Abstract
It is now well appreciated that programmed cell death (PCD) plays critical roles in the life cycle of diverse bacterial species. It is an apparently paradoxical behavior as it does not benefit the cells undergoing PCD. However, growing evidence suggests that PCD can be ‘altruistic’: the dead cells may directly or indirectly benefit survivors through generation of public goods. This property provides a potential explanation on how PCD can evolve as an extreme form of cooperation, though many questions remain to be addressed. From another perspective, as PCD plays a critical role in bacterial pathogenesis, it has been proposed as a potential target for new antibacterial therapy. To this end, understanding the population and evolutionary dynamics resulting from PCD and public-good production may be a key to the success of designing effective antibiotic treatment.
Keywords: Programmed cell death, bacterial cooperation, synthetic biology, antibiotic therapy
Programmed cell death in bacteria
Programmed cell death (PCD) is a genetically encoded process that leads to cell death. It is an essential mechanism in multicellular organisms for proper development and homeostasis. Although PCD has classically been studied in the context of multicellular organisms, it is now widely recognized that bacteria and other single-cell organisms also exhibit PCD in response to environmental stimuli [1-3]. Over the past two decades, PCD has been identified in various bacterial species and found to play critical roles in their survival and pathogenesis [4, 5]. In particular, recent studies discovered that cell death in Escherichia coli also exhibits typical biochemical markers of apoptosis when induced with bactericidal antibiotics [6, 7]. This phenomenon has been termed ‘apoptosis-like death’. The origin and physiological implications of PCD are still a subject of active debate [5]: for instance, is it a trait that is selected for during evolution or a maladaptive byproduct of another trait? In this article, we refer to PCD as any genetic program that can be triggered in response to environmental stimuli (and cell signaling reflecting environmental changes) to cause cell death. In this article, we review commonly observed modes of bacterial PCD and discuss the therapeutic potential of targeting PCD.
A common mechanism to realize PCD in bacteria is through toxin–antitoxin (TA) modules, which are present in almost all free-living bacteria species that have been sequenced [8]. A TA module typically consists of genes encoding a protein toxin and an antitoxin (a protein or an RNA) that neutralizes the toxin by either directly binding to the toxin or inhibiting translation of the toxin [9]. The antitoxin is often less stable than the toxin and has to be constantly produced to inhibit the toxin [9]. Under certain stressful conditions, antitoxins are quickly degraded thus freeing toxins to exert their poisoning effects. The targets of toxins are diverse: they include DNA replication, translation, cell division, and cell wall synthesis [9]. One of the most studied TA modules is the mazEF system, which was first found on chromosomes of E. coli and later in other bacteria [1]. mazF codes for a toxin MazF, an endoribonuclease that cleaves mRNAs; mazE codes for an antitoxin MazE, which can be degraded quickly by the ClpPA serine protease [1]. The mazEF system is activated under various stressful conditions, including amino acid starvation, antibiotic treatment, DNA damage, and oxidative stress [2, 10]. Once induced, it causes PCD in most of the population by increasing the synthesis of ‘death proteins’, while allowing survival of a small sub-population by increasing the synthesis of 'survival proteins’ [11]. Moreover, mazEF-mediated death is regulated by a small peptide called extracellular death factor (EDF) that is secreted by cells [12], suggesting fine tuning of PCD through population density.
PCD can also occur by activation of a prophage, a bacteriophage DNA that is inserted into a bacterial host genome as a result of lysogenic life cycle of bacteriophage. Normally dormant, the prophage can be activated by environmental stresses that damage host DNA [13, 14], which in turn results in host lysis [15-17]. Since prophages-coded genes play critical roles in bacterial evolution and prophages reside in bacterial chromosomes, we consider prophages activation as PCD in this article. Also, this notion is consistent with general definition of PCD. Genomic analyses have identified prophages in many sequenced bacteria; in many cases, a bacterium contains multiple prophages in its genome although many of them have become defective in producing phage particles [18, 19]. A notable example is the food pathogen Shiga toxin-producing E. coli (STEC) O157:H7 strain Sakai, which contains 18 prophage elements [18]. In STEC, the induction of some prophages is accompanied by the expression of phage-encoded Shiga toxin (Stx), often triggered by DNA-damaging agents such as fluoroquinolone antibiotics [20], mitomycin C [21], and neutrophils and their products (e.g. H2O2)[22].
