Abstract
Childhood maltreatment is associated with lasting changes in neuroendocrine regulation, alterations in brain structure and function, and symptoms of “limbic irritability”. Limbic irritability symptoms include somatic, sensory, and behavioral phenomena, and may stem from increased excitatory neurotransmission following maltreatment. In this investigation, we tested the hypotheses that child maltreatment is indirectly associated with depressive and dissociative symptomatology via indicators of limbic irritability, and that variation within FKBP5, a gene involved in glucorticoid receptor functioning, moderates these effects. The sample consisted of high-risk, low-income women (N = 236) living in an inner-city environment. Child maltreatment, limbic irritability, and symptoms of depression and dissociation were measured cross-sectionally using self-report assessments. Haplotype analyses were conducted across four FKBP5 SNPs (rs3800373, rs9296158, rs1360870, rs9470080). Path analysis using bootstrapping procedures was performed to test hypotheses regarding indirect and conditional indirect effects. We found significant indirect effects of maltreatment on depression (β = 0.088, p<0.01) and dissociation (β = 0.105, p<0.01) via limbic irritability. In addition, variation within FKBP5 moderated these significant indirect effects. For individuals with 1–2 copies of the CATT haplotype, the indirect effects of maltreatment on depression (β = 0.137, p<0.01) and dissociation (β = 0.132, p<0.01) via limbic irritability were significant, whereas the indirect paths were not significant for individuals with no copies of this haplotype (depression: β = 0.037, p>0.05; dissociation: β = 0.002, p>0.05). These results add to the growing evidence that child maltreatment may lead to symptoms of internalizing psychopathology through its impact on the limbic system. In addition, this study revealed a potential role of FKBP5 gene variants in contributing to risk for limbic system dysfunction.
Introduction
Childhood maltreatment is one of the most severe forms of stress exposure that can occur during development, and is strongly associated with a broad range of negative mental health outcomes throughout the life course (Cicchetti, 1989; Cicchetti & Valentino, 2006; Cohen, Brown, & Smaile, 2001; Collishaw et al., 2007; Kendler, Kuhn, & Prescott, 2004; Putnam, 1997; Widom, 1999; Widom, Dumont, & Cjaza, 2007). Child maltreatment frequently disrupts the negotiation of stage-salient developmental tasks, including the formation of secure attachment to caregivers, development of an autonomous self, establishment of effective emotion regulation strategies, and maintenance of healthy peer relationships (Briere, 1992; Cicchetti & Lynch, 1995; Cicchetti & Valentino, 2006; Lyons-Ruth & Spielman, 2004; Myers et al., 2002; Trickett & McBride-Chang, 1995). Through probabilistic epigenetic processes, these psychological disruptions often have significant effects on physiological, homeostatic, and psychiatric functioning throughout development (Cicchetti & Toth, 2005; Cicchetti & Valentino, 2006).
Child maltreatment is associated with earlier disorder onset, as well as greater persistence and impairment throughout adulthood (Green et al., 2010; Edwards, Holden, Felitti, & Anda, 2003; McLaughlin et al., 2010a; McLaughlin et al., 2010b). In particular, risk for depressive and dissociative symptoms has been consistently found in reference to childhood traumatic experiences (Cicchetti & Toth, 1998; Macfie, Cicchetti, & Toth, 2001a; Toth, Manly, & Cicchetti, 1992; Widom et al., 2007). Researchers have theorized that unsuccessful resolution of stage-salient tasks during development may cause individuals to develop a depressotypic organization, characterized by dysregulated processes within cognitive, socioemotional, biological, and representational systems (Cicchetti, Rogosch, & Toth, 1997; Cicchetti & Toth, 1995). Deficits stemming from maltreatment may interact across these domains in a transactional manner throughout development to potentiate depressive symptoms. Dissociative symptoms can also be explained through failed attainment of stage-salient tasks, particularly those related to attachment security and self-system development (Macfie et al., 2001a; Putnam, 1997). Inability to attain secure attachment to caregivers during infancy due to harsh, inconsistent, and/or insensitive parenting may lead to disjointed representational models of the self in relation to others (Sroufe & Fleeson, 1986; Macfie, Cicchetti, & Toth, 2001b). Reciprocal interactions between these self-system dysfunctions and difficulties within cognitive and perceptual domains often lead to increased risk for dissociative experiences (Cicchetti & Toth, 1995; Ludwig, 1983; van der Kolk, ver der Hart, & Marmar, 1996).
Research using latent modeling approaches has found that child maltreatment may induce an overall vulnerability toward internalizing or externalizing symptoms rather than risk for a specific psychiatric illness. In fact, this underlying internalizing or externalizing organization was found to fully mediate associations between childhood trauma and psychiatric disorders (Keyes et al., 2012). Therefore, research that is focused on clarifying the core liabilities associated with adverse developmental sequelae may be more informative than identifying outcomes at the disorder level. One current challenge within the field is to better understand the ways in which genetic and neurobiological processes interact with the psychological effects of maltreatment to promote overall psychiatric vulnerability (Cicchetti & Toth, 2005). More extensive investigation into the mediators and moderators of these associations may clarify differential pathways leading to resilient as compared to maladaptive outcomes (Cicchetti, 2010).
One mechanism by which childhood maltreatment may impact the development of depressive and dissociative outcomes is through its effects on the limbic system. Chronic adversity early in life is associated with a “cascade” of negative consequences for stress sensitivity and responsiveness, as well as limbic system development and functioning (Cicchetti & Tucker, 1994; Heim et al., 2000; Heim, Newport, Bonsall, Miller, & Nemeroff, 2001; Lupien, McEwen, Gunnar, & Heim, 2009; Teicher, Andersen, Polcari, Anderson, & Navalta, 2002; Teicher et al., 2003). Although activation of stress-responsive systems may initially lead to adaptive changes and contribute to survival, chronic stress exposure during childhood may derail functioning during sensitive periods, thereby contributing to the development of allostatic overload (Teicher et al., 2003). Under these circumstances, prolonged and repeated stress exposure may result in physiological dysregulation, leading to inefficient homeostatic functioning (McEwen, 1998; McEwen & Stellar, 1993; McEwen & Wingfield, 2003).
According to the cascade model (Teicher et al., 2002), neuroendocrine dysregulation may occur due to the effects of maltreatment on the hypothalamic- pituitary-adrenal (HPA) axis, sympathetic nervous system, and functioning of peptide hormones. Chronic stress exposure is associated with attenuated development of GABA-A, noradrenergic, and glucocorticoid receptors, leading to changes in feedback loops, and resulting in dysregulated release of corticosteroids and noradrenergic hormones in response to stress (Caldji, Francis, Sharma, Plotsky, & Meaney, 2000; Caldji et al., 1998; Liu et al., 1997). These alterations may result in significant health consequences and stress-related psychopathology throughout the life course (Evans, 2003; Heim & Nemeroff; 2001; Lupien et al., 2006; Simeon et al., 2007).
The cascade model is consistent with a developmental psychopathology framework, in elucidating the ways by which reciprocal influences within biological and environmental contexts may influence development (Cicchetti & Valentino, 2006; Teicher et al., 2002). Genetic, neurobiological, experiential, and contextual factors may interact collectively to promote adaptive and maladaptive developmental outcomes (Cicchetti & Tucker, 1994). In understanding the consequences of child maltreatment, developmental considerations such as the timing, chronicity, and severity of stress exposure must be taken into account. Limbic regions of the brain are particularly susceptible to the effects of chronic stress during critical periods of development due to their high density of glucocorticoid receptors (Sanchez, Young, Plotsky, & Insel, 2000). Delayed development and varied sensitivity based on developmental stage may render these regions additionally vulnerable to the consequences of child maltreatment. For instance, projections to the prefrontal cortex remain unmyelinated until adolescence, and hippocampal gray matter overproduction occurs during discrete periods in early and middle childhood (Crews, He, & Hodge, 2007; Gogtay et al., 2006; Spear, 2000). Preclinical research suggests that repeated exposure to stress hormones during development negatively affects neuronal migration, myelination, and neurogenesis, resulting in reduced dendritic spines, gray matter, and white matter connectivity in affected brain regions (Andersen & Teicher, 2004; Meaney, Stewart, & Beatty, 1981; Schapiro, 1971). Therefore, child maltreatment may lead to neurotoxic effects within limbic regions during sensitive periods of development.
Prolonged, stress-induced stimulation of limbic system areas during development may also cause kindling, which is indicated by seizure-like neuronal activity and changes in the excitability and behavior of affected neurons (van der Kolk & Greenberg, 1987; Post, 1992; Teicher et al., 2002). Symptoms of limbic irritability are believed to be manifestations of kindling effects within limbic system regions (Teicher et al., 2002). Limbic irritability symptoms include somatic and perceptual distortions, brief hallucinatory events, motor automatisms, and dissociative symptoms, and are analogous to symptoms that are seen in patients with temporal lobe epilepsy (TLE; Teicher, Glod, Surrey, & Swett, 1993). Elevated symptoms of limbic irritability are strongly associated with childhood traumatic stress, as well as structural and functional alterations within cortico-limbic regions (Anderson, Teicher, Polcari, & Renshaw, 2002; Choi, Jeong, Rohan, Polcari, & Teicher, 2009; Teicher, Samson, Polcari, & McGreenery, 2006; Teicher, Samson, Sheu, Polcari, & McGreenery, 2010). Investigations have found attenuated development within the medial prefrontal cortex, medial frontal gyrus, anterior cingulate gyrus, hippocampus, and corpus callosum in individuals who experienced maltreatment during childhood (Andersen et al., 2008; Bremner et al., 1997; Choi, Jeong, Polcari, Rohan, & Teicher, 2012; Green, Voeller, Gaines, & Kulsie, 1981; Teicher et al., 2004; Tomoda, Navalta, Polcari, Stein, 1997; Sadato, & Tiecher, 2009). There is also evidence of functional impairments within limbic regions for individuals with histories of childhood maltreatment (Anderson et al., 2002; Ito et al., 1993; Ito, Teicher, Glod, & Ackerman, 1998; Teicher et al., 1997). These findings highlight the potential neurotoxic effects of chronic stress on the developing brain. They also lend support to the hypothesis that glucocorticoids have organizational effects on the brain, leading to lasting changes that are evident in adulthood (Gilbertson et al., 2002; Lupien et al., 2009; Lowy, Wittenberg, & Yamamoto, 1995; Seckl, 1998; Welberg & Seckl, 2001).
Comparable dysfunctions within stress-responsive systems and impairments within cortico-limbic regions have been found in individuals with depressive and dissociative symptoms (Fitzgerald, Laird, Maller, & Daskalakis, 2008; Nemeroff & Vale, 2005; Veltman et al., 2005; Yehuda, 2009). Volume reductions within the hippocampus and dorsolateral prefrontal cortex are associated with depression and posttraumatic stress disorder (PTSD; Amico et al., 2011; Chen, Hamilton, & Gotlib, 2010; Gilbertson et al., 2002; MacQueen & Frodl, 2010). Functional abnormalities within the medial prefrontal cortex, insula, and anterior cingulate cortex are consistent findings in PTSD. Evidence that these impairments are more pronounced for individuals who experienced childhood trauma lends support to the argument that these abnormalities may serve as risk factors for the development of depression and/or PTSD (Frodl et al., 2010; Gilbertson et al., 2002; Rao et al., 2010; Vythilingham et al., 2002). These findings collectively support the hypothesis that maltreatment confers psychiatric risk during sensitive periods of development through its effects on the limbic system.
Consistent with a transactional approach to development, the relationship between maltreatment and subsequent psychopathology is not always linear. Although evidence suggests that childhood adversity and trauma are related to limbic system dysfunction, individual differences in developmental pathways and resilience underscore the potential role of genetic factors (Caspi et al., 2003; Gillespie, Phifer, Bradley, & Ressler, 2009; Koenen et al., 2005). In recent years, there has been a growing emphasis on the importance of gene-environment interactions in contributing to psychopathology in the context of maltreatment (Aslund et al., 2009; Caspi et al., 2002; Cicchetti, Rogosch, & Oshri, 2011; Cicchetti, Rogosch, & Sturge-Apple, 2007; Cicchetti, Rogosch, & Thibodeau, 2012; Gillespie et al., 2009; Karg, Burmeister, Shedden, & Sen, 2011; Kim-Cohen et al., 2006; Rutter, Moffitt, & Caspi, 2006). In line with the tenets of developmental psychopathology, this research has emphasized the importance of a multiple levels of analysis approach to examining risk (Cicchetti & Toth, 2009; Cicchetti & Valentino, 2007).
