed] [Google Scholar]
- Collaer M, Hines M. Human behavioral sex differences: a role of gonadal hormones during early development? Psychological Bulletin. 1995;118:55–107. doi: 10.1037/0033-2909.118.1.55. [DOI] [PubMed] [Google Scholar]
- Culbert KM, Breedlove SM, Burt SA, Klump KL. Prenatal hormone exposure and risk for eating disorders: A comparison of opposite- and same-sex twins. Archives of General Psychiatry. 2008;65:329–336. doi: 10.1001/archgenpsychiatry.2007.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Culbert KM, Burt SA, McGue M, Iacono WG, Klump KL. Puberty and the genetic diathesis of disordered eating. Journal of Abnormal Psychology. 2009;118:201–218. doi: 10.1037/a0017207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Culbert KM, Burt SA, Sisk CL, Klump KL. The emergence of sex differences in disordered eating attitudes and behaviors during puberty. doi: 10.1037/a0031791. (submitted). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorn LD, Dahl RE, Woodward HR, Biro F. Defining the boundaries of early adolescence: A user's guide to assessing pubertal status and pubertal timing in research with adolescents. Applied Developmental Science. 2006;10:30–56. [Google Scholar]
- Fairburn CG, Beglin SJ. Assessment of eating disorders: Interview or self-report questionnaire? International Journal of Eating Disorders. 1994;16:363–370. [PubMed] [Google Scholar]
- Gagnidze K, Pfaff DW, Mong JA. Gene expression in neuroendocrine cells during the critical period for sexual differentiation of the brain. Progress in Brain Research. 2010;186:97–111. doi: 10.1016/B978-0-444-53630-3.00007-5. [DOI] [PubMed] [Google Scholar]
- Herman-Giddens ME, Wang L, Koch G. Secondary sexual characteristics in boys: Estimates from the National Health and Nutrition Examination Survey III, 1988–1994. Archives of Pediatric and Adolescent Medicine. 2001:1022–1028. doi: 10.1001/archpedi.155.9.1022. [DOI] [PubMed] [Google Scholar]
- Keski-Rahkonen A, Bulik CM, Neale M, Rose RJ, Rissanen A, Kaprio J. Body dissatisfaction and drive for thinness in young adult twins. International Journal of Eating Disorders. 2005;37:188–199. doi: 10.1002/eat.20138. [DOI] [PubMed] [Google Scholar]
- Klump KL, Burt SA. The Michigan State University Twin Registry (MSUTR): Genetic, environmental and neurobiological influences on behavior across development. Twin Research and Human Genetics. 2006;9:971–977. doi: 10.1375/183242706779462868. [DOI] [PubMed] [Google Scholar]
- Klump KL, Culbert KM. Molecular genetic studies of eating disorders: Current status and future directions. Current Directions in Psychological Science. 2007;16:37–41. doi: 10.1111/j.1467-8721.2007.00471.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klump KL, Gobrogge KL, Perkins P, Thorne D, Sisk CL, Breedlove SM. Preliminary evidence that gonadal hormones organize and activate disordered eating. Psychological Medicine. 2006;36:539–546. doi: 10.1017/S0033291705006653. [DOI] [PubMed] [Google Scholar]
- Klump KL, Keel PK, Sisk CL, Burt SA. Preliminary evidence that estradiol moderates genetic effects on disordered eating attitudes and behaviors during puberty. Psychological Medicine. 2010;40:1745–1754. doi: 10.1017/S0033291709992236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klump KL, McGue M, Iacono WG. Age differences in genetic and environmental influences on eating attitudes and behaviors in female adolescent twins. Journal of Abnormal Psychology. 2000;109:239–251. [PubMed] [Google Scholar]
- Klump KL, McGue M, Iacono WG. Differential heritability of eating attitudes and behaviors in prepubertal versus pubertal twins. International Journal of Eating Disorders. 2003;33:287–292. doi: 10.1002/eat.10151. [DOI] [PubMed] [Google Scholar]
- Klump KL, Perkins P, Burt SA, McGue M, Iacono WG. Puberty moderates genetic influences on disordered eating. Psychological Medicine. 2007;37:627–634. doi: 10.1017/S0033291707000189. [DOI] [PubMed] [Google Scholar]
- Kovacs M. The Children's Depression Inventory (CDI) Psychopharmacology Bulletin. 1985;21:995–998. [PubMed] [Google Scholar]
- Lee PA, editor. Physiology of puberty. Philadelphia, PA: Lippincott Williams & Wilkins; 2001. [Google Scholar]
- Lykken DT, Bouchard TJ, McGue M, Tellegen A. The Minnesota Twin Family Registry: Some initial findings. Acta Gemellogicae et Medicae. 1990;39:35–70. doi: 10.1017/s0001566000005572. [DOI] [PubMed] [Google Scholar]
- Marderosian A, Wu Y, Culbert KM, Burt SA, Nigg JT, Klump KL. Psychometric properties of the Minnesota Eating Behaviors Survey in pre-adolescent and adolescent girls and boys. (in preparation). [Google Scholar]