The molecular mechanisms underlying apoptosis-like death are poorly understood. But a central player appears to be RecA, a multifunctional protein critically involved in DNA repair and maintenance in bacteria. Interestingly, its function resembles that of caspases, cysteine proteases central to the regulation of apoptosis in eukaryotes [23]; it was found that RecA binds specifically to a synthetic caspase substrate [7]. How RecA activation causes apoptosis-like death in bacteria and whether there is a fitness advantage to performing this mode of death are still unknown. Considering its cross-regulation with the mazEF death pathway, it was speculated that the apoptosis-like death is a backup death program ensuring the execution of PCD even when the mazEF pathway is rendered dysfunctional [6]. Another intersection between bacterial PCD and eukaryotic apoptosis has also been found in the Cid/Lrg system of Staphylococcus aureus. It encodes proteins analogous to bacteriophage-encoded holins and antiholins, and when activated causes cell lysis [24]. Interestingly, functional similarity between phage lambda holin and Bax, another core regulator of apoptosis in eukaryotes, has been found [25]. While it remains to be seen how or whether the RecA pathway and Cid/Lrg are related, the two systems suggest a potential evolutionary connection between prokaryotic and eukaryotic PCD.
PCD as an altruistic trait
PCD in bacteria is apparently paradoxical, considering that death offers no direct fitness benefit to its actor (i.e. a bacterial cell that has executed PCD will die). Indeed, the evolutionary origin of PCD remains controversial [5]. One possible explanation is that PCD may be a maladaptive trait resulting from other processes that provide pro-survival function. It was suggested that the primary function of the TA module mazEF is to control the quality of gene expression under stress conditions such as amino acid starvation rather than to mediate PCD [26]. Another explanation, however, is that PCD represents an altruistic trait: sacrifice of some cells in a population can benefit survivors through the generation of public goods [4] (Figure 1A). In other words, PCD represents an extreme form of cooperation, similar to what is observed in animals: sterile workers in social-insect colonies give up their personal reproduction but help their fertile family members [27]. In this case, the evolutionary theory requires that the benefit be enjoyed more by individuals who share the PCD genotype than those who do not (Box 1). An important difference between PCD being maladaptive or altruistic is the evolutionary force that maintains PCD. If PCD is maladaptive, it should be evolutionarily stable as long as the selection on the pro-survival function to which PCD is linked is strong. If PCD represents an altruistic act, it should be lost under conditions in which PCD-mediated public good is not beneficial to the population [5].
Figure 1. Altruistic PCD and its intervention strategies.
(A) Induction of PCD by environmental stress may be coupled with release of public goods that provide direct or indirect benefits to the survivors.
(B) PCD can be artificially induced as an antibacterial treatment strategy, but this increases the amount of public goods released into the environment (indicated by the larger arrow sizes), which can result in overall better bacterial growth.
(C) PCD may be inhibited to decrease the generation of public goods (indicated by the smaller arrow sizes).
(D) A combination approach that both induces PCD and inhibits the function or activities of released public goods.
This notion is consistent with many examples of PCD studied to date (Table 1). For example, PCD mediated by TA modules has been linked to release of nutrients and other molecules necessary for the survival under stressful environments [1, 2, 10], release of virulence factors [28, 29], and structural development of biofilms [30]. Prophages often encode bacterial virulence factors that are expressed as a result of prophage activation [19]. As such, an apparent benefit of prophage-mediated PCD is production of virulence factors [19, 20, 22, 31]. Interestingly, such virulence factors often require cell lysis to be released into the environment. Furthermore, prophage-mediated PCD can also contribute to biofilm development and dispersal [32-35]. We note that public goods resulting from PCD may not necessarily be a tangible physical entity. For example, during biofilm formation in Bacillus subtilis, localized cell death allows spatially focused mechanical forces to initiate wrinkle formation, which is critical for resistance against liquid wetting and gas penetration [30].
Table 1.