Genes that are implicated in the stress-response system have been popular targets for research examining the impact of child maltreatment on psychiatric functioning. The FKBP5 gene is involved in regulating the sensitivity and binding affinity of the glucocorticoid receptor (GR), and therefore has significant implications within the limbic system. FKBP5 is induced by cortisol and acts within a negative feedback loop to promote the transcription of stress-responsive target genes, leading to downstream release of ACTH and plasma cortisol (Davies, Ning, & Sanchez, 2002; Vermeer, Hendriks-Stegeman, van der Burg, van Buul-Offers, & Jansen, 2003). FKBP5 is located on chromosome 6p21 and contains a number of Single Nucleotide Polymorphisms (SNPs) that are associated with differential ability for FKBP5 to be induced by cortisol and bind to the GR. Four of these SNPs (rs3800373, rs9296158, rs1360870, rs9470080), spanning 103 kb of the FKBP5 gene, contain variants that are associated with functional differences at the GR. Genotypes containing minor “high-induction” alleles (C, A, T, T for rs3800373, rs9296158, rs1360870, rs9470080, respectively) are associated with greater expression of FKBP5 and increased binding at the GR, whereas those comprised of major “low-induction” alleles (A, G, C, C) are linked with weaker induction by cortisol and GR receptor binding (Binder et al., 2004; Denny, Valentine, Reynolds, Smith, & Scammell, 2000; Scammel, Denny, Valentine, & Smith, 2001; Wochnik et al., 2005).
Environmental stress is strongly related to differential functioning and regulation of FKBP5 at the GR level. Individuals homozygous for the minor alleles across rs1360870 and rs3800373 exhibited insufficient normalization of cortisol secretion following exposure to acute psychosocial stress (Ising et al., 2008). Under conditions of chronic stress, the minor alleles within these SNPs are not only associated with decreased efficiency within the stress-response system, but also increased risk for depression, PTSD, and other stress-related psychopathology (Appel et al., 2011; Binder et al., 2008; Koenen et al., 2005; Sarapas et al. 2011; Segman et al., 2005). Given that one of the most common findings in both depression and PTSD is impaired signaling at the GR, FKBP5 may play a crucial role in the pathogenesis of these disorders (Gillespie et al., 2009; Koenen, Amstadter, & Nugent, 2009; Mehta & Binder, 2012; Pariante & Lightman, 2008; Pariante & Miller, 2001; Roy, Gorodetky, Yuan, Goldman, & Enoch, 2010; Skelton, Ressler, Norrholm, Jovanovic, & Bradley-Davino, 2012; Xie et al., 2010). Though associations between maltreatment experiences, structural and functional abnormalities, and limbic irritability have been found, no studies to date have examined variation within FKBP5 in relation to limbic irritability and mental health outcomes.
The present investigation examined the associations between child maltreatment, FKBP5 haplotypic variation, limbic irritability, and symptoms of depression and dissociation within a high-risk sample of women exposed to a number of environmental and psychosocial stressors. The first aim was to examine whether limbic irritability mediated the relationship between maltreatment and psychiatric symptoms. We hypothesize that there will be a significant indirect effect of maltreatment on depressive and dissociative symptoms via elevations in limbic irritability symptoms. The second aim was to investigate whether haplotype across four FKBP5 SNPs (rs3800373, rs9296158, rs1360870, rs9470080) moderated these indirect effects. We predict that there will be a significant conditional indirect effect based on haplotypic variation such that limbic irritability will mediate the relationship between maltreatment and symptom outcomes for individuals whose possess one or more copies of the minor allele haplotype (CATT), but that these associations will not be significant for individuals with zero CATT copies.
Method
Design & Sample
The participants in this investigation consisted of 236 low-income women who were initially recruited as part of a larger study examining the impact of maltreatment on children’s functioning. Each woman had a child who was enrolled in a research and recreational summer day camp designed for school-aged maltreated and nonmaltreated children. Mothers of maltreated children were recruited at random through a Department of Human Services (DHS) liaison, who examined Child Protective Services reports to identify children who had been maltreated. The DHS liaison explained the project to identified mothers, who gave informed consent for their contact information to be shared with project staff if they were interested in participating. Consent was also obtained from women to examine DHS records pertaining to the family and for their own participation in completing self-report measures and provision of buccal cells for genotyping. Due to the fact that maltreating families are primarily of low socioeconomic status (National Incidence Study- NIS-4, Sedlak et al., 2010), demographically comparable mothers of nonmaltreated children were identified and recruited by the DHS liaison from a pool of families receiving Temporary Assistance for Needy Families (TANF). Families classified as nonmaltreating did not have any record of CPS or preventive service involvement. They were further interviewed to verify lack of maltreatment within their family (Cicchetti, Toth, & Manly, 2003).
Procedures
In this investigation, adult women were interviewed and administered self-report assessments by trained research assistants. These sessions were held either in research interview rooms or within the participant’s home. Buccal cells also were collected from participants at this time (see DNA Collection, Extraction, and Genotyping procedures).
Measures
Child Trauma Questionnaire – Short Form (CTQ-SF; Bernstein et al., 2003)
The CTQ-SF is a 25-item self-report assessment that was used to measure women’s accounts of their own childhood maltreatment. The CTQ-SF is the most widely used assessment to retrospectively measure childhood maltreatment. Participants are presented with statements reflecting childhood experiences that occurred before the age of 18, and are asked to rate items on a 5-point Likert scale ranging from “never true (1)” to “very often true (5)”. The CTQ-SF measures experiences across five maltreatment domains (physical abuse, physical neglect, emotional abuse, emotional neglect, sexual abuse). Due to high correlations among the five maltreatment subscales (see Table 2), scores on the CTQ-SF were summed, producing a continuous variable ranging from 25–125. The CTQ-SF demonstrates good convergent validity with other self-report and interview measures of child maltreatment, and has moderately high internal consistency (α = 0.66 to 0.92) and test-retest reliability (α = 0.79 to 0.86; Bernstein et al., 2003; Bernstein & Fink, 1994; Hyman, Garcia, Kemp, Mazure, & Sinha, 2005). Internal consistency among items in the current investigation was α = 0.84.
Table 2.
Demographic Characteristics of Sample
| Variable | M (SD) or % |
|---|---|
| Mean Age | |
| Years | 33.8 (6.9) |
| Ethnicity | |
| African American | 53.8% |
| European American | 33.9% |
| Hispanic American | 8.5% |
| Other Biracial | 3.8% |
| Marital Status | |
| Never married | 40.1% |
| Married | 19.8% |
| Livingwith partner | 12.7% |
| No longer married | 27.4% |
| Education | |
| Highest grade completed | 11.7 (2.1) |
| Did not graduate from high school | 42.3% |
| Total Family Income | |
| $1,000s including public assistance | 21.31 (10.1) |
| Family history of receiving public assistance | 95.3% |
Limbic System Checklist – 33 (LSCL-33; Teicher et al., 1993)
The LSCL-33 is a self-report measure containing 33 items that assess for lifetime symptoms of limbic irritability. The LSCL-33 was designed to assess for symptoms of ictal temporal lobe epilepsy (TLE) in individuals without seizure disorders in order to determine whether trauma may produce comparable symptoms to TLE. Item content includes paroxysmal somatic disturbances (e.g. numbness, flushing sensations), brief hallucinatory events (e.g. hearing a voice calling your name), visual disturbances (e.g. seeing patterns, flashing lights), automatisms (e.g. twitching, stuttering), and mneumonic disturbances (e.g. flashbacks, derealization). Each item is rated on a four-point Likert-type scale ranging from “never (0)” to “often (3)” with respect to lifetime frequency. To account for any overlapping content between the LSCL-33 and the Dissociative Experiences Scale (DES), the seven items included within the mneumonic disturbances subscale were removed from the total LSCL-33 score, resulting in a total of 26 items. Items across scales were then summed, producing a continuous variable ranging from 0 to 78. The LSCL-33 has demonstrated high test-retest reliability and discriminates between individuals with and without TLE (Teicher et al., 1993). In this investigation, internal consistency among items was α = 0.92.
Dissociative Experiences Scale (DES-II; Carlson & Putnam, 1993)
The DES consists of 28 questions that assess the frequency of different dissociative experiences within clinical and normative populations. Each item is scored on a scale of 0 to 100 percent, referring to how often each dissociative event is experienced. Scores were averaged to provide a total score, adjusted for skipped items, and divided by 10 to produce a continuous variable ranging from 0 to 10. The DES has high internal consistency (α=0.93), and convergent validity with other measures of dissociation (r = 0.67; Bernstein & Putnam, 1986; van IJzendoorn and Schuengel, 1996). In the current investigation internal consistency was α = 0.95.
Beck Depression Inventory (BDI-II; Beck, Steer, & Brown, 1996)
The BDI-II is a 21-item self-report assessment that is designed to measure current depressive symptoms. Each item is rated on a scale from 0 to 3 with possible scores ranging from 0–63. Total scores of 10–18 indicate mild depression, 19–29 suggest moderate symptoms, and 30 or more represent severe depression. The BDI is a widely-used assessment of depressive symptoms and has high internal consistency (α=0.90) and convergent validity with the Hamilton Depression Rating Scale (r=0.93; Storch, Roberti, & Roth, 2004). Internal consistency in the present investigation was α = 0.88.
DNA Collection, Extraction, and Genotyping
Trained research assistants obtained DNA samples from participants by collecting buccal cells using the Epicentre Catch-All Collection Swabs. If a genotype was undetermined after the first run, then it was repeated up to four times. If the null persisted, then the Whole Genome Amplification reaction was repeated along with genotyping until a genotype was established. The call rate for these SNPs were as follows: rs3800373 = 99.4%; rs9296158 = 99.4%; rs1360780 = 99.4%; rs9470080 = 98.4%. Any samples with one or more missing genotypes were excluded from analyses.
DNA was extracted and prepared for polymerase chain reaction (PCR) amplification using the Epicentre BuccalAmp DNA Extraction Kit (Epicentre, Cat. No. BQ090155C). Genotyping was then performed using an established protocol described by Krämer et al. (2007). SNP genotyping was conducted using Applied Biosystems Custom Taqman SNP Genotyping Assays. The products of these analyses were then analyzed using endpoint allelic discrimination. Genotypes were identified and sequenced with the Beckman-Coulter CEQ8000 semiautomated florescent sequencing system, which utilizes Fragment Analysis Application and associated software. All samples were genotyped twice for quality control. Human DNA from cell lines were purchased from Coriell Cell Repositories for each genotype and used as control samples using DTCS chemistry on an ABI 3130xl. These cell lines and a no template control were run with study samples representing 9% of the total data output. Samples that were not able to be genotyped to a 95% or greater confidence level were repeated under the same procedures.
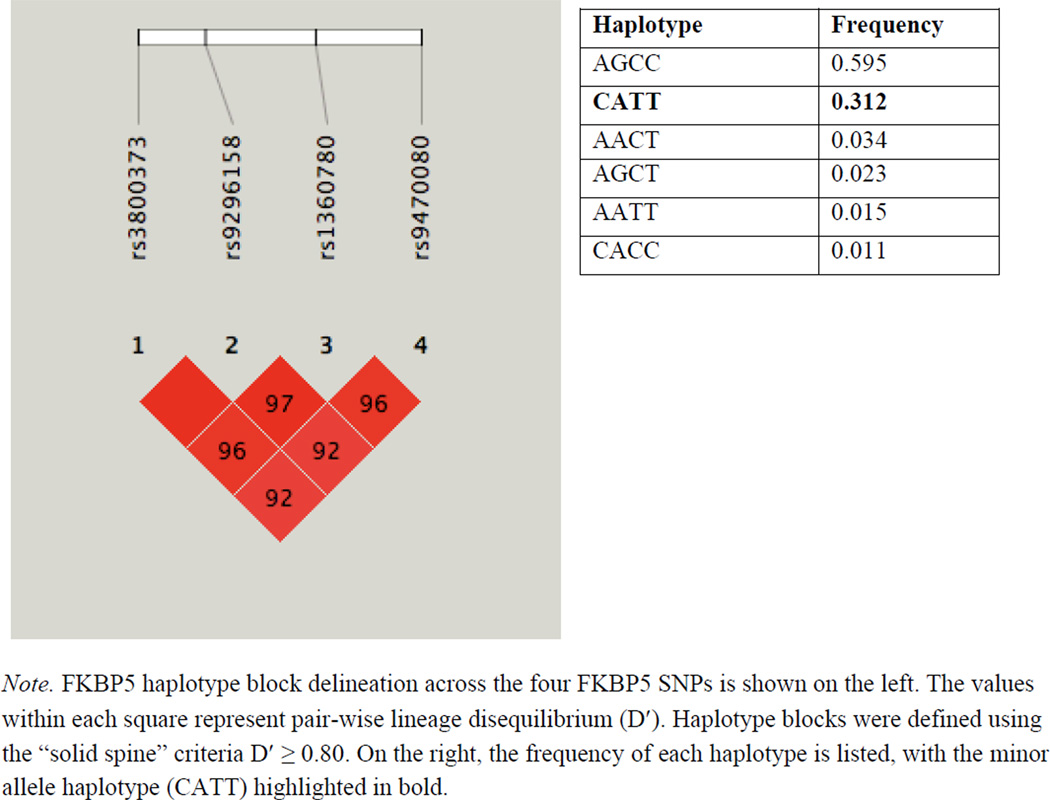
Table 1 presents the location of each SNP within chromosome 6 and the distribution of genotypes within the sample. Haploview software version 4.2 (Barrett, Fry, Maller, & Daly, 2005) was used to determine linkage disequilibrium (LD), Hardy-Weinberg Equilibrium (HWE), and minor allele/haplotype frequencies within the sample. All four SNPs were in HWE (p ≥ 0.28, see Table 1), and analyses indicated statistically significant LD (D′ ≥ 0.92; see Figure 1). Over 90% of the sample was represented by either the AGCC or CATT haplotype (see Figure 1).