- Martin MG, Eaves LJ, Kearsey MJ, Davies P. The power of the classical twin study. Heredity. 1978;40:97–116. doi: 10.1038/hdy.1978.10. [DOI] [PubMed] [Google Scholar]
- Neale MC, Boker SM, Xie G, Maes HH. Mx: Statistical Modeling. 5th Edition. Richmond, VA: Department of Psychiatry; 1999. [Google Scholar]
- Ostlund H, Keller E, Hurd YL. Estrogen receptor gene expression in relation to neuropsychiatric disorders. Annals of the New York Academy of Sciences. 2003;1007:54–63. doi: 10.1196/annals.1286.006. [DOI] [PubMed] [Google Scholar]
- Parent AS, Teilmann G, Juul A, Skakkebaek NE, Toppari J, Bourguignon JP. The timing of normal puberty and the age limits of sexual precocity: Variations around hte world, secular trends, and changes after migration. Endocrine Reviews. 2003:668–693. doi: 10.1210/er.2002-0019. [DOI] [PubMed] [Google Scholar]
- Peeters H, Van Gestel S, Vlietinck R, Derom C, Derom R. Validation of telephone zygosity questionnaire in twins of known zygosity. Behavior Genetics. 1998;28 doi: 10.1023/a:1021416112215. [DOI] [PubMed] [Google Scholar]
- Petersen AC, Crockett L, Richards M, Boxer A. A self-report measure of pubertal status: Reliability, validity, and initial norms. Journal of Youth and Adolescence. 1988;17:117–133. doi: 10.1007/BF01537962. [DOI] [PubMed] [Google Scholar]
- Purcell S. Variance components models for gene-environment interaction in twin analysis. Twin Research. 2002;5:554–571. doi: 10.1375/136905202762342026. [DOI] [PubMed] [Google Scholar]
- Reichborn-Kjennerud T, Bulik CM, Kendler K, Roysamb E, Maes HHM, Tambs K. Gender differences in binge-eating: A population-based twin study. Acta Psychiatrica Scandinavica. 2003;108:196–202. doi: 10.1034/j.1600-0447.2003.00106.x. [DOI] [PubMed] [Google Scholar]
- Reichborn-Kjennerud T, Bulik CM, Tambs K, Harris JR. Genetic and environmental influences on binge eating in the absence of compensatory behaviors: A population-based twin study. International Journal of Eating Disorders 36. 2004;3 doi: 10.1002/eat.20047. [DOI] [PubMed] [Google Scholar]
- Ryan BC, Vandenbergh JG. Intrauterine position effects. Neuroscience and Biobehavioral Reviews. 2002;26:665–678. doi: 10.1016/s0149-7634(02)00038-6. [DOI] [PubMed] [Google Scholar]
- Silberg JL, Bulik CM. The developmental association between eating disorder symptoms and symptoms of depression and anxiety in juvenile twin girls. Journal of Child Psychology and Psychiatry. 2005;46:1317–1326. doi: 10.1111/j.1469-7610.2005.01427.x. [DOI] [PubMed] [Google Scholar]
- Slane JD, Burt SA, Klump KL. Genetic and environmental influences on disordered eating and depressive symptoms. International Journal of Eating Disorders. doi: 10.1002/eat.20867. (in press). [DOI] [PubMed] [Google Scholar]
- Slof-Op't MC, Bartels M, van Furth EF, van Beijsterveldt CEM, Meulenbelt I, Slagboom PE, Boomsma DI. Genetic influences on disordered eating behavior are largely independent of body mass index. Acta Psychiatrica Scandinavica. 2008;117:348–356. doi: 10.1111/j.1600-0447.2007.01132.x. [DOI] [PubMed] [Google Scholar]
- Smith AR, Hawkeswood SE, Joiner TE. The measure of a man: Associations between digit ration and disordered eating in males. International Journal of Eating Disorders. 2010;43:543–548. doi: 10.1002/eat.20736. [DOI] [PubMed] [Google Scholar]
- Suisman JL, Klump KL, editors. Genetic and neuroscientific perspectives on body image. New York: Guildford Press; 2011. [Google Scholar]
- Tholin TS, Rasmussen F, Tynelius P, Karlsson J. Genetic and environmental influences on eating behavior: The Swedish Young Adult Male Twins Study. American Journal of Clinical Nutrition. 2005;81:564–569. doi: 10.1093/ajcn/81.3.564. [DOI] [PubMed] [Google Scholar]
- Thompson JK, Stice E. Mounting evidence for a new risk factor for body-image disturbance and eating pathology. Current Directions in Psychological Science. 2001;10:181–183. [Google Scholar]
- von Ranson KM, Klump KL, Iacono WG, McGue M. Development and validation of Minnesota Eating Behaviors Survey: A brief measure of disordered eating attitudes and behaviors. Eating Behaviors. 2005;6:373–392. doi: 10.1016/j.eatbeh.2004.12.002. [DOI] [PubMed] [Google Scholar]
- Wade GN. Gonadal hormones and behavioral regulation of body weight. Physiol Behav. 1972;8:523–534. doi: 10.1016/0031-9384(72)90340-x. [DOI] [PubMed] [Google Scholar]
- Young JK. Anorexia nervosa and estrogen: Current state of the hypothesis. Neuroscience and Biobehavioral Reviews. 2010;34:1195–1200. doi: 10.1016/j.neubiorev.2010.01.015. [DOI] [PubMed] [Google Scholar]