Programmed cell death in bacteria
| Mode/mechanism | Examples | Benefit | Refs |
|---|---|---|---|
| TA module | MazEF | Nutrient release | [1, 2, 10] |
| PezAT | Pneumolysin (virulence factor) release | [28, 29] | |
| SpoIISAB and NdoAI | Formation of wrinkled biofilm structure | [30, 56] | |
| Prophage | STEC | Stx (virulence factor) production and release | [20, 22, 31] |
| P. aeruginosa PAO1 | Biofilm formation and dispersal | [32-35] | |
| Shewanella oneidensis MR-1 | Biofilm formation | [35] | |
| Apoptosis-like death | RecA-dependent apoptosis-like death | Not known (speculated as a backup death program of MazEF to ensure PCD) | [6, 7, 23] |
The apparent benefit of public goods, however, does not necessarily mean that it is advantageous for a population to perform PCD. Can public goods generated by PCD provide enough benefit to improve overall population fitness? How drastic does PCD have to be to achieve this? A recent study using a synthetic system in E. coli demonstrated conditions under which such altruistic death can indeed improve the overall population growth [36]. This indicates that for clonal populations altruistic death can be evolutionarily favored. For non-clonal populations, however, the public good generated by altruistic death needs to preferentially benefit individuals who share the altruistic genotype (Box 1). The study also revealed a complex interplay between public good production (and its benefit), the degree of programmed death, and the system's temporal dynamics, and highlighted the nontrivial consequences that arise from perturbing such systems parameters [36].
PCD as a target for antibacterial therapy
Since the introduction of antibiotics in the 1940s, antibiotics have become the largest component in our arsenal against bacterial infections. However, decades of overuse of antibiotics are now undermining their own effectiveness. Bacteria have been developing resistance to existing antibiotics at an alarming rate, and there is an urgent need for developing new antibiotics [37] and new treatment strategies by better use of existing antibiotics. The presence of PCD has profound implications for both aspects. On one hand, if antibiotic-mediated killing of pathogens leads to the release of enzymes that degrade the antibiotic (realizing the basic logic of altruistic PCD), the bacterial growth can exhibit non-monotonic responses to the antibiotic doses, whereby the bacteria grow better at a higher dose of antibiotic [36].
On the other hand, elements of PCD can serve as potential drug targets to achieve effective inhibition of bacterial survival. For example, PCD can be artificially induced to promote greater cell death (Figure 1B). This strategy has been proposed for TA systems [38, 39], where the induction can be achieved by disruption or prevention of TA complex formation or enhanced proteolytic degradation of antitoxin [39]. This strategy is analogous to cancer treatment by chemically inducing or promoting apoptosis [40]. While intuitively appealing, however, this strategy could lead to counterintuitive consequences when PCD is coupled with release of public goods. Specifically, induction of PCD will result in faster public good generation in the short term, which can lead to greater fitness benefit for the pathogen in the long term. As a result, triggering death in some cells could in principle lead to apparently better growth in the overall population [36], although it remains to be seen whether the same effect takes place in in vivo systems.
From the perspective that PCD is a cooperative action, it might be more effective to reduce PCD and minimize release of public goods (Figure 1C). This strategy is analogous to inhibition of quorum sensing (QS), a mechanism by which bacteria communicate with each other to coordinate population-level cooperative actions [41]. Inhibition of QS has been shown to be protective against bacterial pathogens in animal models [42, 43], but could in itself cause surprising consequences [44]. Consistent with this notion, a serum from a mouse immunized with autolysin of Streptococcus pneumoniae reduced autolysis in vitro, and the immunization exhibited protection against S. pneumoniae infection [45]. Whether and to what extent the reduced virulence is attributed to reduced autolysis (and reduced release of virulence factors) in vivo requires further study. Alternatively, one can inhibit the effector of PCD-mediated cooperation by directly targeting public goods while allowing PCD itself to occur. Indeed, neutralization of bacterial toxins such as Shiga toxin and botulinum neurotoxin by antibodies has shown promising results in animal studies and clinical trials [46].