Table 1.
Description of FKBP5 SNPs and Hardy-Weinberg Equilibrium Analyses
| SNP ID | Distribution | MAF | Position / Gene H | WE p value |
|---|---|---|---|---|
| rs3800373 | 0.327 | 35650454 / 3′ UTR | 1.00 | |
| AA | 107 (45.3%) | |||
| AC | 104 (44.1%) | |||
| CC | 25 (10.6%) | |||
| rs9296158 | 0.338 | 35675060 / Intron 5 | 0.61 | |
| GG | 94 (39.8%) | |||
| AG | 107 (45.3%) | |||
| AA | 35 (14.8%) | |||
| rs1360780 | 0.378 | 35715549 / Intron 2 | 0.85 | |
| CC | 105 (44.5%) | |||
| CT | 103 (43.6%) | |||
| TT | 28 (11.9%) | |||
| rs9470080 | 0.388 | 35754413 / Intron 1 | 0.28 | |
| CC | 92 (39.0%) | |||
| CT | 104 (44.1%) | |||
| TT | 40 (16.9%) | |||
| CATT haplotype | ||||
| No copies | 113 (47.9%) | |||
| 1–2 copies | 123 (52.1%) |
Note. MAF = minor allele frequency.
Figure 1.
Linkage Disequilibrium across FKBP5 SNPs and haplotype frequencies within the sample.
Each participant was then assigned a haplotype using PHASE version 2.1 software, which employs a Bayesian approach to designate haplotypes based upon population data (Stephens, Smith, & Donnelly, 2001; Stephens & Scheet, 2005). All samples were haplotyped with greater than 98% confidence, with the exception of one sample, which was subsequently excluded from analyses. Following haplotype assignment, individuals with one or more copies of the CATT haplotype were grouped together and compared to individuals with zero copies of this haplotype. This dichotomous variable was utilized in subsequent analyses to examine the joint effects of childhood maltreatment and FKBP5 haplotype variation on symptoms of limbic irritability.
Statistical Analyses
Path analyses were conducted within a Structural Equation Modeling framework using Mplus Version 6.11 statistical software (Muthén & Muthén, 1998–2011, see Figure 1). In the first path analysis, the indirect effects of maltreatment on symptoms of depression and dissociation via limbic irritability were modeled. The total score on the CTQ-SF was entered as a predictor of the LSCL-33 total score, the BDI-II total score, and the average DES symptom score. The BDI-II and DES were entered into the model together as symptom outcomes. Limbic irritability was modeled as an endogenous variable mediating the relationship between maltreatment and the two symptom outcomes. These associations were tested using the product coefficient method, with estimates derived from bootstrapping procedures using 5000 sample replicates (Bollen & Stine, 1990; MacKinnon, 2008; Shrout & Bolger, 2002). Race (black/not black), age, and total income were entered as covariates for all endogenous variables within the model for these and subsequent analyses.
Haplotype was then examined as a moderator of these indirect effects. The total score on the CTQ-SF, FKBP5 haplotype, and their product term were entered as predictors. Prior to creating a product term, scores on the CTQ-SF were first centered. Interactions were then computed. The total LSCL-33 score was entered as a mediating variable between CTQ-SF total score and symptom outcomes on the BDI-II and DES. Confidence intervals were bootstrapped using 10000 samples to account for non-normality. Model fit was determined using the root mean square error of approximation (RMSEA), χ2/df ratio, and the comparative fit index (CFI) as parameters. Adequate fitting models were those in which the RMSEA values were less or equal to 0.08, the χ2/df ratio values were between 1 and 3, and CFI values were greater or equal to 0.90. In the following analyses, the path analytic model was identified and was an excellent fit to the data, χ2 (236) = 204.821, p < 0.00001, RMSEA = 0.000, CFI = 1.00.
Results
Preliminary Results
Demographic characteristics of the sample are presented in Table 2. Correlations among the observed variables are listed in Table 3. Seventy percent of participants (n = 166) in this sample surpassed previously established thresholds for severity within at least one subtype of child maltreatment, whereas thirty percent (n =70) did not endorse experiencing significant maltreatment in childhood. Prior to running path analytic models, analyses of variance (ANOVAs) were conducted to examine whether level of limbic irritability, depressive, or dissociative symptoms differed significantly by haplotype. There were no significant effects of the CATT haplotype on symptoms of depression [F(1, 234) = 1.810, p>0.05)], dissociation [F(1, 234) = 2.600, p>0.05)], or limbic irritability [F(1, 234) = 1.999, p>0.05)]. The relationship between haplotype and total score on the CTQ-SF also was not significant [F(1, 234) = 0.001, p>0.05)], indicating that maltreatment experience did not vary significantly as a function of CATT haplotype.
Table 3.
Correlation Matrix for Observed Variables (N=236).
| Measure | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | Mean (SD) | Range |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. Emotional Abuse | 0.76** | 0.79** | 0.70** | 0.63** | 0.92** | 0.25** | 0.37** | 0.24** | .02 | 11.73 (6.34) | 5 – 25 | |
| 2. Emotional Neglect | 0.60** | 0.73** | 0.50** | 0.83** | 0.12 | 0.23** | 0.17* | −.05 | 11.94 (5.67) | 5 – 25 | ||
| 3. Physical Abuse | 0.67** | 0.64** | 0.87** | 0.19** | 0.34** | 0.21** | −.06 | 9.48(5.77) | 5 – 25 | |||
| 4. Physical Neglect | 0.55** | 0.83** | 0.20** | 0.27** | 0.21** | .02 | 8.49(3.86) | 5 – 25 | ||||
| 5. Sexual Abuse | 0.81** | 0.24** | 0.37** | 0.23** | .06 | 9.86(7.05) | 5 – 25 | |||||
| 6. CTQ total score | .24** | .38** | .25** | −.002 | 51.50 (24.41) | 25 – 125 | ||||||
| 7. LSCL-33 | .44** | .48** | .09 | 14.92 (11.99) | 0 – 64 | |||||||
| 8. BDI-II | .45** | .09 | 11.97 (9.97) | 0 – 45 | ||||||||
| 9. DES | .10 | 1.86(1.57) | 0 – 6.82 | |||||||||
| 10. FKBP5 haplotype |
p<0.05,
p<0.01.
CTQ total score = Childhood Trauma Questionnaire total score (25–125); LSCL-33 = Limbic System Checklist-33 total score, BDI = Beck Depression Inventory-II total score; DES = Dissociative Experiences Scale average score. FKBP5 haplotype coded as zero copies compared to 1–2 copies of the CATT haplotype.
Effect of Child Maltreatment on Depression and Dissociation via Limbic Irritability
In the first set of analyses, indirect effects were modeled to determine whether limbic irritability mediated the relationship between maltreatment and symptoms of depression and dissociation. Table 4 presents the path coefficients and confidence intervals for the direct (path a: from maltreatment to limbic irritability; path b: from limbic irritability to depressive or dissociative symptoms; path c: from maltreatment to depressive or dissociative symptoms) and indirect paths (a*b path: from maltreatment to depressive or dissociative symptoms via limbic irritability). In reference to depression, results indicate that higher self-reported level of maltreatment was associated with significantly greater symptoms of limbic irritability (path a; β = 0.240, p=0.001) and depression (path c; β = 0.285, p<0.001). Greater limbic irritability also was related to higher symptoms of depression (path b; β = 0.367, p<0.001). Further, there was a significant indirect effect of maltreatment on depressive symptoms occurring through limbic irritability (a*b path; β = 0.088, p<0.01; R2 = 0.289).
Table 4.
Standardized and Unstandardized Path Coefficients for Direct and Indirect Effects with Bootstrapped Confidence Intervals
| Direct Effects | B (β) | SE | 95% CI |
|---|---|---|---|
| Maltreatment to LSCL-33 | 0.012 (0.240) | 0.003 (0.066) | [0.005, 0.019]** |
| Maltreatment to BDI-II | 0.116 (0.285) | 0.031 (0.071) | [0.053, 0.177]*** |
| Maltreatment to DES | 0.010 (0.155) | 0.004 (0.061) | [0.002, 0.018]* |
| LSCL-33 to BDI-II | 3.055 (0.367) | 0.549 (0.065) | [2.010, 4.187]*** |
| LSCL-33 to DES | 0.575 (0.438) | 0.087 (0.060) | [0.416, 0.757]*** |
| Indirect Effects | |||
| Maltreatment to BDI via LSCL | 0.036 (0.088) | 0.012 (0.030) | [0.016, 0.065]** |
| Maltreatment to DES via LSCL | 0.007 (0.105) | 0.002 (0.033) | [0.003, 0.012]** |
p<0.05
p<0.01
p<0.001.
SE = standard error of path coefficients; CI = confidence interval of unstandardized path coefficients.
Comparable results were found with respect to dissociative symptoms (see Table 4). Higher dissociative symptoms also were associated with increased symptoms of limbic irritability (path b; β = 0.438, p<0.001), and greater symptoms of maltreatment (path c; β = 0.155, p<0.05). Additionally, the indirect effect of maltreatment on dissociative symptoms via limbic irritability was significant (a*b path; β = 0.105, p<0.01; R2 = 0.293). These results indicate significant indirect associations among maltreatment and depression as well as maltreatment and dissociation via limbic irritability.
Interactive Effects of FKBP5 and Child Maltreatment on Limbic Irritability
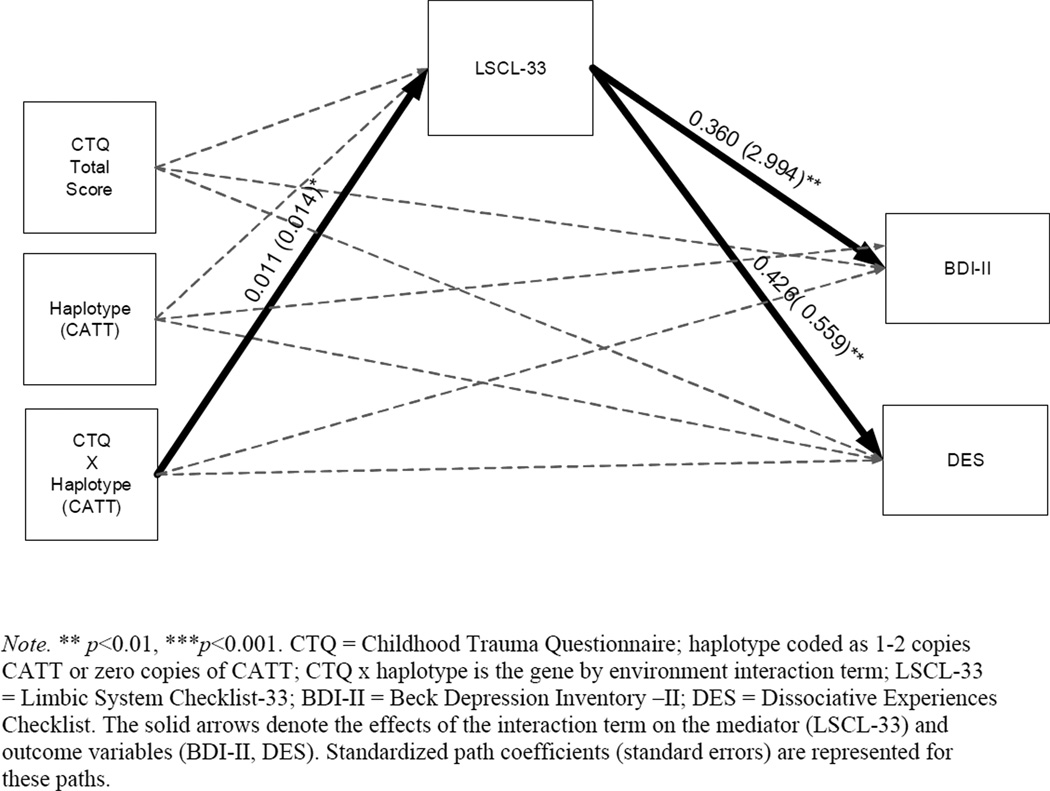
The second set of analyses examined the moderating effect of FKBP5 haplotype on the two established indirect effects. Specifically, we sought to examine whether variation within the CATT haplotype moderated the path from maltreatment to limbic irritability (path a) within the examined indirect effects model. Paths were modeled from maltreatment, haplotype, and their interaction to symptoms of limbic irritability, depression, and dissociation (see Figure 2). The direct effects within this model are presented in Table 5. Consistent with preliminary analyses, the main effect of haplotype was not significantly associated with limbic irritability (β = −0.405, p>0.05). After including haplotype and the interaction term in this model, the main effect of maltreatment on LSCL-33 (β = 0.004, p>0.05) was no longer significant. However, the interaction between maltreatment and CATT haplotype was significantly related to symptoms of limbic irritability (β = 0.354, p<0.05; Figure 2).