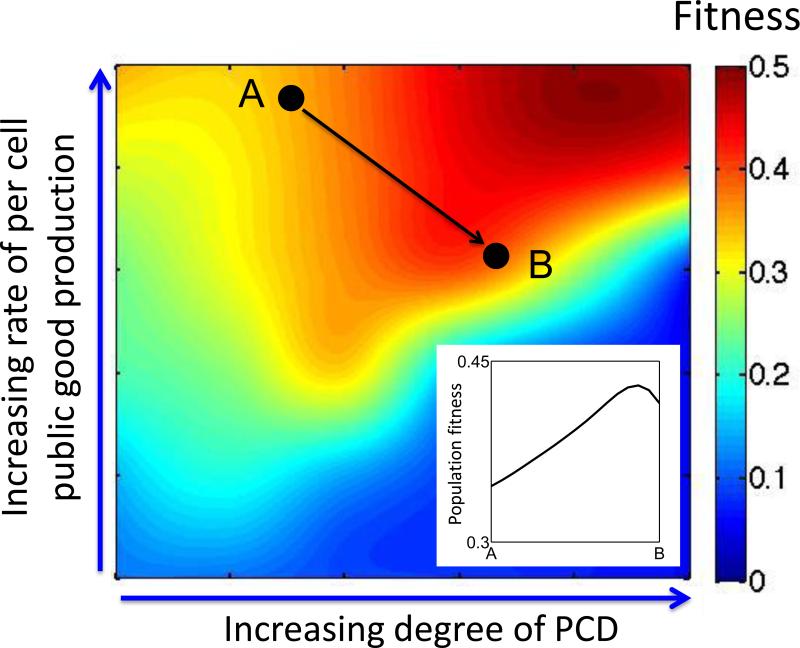
Combining the two aspects above leads to another strategy – inhibition of public goods coupled with proper modulation of PCD (Figure 1D). Again, as noted above, a balance between these two arms of perturbation is critical due to nonlinear coupling of the effects of PCD and public goods. A recent study demonstrated this notion using an engineered altruistic PCD system [36]. The tradeoff between the two factors, together with temporal dynamics of the system, leads to emergence of an optimal degree of PCD (for a given stress level and rate of public-good generation) that maximizes the population fitness. This optimal death rate shifts in a counterintuitive way when the per cell public-good generation is changed [36]. As a result, the population fitness exhibits a complex landscape in terms of the degree of PCD and the rate of public-good generation (Figure 2). Perhaps the most striking realization from this landscape is that, as long as inhibition is incomplete (likely for any drug), simultaneous induction of PCD (greater death) and inhibition of public goods (less benefit from those who die) could lead to overall better growth of the population (Figure 2). Given these complications, proper understanding of the cost-benefit relationship of PCD and public goods is critical for a successful treatment. Moreover, the presence of social cheaters, mutants that do not perform PCD but benefit from public goods generated by the wild type (cooperators), adds further complexity. On one hand, in such a non-clonal situation evolutionary selection usually does not optimize population fitness, and therefore exogenously stimulating PCD could increase overall population growth. On the other, different targeting strategies of PCD may have different consequences to the cooperator-cheater dynamics. Below, we shall discuss this latter aspect in more detail.
Figure 2. Fitness landscape of synthetic altruistic PCD system [36].
Altruistic PCD can lead to a complex fitness landscape as a function of two parameters, the degree of PCD and per cell public good production (redrawn from [36]). The trajectory connecting A and B illustrates a counterintuitive outcome of the intervention strategy depicted in Figure 1D: increased PCD combined with incomplete inhibition of public good can lead to increased growth of the population (inset).
Evolutionary dynamics in targeting PCD
When PCD represents an altruistic trait, effective targeting of PCD also requires a better understanding of the resulting evolutionary dynamics. In particular, altruistic PCD is prone to exploitation by cheaters [47]. If cooperators and cheaters coexist in the same environment (i.e. they equally benefit from public goods), social evolution theory predicts that the cheaters increase in their frequency and the population becomes less cooperative [47]. Notably, in Pseudomonas aeruginosa during lung colonization of mechanically ventilated patients, where QS-mediated cooperation is thought to play a critical role, increased frequency of social cheater (i.e. mutant defective of QS-mediated cooperation) was observed over time [48]. It was also found that the frequency of cheaters was negatively correlated with the onset of ventilator-associated pneumonia, suggesting lower virulence for patients with more cheaters [48]. As such, how PCD is targeted affects the cooperator–cheater dynamics and has an important implication for treatment outcome, and thus should be considered when designing a treatment strategy.
To illustrate this, consider a simple scenario where cooperators and cheaters equally benefit from public goods, and the only fitness difference of the two types is the cost (i.e. whether they perform PCD or not). In this scenario, artificial induction of PCD (Figure 1B and D) would put greater selection for the cheater, thus making the population less virulent. In contrast, PCD inhibition (Figure 1C) essentially closes the fitness gap between cooperator and cheater by making the cooperator act like a cheater, and thus it would favor the maintenance of cooperation. This idea is consistent with the recent clinical study in which QS was inhibited during lung colonization of P. aeruginosa. QS in P. aeruginosa regulates production and secretion of virulence factors, which are energetically costly to produce but are beneficial to nearby individuals. It was found that QS inhibition selected for bacteria that were capable of QS-mediated cooperation [49]. In many natural systems, the situation is likely more complex. For instance, spatial structure may cause patch-like, segregated growth of cooperator and cheater [50], resulting in more concentrated public goods around, and thus more benefit toward, the cooperators. In the presence of population structure, predicting the direction of selection can be challenging. A recent study using a synthetic cooperative system illustrated that the detailed knowledge of how an experimental perturbation affects the cost-benefit structure is critical to understand the direction of selection [51]. On a related note, elucidation of cooperator-cheater dynamics enables a recently proposed ‘Trojan horse’ approach. Engineered cheaters with medically beneficial traits (e.g. modulated PCD, reduced virulence, increased antibiotic sensitivity, etc.) may be introduced to invade a cooperative population [52]. Once such engineered strains take over the infecting pathogens, the population becomes less virulent and more susceptible to other medical interventions.
Concluding remarks
The past four decades have seen an increasing number of examples of multicellular behavior in bacteria. As a unique example, bacterial PCD presents both a challenge for basic biological understanding (in terms of its maintenance and evolution) and potential opportunity for developing effective antibiotic treatment strategies. By recognizing PCD as a potential altruistic trait, we have discussed how PCD can be exploited for combating pathogens (Figure 1B-D). We emphasize that the cooperative nature of PCD requires a deep understanding of cost-benefit relationship of PCD for designing successful treatment strategies. This importance is further underscored by the fact that cooperator-cheater dynamics also plays an important role in determining therapeutic outcome in evolutionary time scales.
As a step forward, it is critical to establish natural or synthetic model systems that enable quantitative studies of the population or evolutionary dynamics resulting from diverse PCD mechanisms, with an emphasis on the network logic, instead of specific molecular components. Such model systems can also facilitate development and testing of different treatment strategies in a controlled manner. This line of research will complement and benefit from ongoing efforts that aim at dissecting the signaling networks underlying PCD: for instance, the latter can provide necessary molecular toolsets to interfere with PCD initiation or the functioning of resulting public goods. A key question is how diverse PCD systems respond to intervention strategies in terms of both short-term dynamics (inhibition of bacterial growth) and long-term dynamics (disruption of population structure). Both are critically important for determining the efficacy of antibacterial treatment in the clinical setting. It would be particularly exciting if unifying principles emerge; if so, the treatment strategies developed for the model systems can be readily applied to real pathogens. For instance, is a particular strategy more likely to be successful regardless of the molecular details of the targeted PCD system? Likewise, is there a particular strategy that tends to favor cheaters more than another strategy? We anticipate that forthcoming years will see considerable progress in addressing these questions, which will likely influence clinical practice in dealing with bacterial pathogens.
Box 1. Evolution of altruistic behaviors.
Altruism is a behavior that reduces fitness of the actor but increases fitness of recipient. Explaining the evolution of altruism has been a challenge because of the negative fitness consequence to the altruist, which would seem to be selected against over time. In 1964, Hamilton formalized a simple rule that describes a condition for the evolution of altruism, known as Hamilton's rule [53, 54]:
b is the fitness benefit to the recipient, c is the fitness cost to the actor, and r measures genetic relatedness between the actor and recipients. For a clonal population (r = 1) where all individuals are altruist, Hamilton's rule simply states that the fitness benefit has to be greater than the fitness cost. This means that altruism is selected when it increases the overall population fitness. For a non-clonal population where altruists and non-altruists coexist (r < 1), however, altruism might not be evolutionarily favored even when it is beneficial for the population. The key factor is to ensure that the fitness benefit of altruistic behavior is preferentially directed towards kin who carry the altruistic gene (i.e. high r). While there could be many mechanisms that realize high relatedness, a previous study experimentally illustrated that strong population bottleneck is one such mechanism [55]. Also, it has been shown that a well-mixed non-clonal population can spatially segregate into clonal patches through simple surface growth [50], suggesting a another generic mechanism to generate a high level of relatedness in nature.
Highlights.
Bacterial programmed cell death (PCD) plays a critical role in pathogenesis and can be altruistic.
Elements of PCD serve as potential drug targets for antibacterial therapy.
Understanding population and evolutionary dynamics is key to effective treatment.