Figure 2.
Path analysis model containing direct and indirect effects.
Table 5.
Standardized and Unstandardized Path Coefficients for Direct Interaction and Conditional Indirect Effects
| Direct Interaction Effects | B (β) | SE | CI |
|---|---|---|---|
| Maltreatment to LSCL-33 | 0.005 (0.004) | 0.004 | [−0.002, 0.013] |
| Haplotype to LSCL-33 | −0.485 (−0.405) | 0.343 | [−1.162, 0.180] |
| GxE interaction to LSCL-33 | 0.014 (0.354) | 0.007 | [0.001, 0.027]* |
| Maltreatment to BDI-II | 0.109 (0.011) | 0.043 | [0.027, 0.195]* |
| Haplotype to BDI-II | 0.255 (0.026) | 2.924 | [−5.647, 5.838] |
| GxE interaction to BDI-II | 0.015 (0.001) | 0.062 | [−0.105, 0.138] |
| Maltreatment to DES | 0.004 (0.003) | 0.005 | [−0.006, 0.013] |
| Haplotype to DES | −0.534 (−0.340) | 0.439 | [−1.388, 0.322] |
| GxE interaction to DES | −0.012 (0.008) | 0.008 | [−0.003, 0.027] |
| Conditional Indirect Effects | B (β) | SE | CI |
| Maltreatment to BDI-II via LSCL-33 | |||
| 1 or 2 copies CATT haplotype | 0.056 (0.137) | 0.020 | [0.025, 0.105]** |
| No copies of CATT haplotype | 0.015 (0.037) | 0.012 | [−0.007, 0.042] |
| Difference | 0.041 (0.101) | 0.023 | [0.004, 0.095]* |
| Maltreatment to DES via LSCL | |||
| 1 or 2 copies CATT haplotype | 0.010 (0.132) | 0.004 | [0.005, 0.019]** |
| No copies of CATT haplotype | 0.003 (0.002) | 0.002 | [−0.001, 0.008] |
| Difference | 0.008 (0.004) | 0.004 | [0.001, 0.017]* |
p<0.05
p<0.01
p<0.001.
SE = standard error of unstandardized path coefficients; CI = confidence interval of unstandardized path coefficients; FKBP5 haplotype coded as zero or 1–2 copies of CATT.
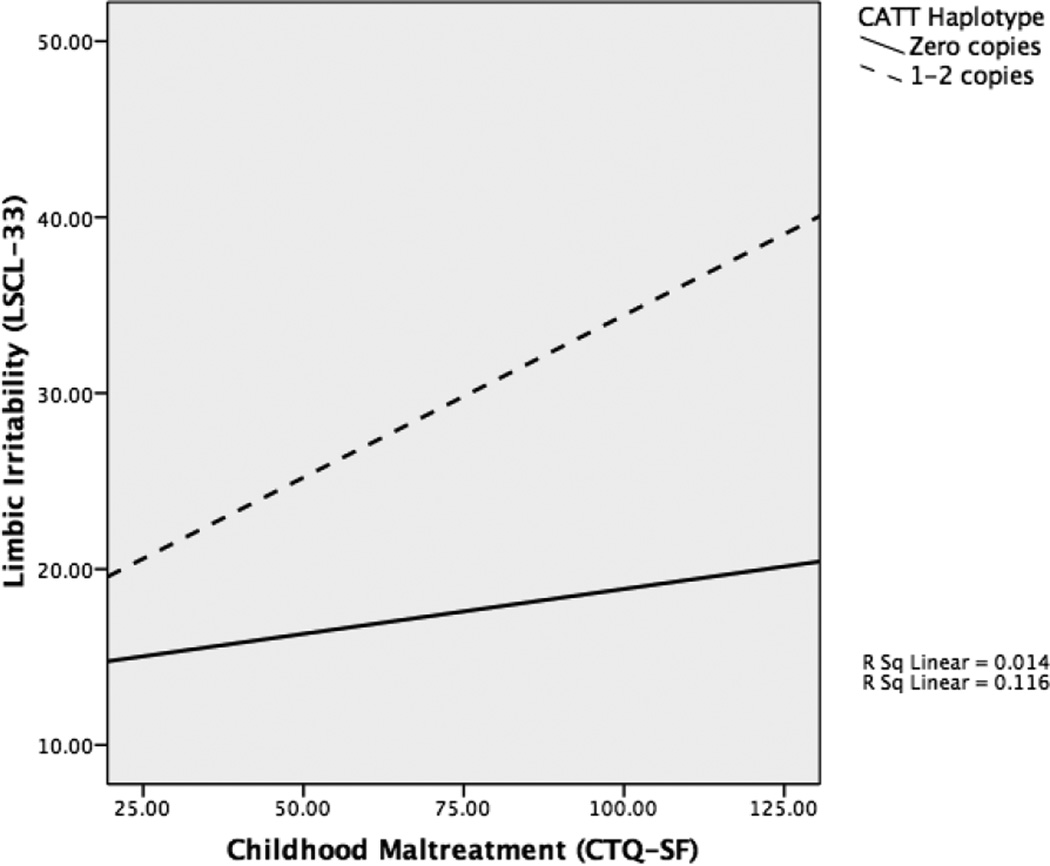
Simple slopes were conducted to clarify this interaction (see Figure 3). These analyses revealed a significant conditional indirect effect of CATT haplotype on the association between maltreatment, limbic irritability, and depression. The indirect path from maltreatment to depression through limbic irritability was significantly different for individuals with 1–2 copies of the CATT haplotype compared to individuals with no copies (β = 0.101, p<0.05, Table 5). Specifically, this mediational path was significant for individuals with 1–2 CATT copies (β = 0.137, p=0.005), whereas this path was not significant for individuals with zero CATT copies (β = 0.037, p>0.05). Therefore, variation within the FKBP5 haplotype moderated the indirect effect of maltreatment on depression via limbic irritability.
Figure 3.
Simple slopes analyses showing the interaction effect of haplotype and child maltreatment on limbic irritability.
Moderation by the CATT haplotype was also found with respect to the indirect effect of maltreatment on dissociative symptoms through limbic irritability. Limbic irritability mediated the association between child maltreatment and dissociation for individuals with 1–2 copies of the CATT haplotype (β = 0.132, p=0.004), but this pathway was not significant for those with zero copies of CATT (β= 0.002, p>0.05, see Table 5). The indirect relationship between maltreatment and dissociation via limbic irritability was significantly different for individuals with one or more copies of CATT compared to individuals without any copies of CATT (β = 0.004, p<0.05). These results indicate that FKBP5 haplotype also moderated the indirect effect of maltreatment on dissociation through limbic irritability.
There were no significant direct effects of the haplotype by maltreatment interaction term on either depressive or dissociative symptoms within this model (β = 0.001, p >0.05; β = 0.008, p >0.05, see Table 5). Therefore, the conditional indirect effect of haplotype was observed specifically for the path between maltreatment and limbic system functioning (path a). Overall, these results indicate a significant conditional indirect path of maltreatment to depression and dissociation via limbic irritability that occurs only in individuals with one or more copies of the CATT haplotype.
Discussion
In the present study, limbic irritability symptoms mediated associations between childhood history of maltreatment and symptoms of depression and dissociation in adulthood. In addition to delineating the role of limbic irritability, we examined the impact of FKBP5 on these significant indirect effects. Consistent with prior research, the main effect of haplotype on symptom outcomes was not significant (Binder et al., 2008; Roy et al., 2010; Xie et al., 2010; Zimmerman, et al., 2011). Analyses revealed a signification interaction between FKBP5 haplotype and exposure to child maltreatment on limbic irritability symptoms. Further, FKBP5 moderated the indirect effects between maltreatment and symptoms of depression and dissociation, as these associations were significant only for women who possessed one or more copies of the CATT haplotype. Although past research suggests that maltreatment is strongly related to greater symptoms of limbic irritability (Anderson et al., 2002; Choi et al., 2009; Teicher et al., 2006; Teicher, et al., 2010), the indirect relationship between these variables was not significant for women with zero copies of the CATT haplotype. Our results are the first to formally examine limbic irritability as an explanatory factor in the relationship between maltreatment and symptom outcomes, and to find differential effects within these associations based upon variation across FKBP5 SNPs.
The results of this investigation are consistent with a number of studies examining gene by environment interactions involving childhood trauma, FKBP5, and adverse outcomes in adulthood. Minor alleles within FKBP5 have been associated with increased symptoms of anxiety and depression following prolonged stress exposure in cancer patents, greater depressive symptoms in individuals who experienced physical abuse, and increased incidence of major depressive disorder over a ten-year period in individuals exposed to childhood trauma (Appel et al., 2011; Kang, Chung, Jeung, Kim, & An, et al., 2012; Zimmerman et al., 2011). In addition, dose-dependent relationships have been revealed with respect to PTSD symptoms, with exposure to greater levels of maltreatment associated with increased symptoms based upon genotype across rs3800373, rs9296158, rs1360870, and rs9470080 (Binder et al., 2008). In particular, among individuals who were exposed to two or more forms of maltreatment, PTSD symptoms were greatest for minor allele homozygotes, followed by minor allele heterozygotes and major allele homozygotes, respectively. Minor allele status across these SNPs has also predicted heightened PTSD severity in an adult sample of 9/11 survivors, and peritraumatic dissociation in children following hospital admission (Koenen et al., 2005; Sarapas et al., 2011).
Few studies to date have examined FKBP5 SNPs using haplotype analyses. Significant main effects have been found for haplotypes containing the minor alleles of rs3800373 and rs1360870 in predicting completed suicide and unipolar depression (Suprianto et al., 2010; Zobel et al., 2010). However, only two investigations have found significant gene by environment interactions using a haplotype comprised of the four FKBP5 SNPs examined in the current study. In a recent investigation, the presence of two CATT haplotype copies predicted aggressive and violent behavior in prison inmates exposed to childhood trauma (Bevilacqua et al., 2012). These findings are consistent with the results of the present investigation, albeit elucidating risk for externalizing rather than internalizing symptomatology. In addition to these findings, Roy et al. (2010) examined interactions between childhood trauma and the yin-ying GACC and CATT haplotypes in predicting suicide attempts in a sample of primarily substance dependent African American individuals. Analyses revealed that the GACC haplotype, which contained the major alleles across all four FKBP5 SNPs, conferred the greatest risk for suicide (Roy et al., 2010). The inconsistency of these results with past research and findings within the present investigation may be due to differences in the sample. The authors state that the level of substance dependency within this sample was severe, and may have contributed to HPA vulnerabilities opposite those associated with PTSD (Roy et al., 2010).
Molecular evidence suggests that traumatic experience and FKBP5 genotype influence depressive and post-traumatic symptoms in different ways. For depressed individuals exposed to trauma, minor alleles predict heightened FKBP5 mRNA expression in lymphocytes, FKBP5 induction by cortisol, and blunted cortisol suppression to dexamethasone (Binder et al., 2004; Binder, 2009). These impairments reflect GR hypersensitivity, which is indicated by underactive negative feedback of cortisol and excessive cortisol release. Alternatively, individuals with PTSD who experienced trauma exhibit opposite impairments, with minor alleles associated with lower FKBP5 expression and induction by cortisol, enhanced negative feedback, and decreased plasma cortisol (Sarapas et al., 2011; Yehuda, Golier, Yang, & Tischler, 2004). The impact of substance dependence on GR functioning may mimic patterns similar to those seen in depression, particularly blunted responsivity of the HPA axis to stress (Roy et al., 2010). However, further research is necessary to elucidate the relationships among childhood traumatic experiences, FKBP5 genotype, and substance dependency to understand these interactions. These results highlight the need for continued investigation into the differential effects of chronic stress and genotype on psychiatric functioning, while accounting for physiological alterations due to existing psychopathology and comorbidities.
Our findings revealed a potential protective effect for individuals with zero copies of the CATT haplotype. These individuals did not exhibit a significant association between maltreatment experience and symptoms of limbic irritability. Binder et al. (2008) also found protective effects of the major alleles within rs3800373, rs9296158, rs1360870, and rs9470080. Individuals who experienced severe maltreatment and were homozygotes for the major alleles across these SNPs exhibited PTSD symptoms comparable to nonmaltreated individuals. Preclinical research has identified physiological changes that may underlie these protective effects. Under stressful conditions, FKBP5 knock-out mice exhibit decreased HPA reactivity and changes in GR expression, which result in enhanced coping behavior (Touma et al., 2011).