Acknowledgements
Related work in the You lab is partially supported by a National Science Foundation CAREER Award (CBET-0953202), a DuPont Young Professorship (LY), a David and Lucile Packard Fellowship (LY), National Institutes of Health (1RO1GM098642), and Office of Naval Research (N00014-12-1-0631). HM acknowledges support of an NSF graduate fellowship; AJL acknowledges support of a Robert Plonsey Fellowship from the Department of Biomedical Engineering at Duke University.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference
- 1.Aizenman E, EngelbergKulka H, Glaser G. An Escherichia coli chromosomal ”addiction module” regulated by 3',5'-bispyrophosphate: A model for programmed bacterial cell death. P Natl Acad Sci Usa. 1996;93:6059–6063. doi: 10.1073/pnas.93.12.6059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hazan R, Sat B, Engelberg-Kulka H. Escherichia coli mazEF-mediated cell death is triggered by various stressful conditions. J Bacteriol. 2004;186(11):3663–9. doi: 10.1128/JB.186.11.3663-3669.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rice KC, Bayles KW. Death's toolbox: examining the molecular components of bacterial programmed cell death. Mol Microbiol. 2003;50(3):729–38. doi: 10.1046/j.1365-2958.2003.t01-1-03720.x. [DOI] [PubMed] [Google Scholar]
- 4.West SA, et al. The social lives of microbes. Annu Rev Ecol Evol S. 2007;38:53–77. [Google Scholar]
- 5.Nedelcu AM, et al. On the paradigm of altruistic suicide in the unicellular world. Evolution. 2011;65(1):3–20. doi: 10.1111/j.1558-5646.2010.01103.x. [DOI] [PubMed] [Google Scholar]
- 6.Erental A, Sharon I, Engelberg-Kulka H. Two programmed cell death systems in Escherichia coli: an apoptotic-like death is inhibited by the mazEF-mediated death pathway. PLoS Biol. 2012;10(3):e1001281. doi: 10.1371/journal.pbio.1001281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dwyer DJ, et al. Antibiotic-induced bacterial cell death exhibits physiological and biochemical hallmarks of apoptosis. Mol Cell. 2012 doi: 10.1016/j.molcel.2012.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pandey DP, Gerdes K. Toxin-antitoxin loci are highly abundant in free-living but lost from host-associated prokaryotes. Nucleic Acids Res. 2005;33(3):966–976. doi: 10.1093/nar/gki201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yamaguchi Y, Inouye M. Regulation of growth and death in Escherichia coli by toxin-antitoxin systems. Nature reviews Microbiology. 2011 doi: 10.1038/nrmicro2651. [DOI] [PubMed] [Google Scholar]
- 10.Engelberg-Kulka H, et al. Bacterial programmed cell death and multicellular behavior in bacteria. PLoS Genetics. 2006;2(10):e135. doi: 10.1371/journal.pgen.0020135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Amitai S, et al. Escherichia coli MazF leads to the simultaneous selective synthesis of both “death proteins” and “survival proteins”. PLoS Genetics. 2009;5(3):e1000390. doi: 10.1371/journal.pgen.1000390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kolodkin-Gal I, et al. A linear pentapeptide is a quorum-sensing factor required for mazEF-mediated cell death in Escherichia coli. Science. 2007;318(5850):652–5. doi: 10.1126/science.1147248. [DOI] [PubMed] [Google Scholar]
- 13.Hall-Asheshov E, Asheshov I. The induction of the lytic cycle in lysogenic bacteria by phagolessin A 58. Journal of general microbiology. 1956;14(1):174–187. doi: 10.1099/00221287-14-1-174. [DOI] [PubMed] [Google Scholar]
- 14.Snyder L, Champness W. Molecular genetics of bacteria. 3rd xvii. ASM Press; Washington, D.C.: p. ed2007.p. 735. [Google Scholar]
- 15.Birge EA. Bacterial and bacteriophage genetics. 4th ed2000 xiv. Springer; New York: p. 559. [Google Scholar]
- 16.Brussow H, Canchaya C, Hardt WD. Phages and the evolution of bacterial pathogens: from genomic rearrangements to lysogenic conversion. Microbiol Mol Biol Rev. 2004;68(3):560–602. doi: 10.1128/MMBR.68.3.560-602.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grodzicker T, Arditti RR, Eisen H. Establishment of repression by lambdoid phage in catabolite activator protein and adenylate cyclase mutants of Escherichia coli. Proceedings of the National Academy of Sciences. 1972;69(2):366–370. doi: 10.1073/pnas.69.2.366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Canchaya C, et al. Prophage genomics. Microbiol Mol Biol Rev. 2003;67(2):238–76. doi: 10.1128/MMBR.67.2.238-276.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brüssow H, Canchaya C, Hardt W-D. Phages and the evolution of bacterial pathogens: from genomic rearrangements to lysogenic conversion. Microbiol Mol Biol Rev. 2004;68(3):560–602. doi: 10.1128/MMBR.68.3.560-602.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang X, et al. Quinolone antibiotics induce Shiga toxin-encoding bacteriophages, toxin production, and death in mice. Journal of Infectious Diseases. 2000;181(2):664–670. doi: 10.1086/315239. [DOI] [PubMed] [Google Scholar]
- 21.Hull A, et al. Mitomycin immunoblot colony assay for detection of Shiga-like toxin-producing Escherichia coli in fecal samples: comparison with DNA probes. Journal of clinical microbiology. 1993;31(5):1167–1172. doi: 10.1128/jcm.31.5.1167-1172.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wagner PL, Acheson DWK, Waldor MK. Human neutrophils and their products induce Shiga toxin production by enterohemorrhagic Escherichia coli. Infection and immunity. 2001;69(3):1934–1937. doi: 10.1128/IAI.69.3.1934-1937.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hengartner MO. The biochemistry of apoptosis. Nature. 2000;407(6805):770–776. doi: 10.1038/35037710. [DOI] [PubMed] [Google Scholar]
- 24.Bayles KW. The biological role of death and lysis in biofilm development. Nat Rev Microbiol. 2007;5(9):721–6. doi: 10.1038/nrmicro1743. [DOI] [PubMed] [Google Scholar]
- 25.Pang X, et al. Active Bax and Bak are functional holins. Gene Dev. 2011;25(21):2278–2290. doi: 10.1101/gad.171645.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pandey DP, Gerdes K. Toxin–antitoxin loci are highly abundant in free-living but lost from host-associated prokaryotes. Nucleic Acids Research. 2005;33(3):966–976. doi: 10.1093/nar/gki201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gardner A, Kümmerli R. Social evolution: this microbe will self-destruct. Curr Biol. 2008;18(21):R1021–3. doi: 10.1016/j.cub.2008.09.003. [DOI] [PubMed] [Google Scholar]
- 28.Khoo SK, et al. Molecular and structural characterization of the PezAT chromosomal toxin-antitoxin system of the human pathogen Streptococcus pneumoniae. J Biol Chem. 2007;282(27):19606–19618. doi: 10.1074/jbc.M701703200. [DOI] [PubMed] [Google Scholar]
- 29.Mutschler H, et al. A novel mechanism of programmed cell death in bacteria by toxin-antitoxin systems corrupts peptidoglycan synthesis. PLoS biology. 2011;9(3):e1001033. doi: 10.1371/journal.pbio.1001033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Asally M, et al. Localized cell death focuses mechanical forces during 3D patterning in a biofilm. P Natl Acad Sci Usa. 2012;109(46):18891–18896. doi: 10.1073/pnas.1212429109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lainhart W, Stolfa G, Koudelka GB. Shiga toxin as a bacterial defense against a eukaryotic predator, Tetrahymena thermophila. J Bacteriol. 2009;191(16):5116–22. doi: 10.1128/JB.00508-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Webb JS, et al. Cell death in Pseudomonas aeruginosa biofilm development. J Bacteriol. 2003;185(15):4585–92. doi: 10.1128/JB.185.15.4585-4592.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Webb JS, Givskov M, Kjelleberg S. Bacterial biofilms: prokaryotic adventures in multicellularity. Curr Opin Microbiol. 2003;6(6):578–85. doi: 10.1016/j.mib.2003.10.014. [DOI] [PubMed] [Google Scholar]
- 34.Rice SA, et al. The biofilm life cycle and virulence of Pseudomonas aeruginosa are dependent on a filamentous prophage. The ISME Journal. 2009;3(3):271–282. doi: 10.1038/ismej.2008.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gödeke J, et al. Phage-induced lysis enhances biofilm formation in Shewanella oneidensis MR-1. The ISME Journal. 2011;5(4):613–626. doi: 10.1038/ismej.2010.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tanouchi Y, et al. Programming stress-induced altruistic death in engineered bacteria. Mol Syst Biol. 2012;8:626. doi: 10.1038/msb.2012.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Falconer SB, Brown ED. New screens and targets in antibacterial drug discovery. Curr Opin Microbiol. 2009;12(5):497–504. doi: 10.1016/j.mib.2009.07.001. [DOI] [PubMed] [Google Scholar]