Evidence suggests that there may be differential protective effects for both minor and major alleles depending on stress exposure. In one investigation, the minor homozygous genotype within rs9470080 conferred the lowest risk for PTSD symptoms in individuals with no exposure to childhood trauma, but was associated with the highest risk for those who did experience childhood adversity (Xie et al., 2010). Similarly, rs3800373 minor allele homozygotes with trauma exposure exhibited greater risk for developing a major depressive episode, whereas this genotype was protective for individuals who did not experience trauma (Zimmerman et al., 2011). It may be that the minor alleles are adaptive in allowing for a hyperresponsive and efficient HPA axis in the context of mild stress, but pose significant consequences for dysfunction when stress is chronic or occurs early in development.
Research has consistently identified emotional memory, processing, and regulation as areas of functioning that are adversely affected by child maltreatment (Kim & Cicchetti, 2010; Kim-Spoon, Rogosch, & Cicchetti, in press; Shields & Cicchetti, 1997, 1998, 2001; Shields, Ryan, & Cicchetti, 2001; Teicher et al., 2002). Several lines of research have revealed that children and adults with maltreatment histories display exaggerated defensive responding and hypervigilance to threat-related emotional stimuli (Cicchetti & Curtis, 2005; Ito et al., 1993, 1998; Jovanovic et al., 2009; Orr et al., 1998; Pollack, Klorman, Thatcher, & Cicchetti, 2001). These findings may highlight a tendency toward negative emotional processing and attentional biases (Dannlowski et al., 2007). Most recently, investigators using functional imaging have found that adults who experienced childhood maltreatment exhibited decreased hippocampal volume and hyperresponsive amygdala activation to angry and fearful facial expressions compared to individuals without maltreatment histories (Dannlowski et al., 2012). McCrory and colleagues (2011) also found that non-symptomatic children with documented histories of family violence exposure exhibited greater activation within the right amygdala and anterior insula in response to viewing angry versus neutral faces compared to children who were not exposed to violence.
Therefore, research suggests that child maltreatment may lead to lasting structural and functional consequences within neurobiological systems that are crucial for emotional functioning. Abnormal activation patterns within limbic structures following maltreatment are consistent with those seen in Major Depressive Disorder (MDD) and PTSD, again suggesting a potential mediational role of limbic system impairments in the relationship between child maltreatment and psychiatric illness (Etkin & Wagner; 2007; Dannlowski et al., 2007; Dannlowski et al., 2012). Limbic system impairments may be present in childhood, persist into adulthood, and serve as an indication of risk for developing depressive and/or posttraumatic symptoms (Gibb, Uhrlass, Grassia, Benas, & McGeary, 2010). Research examining genetic moderation of emotional processing in stress-exposed populations has also revealed differences as a function of genotype, highlighting the importance of examining these associations from multiple levels of analyses (Bradley et al., 2011; Johnson, Gibb, & McGeary, 2010). The results within the present investigation provide a bridge between molecular and functional studies by showing a means by which maltreatment may affect stress-sensitivity and brain development through its impact on limbic circuits.
This study has several strengths. The use of a haplotype enabled us to examine interactions across four FKBP5 SNPs that were in high linkage disequilibrium. We utilized rigorous statistical methods by which to model the effect of haplotype on symptom outcomes, and formally tested for each conditional indirect effect using bootstrapping procedures. In addition, we used a non-invasive self-report checklist to measure limbic irritability. Although functional imaging research is useful in understanding the impact of maltreatment on the brain, the LSCL-33 provides a reliable and valid means by which to measure these effects in adults (Teicher et al., 1993). Our results show that this measure may be useful within community clinics and primary care facilities to assess for potential limbic system abnormalities and risk for psychopathology. A significant strength of this study was that we used a high-risk, underserved sample of women in which to examine these interactions. Over 65% of participants in this investigation were of racial/ethnic minority status and all were low-income. Depressive and dissociative outcomes are highly prevalent in women, particularly for individuals who experienced child maltreatment. Given that 70% of the women in our sample reported significant experiences of child maltreatment, these results underscore the importance of investigating these associations in a female sample (Cicchetti & Valentino, 2006; Kessler, Chiu, Demler, & Walters, 2005).
Half of the women in this investigation were involved with child protective services with their own children. Intergenerational transmission of maltreatment is associated with poverty, unemployment, and mental illness, and poses serious public health concerns (Cicchetti & Valentino, 2006; Dixon, Browne, & Hamilton-Giachritsis, 2005). Therefore, understanding the impact of maltreatment and genotype on limbic irritability in this sample may clarify means by which to identify individuals who are at-risk for perpetuating this cycle. Mothers who exhibit elevated symptoms of limbic irritability may benefit from services that particularly address difficulties in emotion regulation (Kim & Cicchetti, 2010; Kim-Spoon et al., in press; Shields & Cicchetti, 1997; 1998; 2001). Further, research has indicated that the minor allele genotypes within FKBP5 SNPs are associated with greater response to antidepressant medication and increased remission of depression (Binder et al., 2004; Horstmann et al., 2010; Lekman, Laje, Charney, Rush, & Wilson, 2008). Therefore, these women may respond differentially to pharmacological treatment. Continued work in this area is crucial for understanding ways in which to intervene for individuals who experienced childhood trauma and evidence symptoms of limbic irritability.
This investigation was limited by the use of a cross-sectional design, mono-informant measurement strategy, and retrospective assessment to measure child maltreatment. The CTQ-SF assesses for perceptions of maltreatment that occurred prior to age 18. Therefore, we did not take into account any other traumatic events during late adolescence or adulthood that may have impacted limbic system functioning. We attempted to control for any covariation among self-reports by removing seven LSCL-33 items from analyses that overlapped in content with those on the DES. However, additional investigations using longitudinal designs and better controls for other environmental stressors are crucial to decrease possible respondent bias and elucidate the effects of different types of traumatic experiences. Longitudinal studies also could reveal the developmental stage at which limbic irritability symptoms emerge, as findings have been inconsistent regarding whether impairments are detectable in childhood (Bremner et al., 1997; DeBellis et al., 1999; Stein, 1997). The consensus is that these symptoms appear after the age of 18 due to delayed maturation within limbic system regions (Andersen et al., 2009; Teicher et al., 2002). However, evidence of EEG and fMRI abnormalities in childhood following maltreatment support the potential for earlier detection (Ito et al., 1993; Ito et al., 1998; McCrory et al., 2011; Teicher et al., 1997; Teicher et al., 2004). Though robust mediational associations and interactive effects were found in the present study, analyses were limited by the sample size. It is also unclear whether gender could play a role in these associations, as the present sample was exclusively female. Additionally, interactions between child maltreatment and FKBP5 may differ in a clinical population, in which symptoms are more severe. However, the utilization of a less symptomatic community sample is helpful in disentangling neurobiological risk factors from impairments resulting from the disorder itself.
This is the first study to date examining the differential impact of maltreatment on limbic irritability with respect to FKBP5 haplotype. These findings emphasize the effects of chronic stress, haplotypic risk, and subsequent GR inefficiency on limbic system circuits. Further, they suggest that dysregulation at the GR level may have lasting effects within temporal-limbic regions, lead to bizarre sensory, somatic, and behavioral symptoms of limbic irritability, and increase risk for development of depressive and dissociative symptoms. Understanding these associations has significant implications given that symptoms of limbic irritability are believed to be manifestations of the effects of early stress on the developing brain. These findings highlight the potential for both preventative and therapeutic effort at the GR level for women who experienced trauma during childhood.
Acknowledgments
This research was supported by funding from the National Institute of Mental Health (MH083979) and the Spunk Fund, Inc.
References
- Amico F, Meisenzahl E, Koutsouleris N, Reiser M, Moller HJ, Frodl T. Structural MRI correlates for vulnerability and resilience to major depressive disorder. Journal of Psychiatry and Neuroscience. 2011;36:15–22. doi: 10.1503/jpn.090186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen SL, Teicher MH. Delayed effects of early stress on hippocampal development. Neuropsychopharmacology. 2004;29:1988–1993. doi: 10.1038/sj.npp.1300528. [DOI] [PubMed] [Google Scholar]
- Andersen SL, Tomada A, Vincow ES, Valente E, Polcari A, Teicher MH. Preliminary evidence for sensitive periods in the effect of childhood sexual abuse on regional brain development. Journal of Neuropsychiatry and Clinical Neuroscience. 2008;20(3):292–301. doi: 10.1176/appi.neuropsych.20.3.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson CM, Teicher MH, Polcari A, Renshaw PF. Abnormal T2 relaxation time in the cerebellar vermis of adults sexually abused in childhood: potential role of the vermis in stress-enhanced risk for drug abuse. Psychoneuroendocrinology. 2002;27:231–244. doi: 10.1016/s0306-4530(01)00047-6. [DOI] [PubMed] [Google Scholar]
- Appel K, Schwahn C, Mahler J, Schulz A, Spitzer C, Fenske K, et al. Moderation of adult depression by a polymorphism in the FKBP5 gene and childhood physical abuse in the general population. Neuropsychopharmacology. 2011;36:1982–1991. doi: 10.1038/npp.2011.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aslund C, Leppert J, Comasco E, Nordquist N, Oreland L, Nilsson KW. Impact of the interaction between the 5HTTLPR polymorphism and maltreatment on adolescent depression. A population-based study. Behavioral Genetics. 2009;39(5):5240531. doi: 10.1007/s10519-009-9285-9. [DOI] [PubMed] [Google Scholar]
- Barrett JC, Fry B, Maller J, Daly MJ. Haploview analysis and visualization of LD and haplotype maps. Bioinformatics. 2005;21:263–265. doi: 10.1093/bioinformatics/bth457. [DOI] [PubMed] [Google Scholar]
- Beck AT, Steer RA, Brown GK. Manual for the Beck Depression Inventory – II. San Antonio, TX: Psychological Corporation; 1996. [Google Scholar]
- Bernstein DP, Fink L. Childhood trauma questionnaire: A retrospective self-report manual. New York: The Psychological Corporation; 1994. [Google Scholar]
- Bernstein DP, Putnam FW. Development, reliability, and validity of a dissociation scale. Journal of Nervous and Mental Disease. 1986;174(12):727–735. doi: 10.1097/00005053-198612000-00004. [DOI] [PubMed] [Google Scholar]
- Bernstein DP, Stein JA, Newcomb MD, Walker E, Pogge D, Ahluvalia T, et al. Development and validation of a brief screening version of the Childhood Trauma Questionnaire. Child Abuse & Neglect. 2003;27(2):169–90. doi: 10.1016/s0145-2134(02)00541-0. [DOI] [PubMed] [Google Scholar]
- Binder EB. The role of FKBP5, a co-chaperone of the glucocorticoid receptor in the pathogenesis and therapy of affective and anxiety disorders. Psychoneuroendocrinology. 2009;34(1):S186–S195. doi: 10.1016/j.psyneuen.2009.05.021. [DOI] [PubMed] [Google Scholar]
- Binder EB, Bradley RC, Liu W, Epstein MP, Deveau TC, Mercer KB, et al. Association of FKBP5 polymorphisms and childhood abuse with risk of posttraumatic stress disorder symptoms in adults. JAMA. 2008;299(11):1291–1305. doi: 10.1001/jama.299.11.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder EB, Salyakina D, Lichtner P, Wochnik GM, Ising M, Putz B, et al. Polymorphisms in FKBP5 are associated with increased recurrence of depressive episodes and rapid response to antidepressant treatment. Nature Genetics. 2004;36(12):1319–1325. doi: 10.1038/ng1479. [DOI] [PubMed] [Google Scholar]
- Bollen KA, Stine R. Direct and indirect effects: classical and bootstrap estimates of variability. Sociological Methodology. 1990;20:115–140. [Google Scholar]
- Bradley B, Westen D, Mercer KB, Binder EB, Jovanovic T, Crain D, et al. Association between childhood maltreatment and adult emotional dysregulation in a low-income, urban, African American sample: moderation by oxytocin receptor gene. Development and Psychopathology. 2011;23(2):439–452. doi: 10.1017/S0954579411000162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremner JD, Licinio J, Darnell A, Krystal JH, Owens MJ, et al. Elevated CSF corticotropin-releasing factor concentrations in posttraumatic stress disorder. The American Journal of Psychiatry. 1997;154:624–629. doi: 10.1176/ajp.154.5.624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briere J. Child abuse trauma: theory and treatment of the lasting effects. Newbury Park: CA: SAGE; 1992. [Google Scholar]
- Caldji C, Francis D, Sharma S, Plotsky PM, Meaney MJ. The effects of early rearing environment on the development of GABAA and central benzodiazepine receptor levels and novelty-induced fearfulness in the rat. Neuropsychopharmacology. 2000;22:219–229. doi: 10.1016/S0893-133X(99)00110-4. [DOI] [PubMed] [Google Scholar]
- Caldji C, Tannenbaum B, Sharma S, Francis D, Plotsky PM, Meaney MJ. Maternal care during infancy regulates the development of neural systems mediating the expression of fearfulness in the rat. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:5335–5340. doi: 10.1073/pnas.95.9.5335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson EB, Putnam FW. An update on the dissociative experiences scale. Dissociation. 1993;6(1):16–27. [Google Scholar]
- Caspi A, McClay J, Moffitt TE, Martin J, Craig IW, Taylor A. Role of genotype in the cycle of violence in maltreated children. Science. 2002;297(5582):851–854. doi: 10.1126/science.1072290. 2. [DOI] [PubMed] [Google Scholar]
- Caspi A, Sugden K, Moffitt TE, Taylor A, Craig IW, Harrington H, et al. Influence of life stress on depression: moderation by a polymorphism in the 5-HT gene. Science. 2003;301:386–389. doi: 10.1126/science.1083968. [DOI] [PubMed] [Google Scholar]
- Chen MC, Hamilton JP, Gotlib IH. Decreased hippocampal volume in healthy girls at risk for depression. Archives of General Psychiatry. 2010;67:270–276. doi: 10.1001/archgenpsychiatry.2009.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi J, Jeong B, Polcari A, Rohan ML, Teicher MH. Reduced fractional anisotrophy in the visual limbic pathway of young adults witnessing domestic violence in childhood. Neuroimage. 2012;59(2):1071–1079. doi: 10.1016/j.neuroimage.2011.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi J, Jeong B, Rohan ML, Polcari AM, Teicher MH. Preliminary evidence for white matter tract abnormalities in young adults exposed to parental verbal abuse. Biological Psychiatry. 2009;65:227–234. doi: 10.1016/j.biopsych.2008.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cicchetti D. How research on child maltreatment has informed the study of child development: Perspectives from developmental psychopathology. In: Cicchetti D, Carlson V, editors. Child maltreatment: Theory and research on the causes and consequences of child abuse and neglect. New York: Cambridge University Press; 1989. pp. 377–431. [Google Scholar]
- Cicchetti D. Resilience under conditions of extreme stress: A multilevel perspective. World Psychiatry. 2010;9:145–154. doi: 10.1002/j.2051-5545.2010.tb00297.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cicchetti D, Curtis WJ. An event-related potential study of the processing of affective facial expressions in young children who experienced maltreatment during the first year of life. Development and Psychopathology. 2005;17(3):641–677. doi: 10.1017/S0954579405050315. [DOI] [PubMed] [Google Scholar]
- Cicchetti D, Lynch M. Failures in the expectable environment and their impact on individual development: The case of child maltreatment. In: Cicchetti D, Cohen DJ, editors. Developmental psychopathology: Vol. 2.Risk, disorder and adaptation. Vol. 2. New York: Wiley; 1995. pp. 32–71. [Google Scholar]
- Cicchetti D, Rogosch FA, Oshri A. Interactive effects of corticotropin releasing hormone receptor 1, serortonin transporter linked polymorphic region, and child maltreatment on diurnal cortisol regulation and internalizing symptomatology. Development and Psychopathology. 2011;23(4):1125–1138. doi: 10.1017/S0954579411000599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cicchetti D, Rogosch FA, Sturge-Apple ML. Interaction of child maltreatment and 5-HT polymorphisms: suicidal ideation among children from low-SES backgrounds. Journal of Pediatric Psychology. 2010;35(5):536–546. doi: 10.1093/jpepsy/jsp078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cicchetti D, Rogosch FA, Thibodeau EL. The effects of child maltreatment on early signs of antisocial behavior: Genetic moderation by Tryptophan Hydroxylase, Serotonin Transporter, and Monoamine Oxidase-A-Genes. Development and Psychopathology. 2012;24(3) doi: 10.1017/S0954579412000442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cicchetti D, Rogosch FA, Toth SL. Ontonogenesis, depressotypic organization, and the depressive spectrum. In: Luthar SS, Burack JA, Cicchetti D, Weisz JR, editors. Developmental Psychopathology: Perspective on adjustment, risk, and disorder. Cambridge, U.K: Cambridge University Press; 1997. pp. 273–349. [Google Scholar]
- Cicchetti D, Toth SL. Child maltreatment. Annual Review of Clinical Psychology. 2005;1:409–438. doi: 10.1146/annurev.clinpsy.1.102803.144029. [DOI] [PubMed] [Google Scholar]
- Cicchetti D, Toth SL. Developmental psychopathology and disorders of affect. In: Cicchetti D, Cohen DJ, editors. Developmental psychopathology: Risk, disorder, and adaptation. Vol. 2. New York: Wiley; 1995. pp. 369–420. [Google Scholar]
- Cicchetti D, Toth SL. The development of depression in children and adolescents. American Psychologist. 1998;53(2):221–241. doi: 10.1037//0003-066x.53.2.221. [DOI] [PubMed] [Google Scholar]
- Cicchetti D, Toth SL. The past achievements and future promises of developmental psychopathology: The coming of age of a discipline. Journal of Child Psychology and Psychiatry. 2009;50:16–25. doi: 10.1111/j.1469-7610.2008.01979.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cicchetti D, Toth S, Manly JT. Maternal Maltreatment Interview. Unpublished manuscript: 2003. [Google Scholar]
- Cicchetti D, Tucker D. Development and self-regulatory structures of the mind. Development and Psychopathology. 1994;6:533–549. [Google Scholar]
- Cicchetti D, Valentino K. An ecological-transactional perspective on child maltreatment: failure of the average expectable environment and its influences on child development. In: Cicchetti D, Cohen D, editors. Development and Psychopathology: Vol. 3. Risk, disorder, and adaptation. 2nd ed. New York: Wiley; 2006. pp. 129–201. [Google Scholar]
- Cicchetti D, Valentino K. Toward the application of a multiplelevels-of-analysis perspective to research in development and psychopathology. In: Masten AS, editor. Minnesota Symposia on Child Psychology: Vol. 34. Multilevel dynamics in developmental psychopathology. Mahwah, NJ: Erlbaum; 2007. pp. 243–284. [Google Scholar]
- Cohen P, Brown J, Smaile E. Child abuse and neglect and development of mental disorders in the general population. Development and Psychopatholog. 2001;13:981–999. [PubMed] [Google Scholar]
- Collishaw S, Pickles A, Messer J, Rutter M, Shearer C, Maughan B. Resilience to adult psychopathology following childhood maltreatment: Evidence from a community sample. Child Abuse & Neglect. 2007;31:211–229. doi: 10.1016/j.chiabu.2007.02.004. [DOI] [PubMed] [Google Scholar]
- Crews F, He J, Hodge C. Adolescent cortical development: a critical period of vulnerability for addiction. Pharmacology Biochemistry and Behavior. 2007;86:189–199. doi: 10.1016/j.pbb.2006.12.001. [DOI] [PubMed] [Google Scholar]
- Dannlowski U, Ohrmann P, Bauer J, Kugel H, Arolt V, Heindel W, et al. Amygdala reactivity to masked negative faces is associated with automatic judgmental bias in major depression: A 3T fMRI study. Journal of Psychiatry and Neuroscience. 2007;32:423–439. [PMC free article] [PubMed] [Google Scholar]
- Dannlowski U, Stuhrmann A, Beutelmann V, Zwanzger P, Lenzen T, Grotegerd D, et al. Limbic scars: Long-term consequences of childhood maltreatment revealed by functional and structural magnetic resonance imaging. Biological Psychiatry. 2012;71:286–293. doi: 10.1016/j.biopsych.2011.10.021. [DOI] [PubMed] [Google Scholar]
- Davies TH, Ning YM, Sanchez ER. A new first step in activation of steroid receptors: hormone induced switching of FKBP51 and FKBP52 immunophilins. Journal of Biological Chemistry. 2002;277(7):4597–4600. doi: 10.1074/jbc.C100531200. [DOI] [PubMed] [Google Scholar]
- DeBellis MD, Keshavan MS, Clark DB, Case BJ, Giedd JN, Boring AM, et al. Developmental traumatology. Part II: brain development. Biological Psychiatry. 1999;45(10):1271–1284. doi: 10.1016/s0006-3223(99)00045-1. [DOI] [PubMed] [Google Scholar]
- Denny WB, Valentine DL, Reynolds PD, Smith DF, Scammel JG. Squirrel monkey immunophilin FKBP51 is a potent inhibitor of glucocorticoid receptor binding. Endocrinology. 2000;141(11):4107–4113. doi: 10.1210/endo.141.11.7785. [DOI] [PubMed] [Google Scholar]
- Dixon L, Browne K, Hamilton-Giachritsis C. Risk factors of parents abused as children: A mediational analysis of the intergenerational continuity of child maltreatment (Part 1) Journal of Child Psychology and Psychiatry. 2005;46(1):47–57. doi: 10.1111/j.1469-7610.2004.00339.x. [DOI] [PubMed] [Google Scholar]
- Edwards V, Holden G, Felitti V, Anda R. Relationship between multiple forms of childhood maltreatment and adult mental health in community respondents: Results from the Adverse Childhood Experiences study. American Journal of Psychiatry. 2003;160:1453–1460. doi: 10.1176/appi.ajp.160.8.1453. [DOI] [PubMed] [Google Scholar]
- Etkin A, Wagner TD. Functional neuroimagining of anxiety: a meta-analysis of emotional processing in PTSD, social anxiety disorder, and specific phobia. American Journal of Psychiatry. 2007;164(1):1476–1488. doi: 10.1176/appi.ajp.2007.07030504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans GW. A multimethodological analysis of cumulative risk and allostatic load among rural children. Developmental Psychology. 2003;39:924–933. doi: 10.1037/0012-1649.39.5.924. [DOI] [PubMed] [Google Scholar]
- Fitzgerald PB, Laird AR, Maller J, Daskalakis ZJ. A meta-analytic study of changes in brain activation in depression. Human Brain Mapping. 2008;29:683–695. doi: 10.1002/hbm.20426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frodl T, Reinhold E, Koutsouleris N, Donohoe G, Bondy B, Reiser M, et al. Childhood stress, serotonin transporter gene and brain structures in major depression. Neuropsychopharmacology. 2010;35:1383–1390. doi: 10.1038/npp.2010.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibb BE, Uhrlass DJ, Grassia M, Benas JS, McGeary J. Children’s inferential styles, 5-HTTLPR genotype, and maternal expressed emotion-criticism: an integrated model for the intergenerational transmission of depression. Journal of Abnormal Psychology. 2010;118(4):734–745. doi: 10.1037/a0016765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbertson MW, Shenton ME, Ciszewski A, Kasai K, Lasko NB, Orr SP, et al. Smaller hippocampal volume predicts pathologic vulnerability to psychological trauma. Nature Neuroscience. 2002;5:1242–1247. doi: 10.1038/nn958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillespie CF, Phifer J, Bradley B, Ressler KJ. Risk and resilience: genetic and environmental influences on development of the stress response. Depression and Anxiety. 2009;26(11):984–992. doi: 10.1002/da.20605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gogtay N, Nugent TF, Herman DH, Ordonez A, Greenstein D, Hayashi KM, et al. Dynamic mapping of normal human hippocampal development. Hippocampus. 2006;16:664–672. doi: 10.1002/hipo.20193. [DOI] [PubMed] [Google Scholar]
- Green A, Voeller K, Gaines R, Kulsie J. Neurological impairment in maltreated children. Child Abuse & Neglect. 1981;5:129–134. [Google Scholar]