- 38.Engelberg-Kulka H, et al. Bacterial programmed cell death systems as targets for antibiotics. Trends Microbiol. 2004;12(2):66–71. doi: 10.1016/j.tim.2003.12.008. [DOI] [PubMed] [Google Scholar]
- 39.Williams JJ, Hergenrother PJ. Artificial activation of toxin-antitoxin systems as an antibacterial strategy. Trends Microbiol. 2012;20(6):291–298. doi: 10.1016/j.tim.2012.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fesik SW. Promoting apoptosis as a strategy for cancer drug discovery. Nat. Rev. Cancer. 2005;5(11):876–885. doi: 10.1038/nrc1736. [DOI] [PubMed] [Google Scholar]
- 41.Rasmussen TB, Givskov M. Quorum-sensing inhibitors as anti-pathogenic drugs. Int J Med Microbiol. 2006;296(2-3):149–61. doi: 10.1016/j.ijmm.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 42.Hentzer M, Givskov M. Pharmacological inhibition of quorum sensing for the treatment of chronic bacterial infections. J. Clin. Invest. 2003;112(9):1300–7. doi: 10.1172/JCI20074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Swem LR, et al. A quorum-sensing antagonist targets both membrane-bound and cytoplasmic receptors and controls bacterial pathogenicity. Molecular cell. 2009;35(2):143–153. doi: 10.1016/j.molcel.2009.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pai A, Tanouchi Y, You L. Optimality and robustness in quorum sensing (QS)-mediated regulation of a costly public good enzyme. P Natl Acad Sci Usa. 2012;109(48):19810–19815. doi: 10.1073/pnas.1211072109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Berry AM, et al. Contribution of autolysin to virulence of Streptococcus pneumoniae. Infect Immun. 1989;57(8):2324–30. doi: 10.1128/iai.57.8.2324-2330.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cegelski L, et al. The biology and future prospects of antivirulence therapies. Nat Rev Microbiol. 2008;6(1):17–27. doi: 10.1038/nrmicro1818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.West SA, et al. Social evolution theory for microorganisms. Nat Rev Microbiol. 2006;4(8):597–607. doi: 10.1038/nrmicro1461. [DOI] [PubMed] [Google Scholar]
- 48.Köhler T, Buckling A, van Delden C. Cooperation and virulence of clinical Pseudomonas aeruginosa populations. P Natl Acad Sci Usa. 2009;106(15):6339–6344. doi: 10.1073/pnas.0811741106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Köhler T, et al. Quorum sensing inhibition selects for virulence and cooperation in Pseudomonas aeruginosa. PLoS Pathogens. 2010;6(5):e1000883. doi: 10.1371/journal.ppat.1000883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hallatschek O, et al. Genetic drift at expanding frontiers promotes gene segregation. P Natl Acad Sci Usa. 2007;104(50):19926–30. doi: 10.1073/pnas.0710150104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chuang JS, Rivoire O, Leibler S. Cooperation and Hamilton's rule in a simple synthetic microbial system. Mol Syst Biol. 2010;6:398. doi: 10.1038/msb.2010.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Brown SP, et al. Social evolution in micro-organisms and a Trojan horse approach to medical intervention strategies. Philos Trans R Soc Lond, B, Biol Sci. 2009;364(1533):3157–68. doi: 10.1098/rstb.2009.0055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hamilton WD. The genetical evolution of social behaviour. II. J Theor Biol. 1964;7(1):17–52. doi: 10.1016/0022-5193(64)90039-6. [DOI] [PubMed] [Google Scholar]
- 54.Hamilton WD. The genetical evolution of social behaviour. I. J Theor Biol. 1964;7(1):1–16. doi: 10.1016/0022-5193(64)90038-4. [DOI] [PubMed] [Google Scholar]
- 55.Chuang JS, Rivoire O, Leibler S. Simpson's paradox in a synthetic microbial system. Science. 2009;323(5911):272–5. doi: 10.1126/science.1166739. [DOI] [PubMed] [Google Scholar]
- 56.Epstein AK, et al. Bacterial biofilm shows persistent resistance to liquid wetting and gas penetration. P Natl Acad Sci Usa. 2011;108(3):995–1000. doi: 10.1073/pnas.1011033108. [DOI] [PMC free article] [PubMed] [Google Scholar]