- Green JG, McLaughlin KA, Berglund PA, Gruber MJ, Sampson NA, Zaslavsky AM, et al. Childhood adversities and adult psychiatric disorders in the national comorbidity survey replication 1: Associations with first onset of DSM-IV disorders. Archives of General Psychiatry. 2010;67(2):113–123. doi: 10.1001/archgenpsychiatry.2009.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heim C, Nemeroff CB. The role of childhood trauma in the neurobiology of mood and anxiety disorders: preclinical and clinical studies. Biological Psychiatry. 2001;49:1023–1039. doi: 10.1016/s0006-3223(01)01157-x. [DOI] [PubMed] [Google Scholar]
- Heim C, Newport DJ, Bonsall R, Miller AH, Nemeroff CB. Altered pituitary-adrenal axis responses to provocative challenge tests in adult survivors of childhood abuse. American Journal of Psychiatry. 2001;158:575–581. doi: 10.1176/appi.ajp.158.4.575. [DOI] [PubMed] [Google Scholar]
- Heim C, Newport DJ, Heit S, Graham YP, Wilcox M, Bonsall R, et al. Pituitary-adrenal and autonomic responses to stress in women after sexual and physical abuse in childhood. JAMA. 2000;284(5):592–597. doi: 10.1001/jama.284.5.592. [DOI] [PubMed] [Google Scholar]
- Horstmann S, Lucae S, Menke A, Hennings JM, Ising M, Roeske D, et al. Polymorphisms in GRIK4, HTA21, and FKBP5 Show Interactive effects in predicting remission to antidepressant treatment. Neuropsychopharmacology. 2010;35:727–740. doi: 10.1038/npp.2009.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyman SM, Garcia M, Kemp K, Mazure CM, Sinha R. A gender specific psychometric analysis of the early trauma inventory short form in cocaine dependent adults. Addictive Behavior. 2005;30(4):847–852. doi: 10.1016/j.addbeh.2004.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ising M, Depping AM, Siebertz A, Lucae S, Unschuld PG, Kloiber, et al. Polymorphisms in the FKBP5 gene region modulate recovery from psychosocial stress in healthy controls. European Journal of Neuroscience. 2008;28:389–398. doi: 10.1111/j.1460-9568.2008.06332.x. [DOI] [PubMed] [Google Scholar]
- Ito Y, Teicher MH, Glod CA, Harper D, Magnus E, Gelbard HA. Increased prevalence of electrophysiological abnormalities in children with psychological, physical, and sexual abuse. Neuropsychiatric Clinical Neuroscience. 1993;5:401–408. doi: 10.1176/jnp.5.4.401. [DOI] [PubMed] [Google Scholar]
- Ito Y, Teicher MH, Glod CA, Ackerman E. Preliminary evidence for aberrant cortical development in abused children: a quantitative EEG study. Neuropsychiatric Clinical Neuroscience. 1998;10:298–307. doi: 10.1176/jnp.10.3.298. [DOI] [PubMed] [Google Scholar]
- Johnson AL, Gibb BE, McGeary J. Reports of childhood physical abuse, 5-HTTLPR genotype, and women’s attentional biases for angry faces. Cognitive Therapy Research. 2010;34:380–387. doi: 10.1007/s10608-009-9269-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jovanovic T, Blanding NQ, Norrholm SD, Duncan E, Bradley B, & Ressler KJ. Childhood abuse is associated with increased startle reactivity in adulthood. Depression and Anxiety. 2009;26(110):1018–1026. doi: 10.1002/da.20599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang JI, Chung HC, Jeung HC, Kim SJ, An SK. FKBP5 polymorphisms as vulnerability to anxiety and depression in patients with advanced gastric cancer: A controlled and prospective study. Psychoneuroendocrinology. doi: 10.1016/j.psyneuen.2012.02.017. In press. [DOI] [PubMed] [Google Scholar]
- Karg K, Burmeister M, Shedden K, Sen S. The serotonin transporter promoter variant (5-HTTLPR), stress, and depression meta-analysis revisited: evidence of genetic moderation. Archives of General Psychiatry. 2011;68(5):444–454. doi: 10.1001/archgenpsychiatry.2010.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendler KS, Kuhn JW, Prescott CA. Childhood sexual abuse, stressful life events, and risk for major depression in women. Psychological Medicine. 2004;34:1475–1482. doi: 10.1017/s003329170400265x. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Chiu WT, Demler O, Walters EE. Prevalence, Severity, and Comorbidity of Twelve-month DSM-IV Disorders in the National Comorbidity Survey Replication (NCS-R) Archives of General Psychiatry. 2005;62(6):617–627. doi: 10.1001/archpsyc.62.6.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keyes KM, Eaton NR, Krueger RF, McLaughlin KA, Wall MM, Grant BF, et al. Child maltreatment and the structure of common psychiatric disorders. The British Journal of Psychiatry. 2012;200:107–115. doi: 10.1192/bjp.bp.111.093062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Cicchetti D. Longitudinal pathways linking child maltreatment, emotion regulation, peer relations, and psychopathology. Journal of Child Psychology & Psychiatry. 2010;51(6):706–716. doi: 10.1111/j.1469-7610.2009.02202.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim-Cohen J, Caspi A, Taylor A, Williams B, Newcombe R, Craig IW, et al. MAOA, maltreatment, and gene-environment interaction predicting children’s mental health: new evidence and a meta-analysis. Molecular Psychiatry. 2006;11(10):903–913. doi: 10.1038/sj.mp.4001851. [DOI] [PubMed] [Google Scholar]
- Kim-Spoon J, Cicchetti D, Rogosch FA. A longitudinal study of emotion regulation, negative emotionality, and internalizing symptomatology in maltreated and nonmaltreated children. Child Development. in press doi: 10.1111/j.1467-8624.2012.01857.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenen KC, Saxe G, Purcell S, Smoller JW, Bartholomew D, Miller A, et al. Polymorphisms in FKBP5 are associated with peritraumatic dissociation in medically injured children. Molecular Psychiatry. 2005;10:1058–1059. doi: 10.1038/sj.mp.4001727. [DOI] [PubMed] [Google Scholar]
- Koenen KC, Amstadter AB, Nugent NR. Gene-environment interaction in posttraumatic stress disorder: an update. Journal of Traumatic Stress. 2009;22(5):416–426. doi: 10.1002/jts.20435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krämer UM, Cunillera T, Ca`mara E, Marco-Pallare´s J, Cucurell D, Nager W, et al. The impact of catechol-O-methyltransferase and dopamine D4 receptor genotypes on neurophysiological markers of performance monitoring. Journal of Neuroscience. 2007;27:14190–14198. doi: 10.1523/JNEUROSCI.4229-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lekman M, Laje G, Charney D, Rush AJ, Wilson AF, Sorant AJ, et al. The FKBP5-gene in depression and treatment response – an association study in the Sequenced Treatment Alternatives to Relieve Depression (STAR*D) cohort. Biological Psychiatry. 2008;63:1103–1110. doi: 10.1016/j.biopsych.2007.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu D, Diorio J, Tannenbaum B, Caldji C, Francis D, Freedman A, et al. Maternal care, hippocampal glucocorticoid receptors, and hypothalamic–pituitary–adrenal responses to stress. Science. 1997;277:1659–1662. doi: 10.1126/science.277.5332.1659. [DOI] [PubMed] [Google Scholar]
- Lowy MT, Wittenberg L, Yamamoto BK. Effect of acute stress on hippocampal glutamate levels and spectrin proteolysis in young and aged rats. Journal of Neurochemistry. 1995;65:268–274. doi: 10.1046/j.1471-4159.1995.65010268.x. [DOI] [PubMed] [Google Scholar]
- Ludwig AM. The psychobiological functions of dissociation. American Journal of Clinical Hypnosis. 1983;26:93–99. doi: 10.1080/00029157.1983.10404149. [DOI] [PubMed] [Google Scholar]
- Lupien SJ, McEwen BS, Gunnar MR, Heim C. Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nature Reviews Neuroscience. 2009;10:434–445. doi: 10.1038/nrn2639. [DOI] [PubMed] [Google Scholar]
- Lupien SJ, Ouellet-Morin I, Hupbach A, Tu MT, Buss C, Walker D, et al. Beyond the stress concept: Allostatic load—A developmental biological and cognitive perspective. In: Cicchetti D, Cohen D, editors. Developmental psychopathology: Vol. 2. Developmental neuroscience. 2nd ed. New York: Wiley; 2006. pp. 578–628. [Google Scholar]
- Lyons-Ruth K, Spielman E. Disorganized infant attachment strategies and helpless-fearful profiles of parenting: integrating attachment research with clinical intervention. Infant Mental Health Journal. 2004;25(4):318–335. doi: 10.1002/imhj.20008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macfie J, Cicchetti D, Toth SL. Dissociation in maltreated versus nonmaltreated preschool-aged children. Child Abuse & Neglect. 2001a;25:1253–1267. doi: 10.1016/s0145-2134(01)00266-6. [DOI] [PubMed] [Google Scholar]
- Macfie J, Cicchetti D, Toth SL. The development of dissociation in maltreated preschool-aged children. Development and Psychopathology. 2001b;13:233–254. doi: 10.1017/s0954579401002036. [DOI] [PubMed] [Google Scholar]
- MacKinnon DP. An introduction to statistical mediation analysis. New York, NY: Lawrence Erlbaum Associates; 2008. [Google Scholar]
- MacQueen GM, Frodl T. The hippocampus in major depression: Evidence for the convergence of the bench and bedside in psychiatric research? Molecular Psychiatry. 2010;16:252–264. doi: 10.1038/mp.2010.80. [DOI] [PubMed] [Google Scholar]
- McCrory EJ, De Brito SA, Sebastian CL, Mechelli A, Bird G, Kelly PA, et al. Heightened neural reactivity to threat in child victims of family violence. Current Biology. 2011;21(23):R947–948. doi: 10.1016/j.cub.2011.10.015. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Stress, adaptation, and disease. Allostasis and allostatic load. Annals of the New York Academy of Sciences. 1998;840:33–44. doi: 10.1111/j.1749-6632.1998.tb09546.x. [DOI] [PubMed] [Google Scholar]
- McEwen BS, Stellar E. Stress and the individual: Mechanisms leading to disease. Archives of Internal Medicine. 1993;153:2093–2101. [PubMed] [Google Scholar]
- McEwen BS, Wingfield JC. The concept of allostasis in biology and biomedicine. Hormones and Behavior. 2003;43:2–15. doi: 10.1016/s0018-506x(02)00024-7. [DOI] [PubMed] [Google Scholar]
- McLaughlin KA, Green JG, Gruber MJ, Sampson NA, Zaslavsky AM, Kessler RC. Childhood adversities and adult psychiatric disorders in the national comorbidity survey replication II: associations with persistence of DSM-IV disorders. Archives of General Psychiatry. 2010a;67(2):124–132. doi: 10.1001/archgenpsychiatry.2009.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin KA, Green JG, Gruber MJ, Sampson NA, Zaslavsky AM, Kessler RC. Childhood adversities and adult psychiatric disorders in the national comorbidity survey replication (NCR) III: associations with functional impairment related to DSM-IV disorders. Psychological Medicine. 2010b;40(5):847–859. doi: 10.1017/S0033291709991115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meaney MJ, Stewart J, Beatty WW. The effects of gluococorticoids during the neonatalperiod on the development of social play in juvenile rats. Hormones and Behavior. 1981;16:475–91. doi: 10.1016/0018-506x(82)90054-x. [DOI] [PubMed] [Google Scholar]
- Mehta D, Binder EB. Gene x environment vulnerability factors for PTSD: The HPA-axis. Neuropharmacology. 2012;62(2):654–662. doi: 10.1016/j.neuropharm.2011.03.009. [DOI] [PubMed] [Google Scholar]
- Muthén B. Applications of causally defined direct and indirect effects in mediation analysis using SEM in Mplus. Submitted for publication: 2011. [Google Scholar]
- Myers J, Berliner L, Briere L, Hendrix C, Jenny C, Reid TA. The APSAC handbook on child maltreatment. Thousand Oaks, CA: SAGE; 2002. [Google Scholar]
- Nemeroff CB, Vale WW. The neurobiology of depression: inroads to treatment and new drug discovery. Journal of Clinical Psychiatry. 2005;66(7):5–13. [PubMed] [Google Scholar]
- Orr SP, Lasko NB, Metzger LJ, Berry NJ, Ahern CE, Pitman RK. Psychophysiological assessment of women with posttraumatic stress disorder resulting from childhood sexual abuse. Journal of Consulting and Clinical Psychology. 1998;66(6):906–913. doi: 10.1037//0022-006x.66.6.906. [DOI] [PubMed] [Google Scholar]
- Pariante CM, Lightman SL. The HPA axis in major depression: classical theories and new developments. Trends in Neurosciences. 2008;31(9):464–468. doi: 10.1016/j.tins.2008.06.006. [DOI] [PubMed] [Google Scholar]
- Pariante CM, Miller AH. Glucocorticoid receptors in major depression: relevance to pathophysiology and treatment. Biological Psychiatry. 2001;49(5):391–404. doi: 10.1016/s0006-3223(00)01088-x. [DOI] [PubMed] [Google Scholar]
- Pollak SD, Klorman R, Thatcher JE, Cicchetti D. P3b reflects maltreated children’s reactions to facial displays of emotion. Psychophysiology. 2001;38(2):267–274. [PubMed] [Google Scholar]
- Post RM. Transduction of psychosocial stress into the neurobiology of recurrent affective disorder. American Journal of Psychiatry. 1002;149:999–1010. doi: 10.1176/ajp.149.8.999. [DOI] [PubMed] [Google Scholar]
- Putnam FW. Dissociation in children and adolescents: A developmental perspective. New York: Guilford Press; 1997. [Google Scholar]
- Rao H, Betancourt L, Giannetta JM, Brodsky NL, Korczykowski M, Avants BB, et al. Early parental care is important for hippocampal maturation: evidence from brain morphology in humans. Neuroimage. 2010;49:1144–1150. doi: 10.1016/j.neuroimage.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy A, Gilman SE, Brslau J, Breslau N, Koenen KC. Race-ethnic differences in exposure to traumatic events, development of posttraumatic stress disorder, and treatment seeking in the United States population. Psychological Medicine. 2010;29:1–13. doi: 10.1017/S0033291710000401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutter M, Moffitt TE, Caspi A. Gene-environment interplay and psychopathology: multiple varieties but real effects. Journal of Child Psychology and Psychiatry. 2007;47(3–4):226–261. doi: 10.1111/j.1469-7610.2005.01557.x. [DOI] [PubMed] [Google Scholar]
- Sanchez MM, Young LJ, Plotsky PM, Insel TR. Distribution of corticosteroid receptors in the rhesus brain: relative absence of glucocorticoid receptors in the hippocampal formation. Journal of Neuroscience. 2000;20:4657–4668. doi: 10.1523/JNEUROSCI.20-12-04657.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarapas C, Cai G, Bierer LM, Golier JA, Galea S, Ising M, et al. Genetic markers for PTSD risk and resilience among survivors of the World Trade Center attacks. Disease Markers. 2011;30:101–110. doi: 10.3233/DMA-2011-0764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scammell JG, Denny WB, Valentine DL, Smith DF. Overexpression of the FK506-binding immunophilin FKBP51 is the common cause of glucocorticoid resistance in three New World primates. General and Comparative Endocrinology. 2001;124(2):152–165. doi: 10.1006/gcen.2001.7696. [DOI] [PubMed] [Google Scholar]
- Schapiro S. Hormonal and environmental influences on rat brain and behavior. In: Sterman MB, McGinty OJ, editors. Brain development and behavior. New York: Academic Press; 1971. pp. 307–34. [Google Scholar]
- Simeon D, Knutelska M, Yehuda R, Putnam F, Schmeidler J, Smith LM. Hypothalamic-pituitary-adrenal axis dysfunction in dissociative disorders, post-traumatic stress disorder, and health volunteers. Biological Psychiatry. 2007;61(8):966–973. doi: 10.1016/j.biopsych.2006.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seckl JR. Physiologic programming of the fetus. Clinical Perinatology. 1998;25(4):939–962. [PubMed] [Google Scholar]
- Sedlak AJ, Mettenburg J, Basena M, Petta I, McPherson K, Greene A, et al. Fourth National Incidence Study of Child Abuse and Neglect (NIS-4): Report to Congress, Executive Summary. Washington DC: U.S. Department of Health and Human Services, Administration for Children and Families; 2010. [Google Scholar]
- Segman RH, Shefi N, Goltser-Dubner T, Friedman N, Kaminski N, Shalev AY. Peripheral blood mononuclear cell gene expression profiles identify emergent post-traumatic stress disorder among trauma survivors. Molecular Psychiatry. 2005;10(5):500–513. doi: 10.1038/sj.mp.4001636. [DOI] [PubMed] [Google Scholar]
- Shields A, Cicchetti D. Emotion regulation among school-age children: the development and validation of a new criterion Q-sort scale. Development and Psychopathology. 1997;33(6):906–916. doi: 10.1037//0012-1649.33.6.906. [DOI] [PubMed] [Google Scholar]
- Shields A, Cicchetti D. Reactive aggression among maltreated children: the contributions of attention and emotion dysregulation. Journal of Clinical Child Psychology. 1998;27(4):381–395. doi: 10.1207/s15374424jccp2704_2. [DOI] [PubMed] [Google Scholar]
- Shields A, Cicchetti D. Parental maltreatment and emotion dysregulation as risk factors for bullying and victimization in middle childhood. Journal of Clinical Child Psychology. 2001;30(3):349–363. doi: 10.1207/S15374424JCCP3003_7. [DOI] [PubMed] [Google Scholar]
- Shields A, Ryan RM, Cicchetti D. Narrative representations of caregivers and emotion regulation as predictors of maltreated children’s rejection by peers. Development and Psychopathology. 2001;37(3):321–337. doi: 10.1037//0012-1649.37.3.321. [DOI] [PubMed] [Google Scholar]
- Shrout PE, Bolger N. Mediation in experimental and nonexperimental studies: new procedures and recommendation. Psychological Methods. 2002;7(4):422–445. [PubMed] [Google Scholar]
- Skelton K, Ressler KJ, Norrholm SD, Jovanovic T, Bradley-Davino B. PTSD and gene variants: New pathways and new thinking. Neuropharmacology. 2012;62:628–637. doi: 10.1016/j.neuropharm.2011.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear L. The adolescent brain and age-related behavioral manifestations. Neuroscience & Biobehavioral Reviews. 2000;24:417–463. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- Sroufe LA, Fleeson J. Attachment and the construction of relationships. In: Hartup NW, Rubin Z, editors. Relationships and development. Hillsdale, NJ: Erlbaum; 1986. pp. 273–308. [Google Scholar]
- Stein MB. Hippocampal volume in women victimized by childhood sexual abuse. Psychological Medicine. 1997;27:951–959. doi: 10.1017/s0033291797005242. [DOI] [PubMed] [Google Scholar]
- Stephens M, Smith N, Donnelly P. A new statistical method for haplotype reconstruction from population data. American Journal of Human Genetics. 2001;68:978–989. doi: 10.1086/319501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens M, Scheet P. Accounting for decay of linkage disequilibrium in haplotype interference and missing-data imputation. American Journal of Human Genetics. 2005;76:449–462. doi: 10.1086/428594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storch EA, Roberti JW, Roth DA. Factor structure, concurrent validity, and internal consistency of the Beck Depression Inventory- Second Edition in a sample of college students. Depression and Anxiety. 2004;19(3):187–189. doi: 10.1002/da.20002. [DOI] [PubMed] [Google Scholar]
- Teicher MH, Andersen SL, Polcari A, Anderson CM, Navalta CP. Developmental neurobiology of childhood stress and trauma. Psychiatric Clinics of North America. 2002;25:397–426. doi: 10.1016/s0193-953x(01)00003-x. [DOI] [PubMed] [Google Scholar]
- Teicher MH, Andersen SL, Polcari A, Anderson CM, Navalta CP, Kim DM. The neurobiological consequences of early stress and childhood maltreatment. Neuroscience and Biobehavioral Reviews. 2003;27:33–44. doi: 10.1016/s0149-7634(03)00007-1. [DOI] [PubMed] [Google Scholar]
- Teicher MH, Dumont N, LIto Y, Vaituzis C, Gledd JN, Andersen SL. Childhood neglect is associated with reduced corpus callosum area. Biological Psychiatry. 2004;15(56):80–85. doi: 10.1016/j.biopsych.2004.03.016. [DOI] [PubMed] [Google Scholar]
- Teicher MH, Glod CA, Surrey J, Swett C. Early childhood abuse and limbic system ratings in adult psychiatric outpatients. Journal of Neuropsychiatry. 1993;5:301–306. doi: 10.1176/jnp.5.3.301. [DOI] [PubMed] [Google Scholar]
- Teicher MH, Ito Y, Glod CA, Anderson SL, Dumont N, Ackerman E. Preliminary evidence for abnormal cortical development in physically and sexually abused children using EEG coherence and MRI. Annals of the New York Academy of Science. 1997;821:160–175. doi: 10.1111/j.1749-6632.1997.tb48277.x. [DOI] [PubMed] [Google Scholar]
- Teicher MH, Samson JA, Polcari A, McGreenery CE. Sticks, stones, and hurtful words: relative effects of various forms of childhood maltreatment. American Journal of Psychiatry. 2006;163(3):993–1000. doi: 10.1176/ajp.2006.163.6.993. [DOI] [PubMed] [Google Scholar]
- Teicher MH, Samson JA, Sheu Y, Polcari A, McGreenery CE. Hurtful words: association of exposure to peer verbal abuse with elevated psychiatric symptom scores and corpus callosum abnormalities. American Journal of Psychiatry. 2010;167(12):1464–1471. doi: 10.1176/appi.ajp.2010.10010030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomoda A, Navalta CP, Polcari A, Sadato N, Teicher MH. Childhood sexual abuse is associated with reduced gray matter volume in visual cortex of young women. Biological Psychiatry. 2009;66:642–648. doi: 10.1016/j.biopsych.2009.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toth SL, Manly JT, Cicchetti D. Child maltreatment and vulnerability to depression. Development and Psychopathology. 1992;4:97–112. [Google Scholar]
- Touma C, Gassen NC, Herrman L, Cheung-Flynn J, Bull DR, Ionescu IA, et al. FK506 binding protein 5 shapes stress responsiveness: modulation of neuroendocrine reactivity and coping behavior. Biological Psychiatry. 2011;15(70):928–936. doi: 10.1016/j.biopsych.2011.07.023. [DOI] [PubMed] [Google Scholar]
- Trickett PK, McBridge-Chang C. The developmental impact of different types of child abuse and neglect. 1995;(15):311–337. [Google Scholar]
- van der Kolk B, Greenberg MS. The psychobiology of the trauma response: hyperarousal, constriction, and addiction to traumatic reexposure. In: van der Kolk B, editor. Psychological Trauma. Washington, D.C: American Psychiatric Press; 1987. pp. 63–87. [Google Scholar]
- van der Kolk BA, van der Hart OV, Marmar CR. Dissociation and information processing in posttraumatic stress disorder. In: van der Kolk BA, McFarlane AC, Weisaeth L, editors. Traumatic Stress: The effects of overwhelming experience on mind, body, and society. New York: Guilford Press; 1996. pp. 303–327. [Google Scholar]
- van Ijendoorn M, Schuengel C. The measurement of dissociation in normal and clinical populations: meta-analytic validation of the Dissociative Experiences Scale (DES) Clinical Psychology Review. 1996;16:362–382. [Google Scholar]
- Velders FP, Kunigas M, Kumari M, Dekker MJ, Uitterlinden AG, Kirschbaum C, et al. Genetics of cortisol secretion and depressive symptoms: A candidate gene and genome wide association approach. Psychoneuroendocrinology. 2011;36:1053–1061. doi: 10.1016/j.psyneuen.2011.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veltman DJ, de Ruiter MB, Rombouts SA, Lazeron RH, Barkhof F, van Dyck R, et al. Neurophysiological correlates of increased verbal working memory in high-dissociative participants: A functional MRI study. Psychological Medicine. 2005;35:175–185. doi: 10.1017/s0033291704002971. [DOI] [PubMed] [Google Scholar]
- Vermeer H, Hendriks-Stegeman BI, van der Burg B, van Buul-Offers SC, Jansen M. Glucocorticoid-induced increase in lymphocytic FKBP51 messenger ribonucleic acid expression. Journal of Clinical Endocrinology and Metabolism. 2003;88(1):277–284. doi: 10.1210/jc.2002-020354. [DOI] [PubMed] [Google Scholar]
- Vythilingam M, Heim C, Newport J, Miller AH, Anderson E, Bronen R, et al. Childhood trauma is associated with smaller hippocampal volume in women with major depression. American Journal of Psychiatry. 2002;159:2072–2080. doi: 10.1176/appi.ajp.159.12.2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welberg LA, Seckl JR. Prenatal stress, glucocortioicds and the programming of the brain. Journal of Neuroendocrinology. 2001;13(2):113–128. doi: 10.1046/j.1365-2826.2001.00601.x. [DOI] [PubMed] [Google Scholar]
- Widom CS. Post-traumatic stress disorder in abused and neglected children grown up. American Journal of Psychiatry. 1999;156:1223–1229. doi: 10.1176/ajp.156.8.1223. [DOI] [PubMed] [Google Scholar]
- Widom CS, DuMont K, Czaja SJ. A prospective investigation of major depressive disorder and comorbidity in abused and neglected children grown up. Archives of General Psychiatry. 2007;64:49–56. doi: 10.1001/archpsyc.64.1.49. [DOI] [PubMed] [Google Scholar]
- Wochnik GM, Ruegg J, Abel GA, Schmidt U, Holsboer F, Rein T, et al. FK506-binding proteins 51 and 52 differentially regulate dynein interaction and nuclear translocation of the glucocorticoid receptor in mammalian cells. Journal of Biological Chemistry. 2005;280(6):4609–4616. doi: 10.1074/jbc.M407498200. [DOI] [PubMed] [Google Scholar]
- Xie P, Kianzler HR, Poling J, Stein MB, Anton RF, Farrer LA, et al. Interaction of FKBP5 with childhood adversity on risk for posttraumatic stress disorder. Neuropsychopharmacology. 2010;35:1684–1694. doi: 10.1038/npp.2010.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yehuda R. Status of glucocorticoid alterations in post-traumatic stress disorder. Annals of the New York Academy of Science. 2009;1179:56–69. doi: 10.1111/j.1749-6632.2009.04979.x. [DOI] [PubMed] [Google Scholar]
- Yehuda R, Golier JA, Yang R, Tischler L. Enhanced sensitivity to glucocorticoids in peripheral mononuclear leukocytes in posttraumatic stress disorder. Biological Psychiatry. 2004;55:1110–1116. doi: 10.1016/j.biopsych.2004.02.010. [DOI] [PubMed] [Google Scholar]
- Zimmermann P, Bruckl T, Nocon A, Pfister H, Binder E, et al. Interaction of FKBP5 gene variants and adverse life events in predicting depression onset: results form a 10-year prospective community sample. American Journal of Psychiatry. 2011;168(10):1107–1116. doi: 10.1176/appi.ajp.2011.10111577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zobel A, Schuhmacher A, Jessen F, Hofels S, von Widdern O, Metten M, et al. DNA sequence variants of the FKBP5 gene are associated with unipolar depression. International Journal of Neuropsychopharmacology. 2010;13:649–660. doi: 10.1017/S1461145709991155. [DOI] [PubMed] [Google Scholar]