Abstract
Introduction and objective
The cost-efficiency gains achieved from moving procedures to ASCs and the office may be mitigated if the quality of surgical care at these facilities is not comparable to that of the hospital. Motivated by this, we assessed short-term morbidity and mortality for patients by location of care.
Methods
Using a national sample of Medicare claims (1998 – 2006), we identified elderly beneficiaries who underwent one of 22 common outpatient urologic procedures. After determining the facility type at which each procedure was performed, we then measured 30-day mortality, unexpected admissions, and postoperative complications. Finally, we fit multivariable logistic regression models to evaluate the association between occurrence of an adverse event and the ambulatory setting where surgical care was delivered.
Results
Over the study period, there was a substantial increase in the frequency of nonhospital-based outpatient surgery. Compared to ASCs and the office, hospitals treated more women (P<0.001). Their patients also tended to be less healthy (P<0.001). While patients experienced fewer postoperative complications following surgery at an ASC, procedures performed outside the hospital were associated with a higher likelihood of a same-day admission [ASC: OR, 6.96 (95% CI, 4.44 – 10.90); office: OR, 3.64 (95% CI, 2.48 – 5.36)]. However, it is important to note that, with case-mix adjustment, the probability of any adverse event was exceedingly low across all ambulatory settings.
Conclusions
These data indicate that small, but measurable variation in surgical quality exists by location of care delivery.
Introduction
Of the 72 million surgical procedures performed annually in the United States, over 70% occur in an outpatient setting.1 Although most occur in hospital outpatient departments,2 improved technology and anesthesia have facilitated movement of many procedures away from the more expensive hospital environment to other outpatient facilities, including freestanding ASCs and the office. In 2009 alone, nearly 16 million surgical procedures were performed at these facilities.3, 4 While the trend towards delivery of surgical care in less resource-intensive practice settings may be associated with economic advantages, effects of this migration on surgical quality are uncertain.
In fact, there are at least two reasons to believe that surgical quality may vary by location of care. On one hand, hospitals have access to more organizational resources (e.g., intensive care services and the ability to immediately transfer patients to other clinical departments) than other outpatient facilities,5 which may help their performance. On the other hand, ASCs and the office tend to have a “limited-service” focus and are more likely than hospitals to specialize on certain types of procedures.6 Insofar as this specialization allows them to secure high procedural volumes, ASCs and the office may enjoy improved outcomes.7, 8
To better understand the influence of location of care on surgical quality, we used national Medicare claims to examine risk-adjusted morbidity and mortality among patients undergoing common outpatient urologic procedures performed in hospitals, ASCs, and the office. The findings reported herein serve to inform policymakers and payers on the appropriateness of movement of patients and procedures from the more expensive hospital setting to lower-intensity outpatient facilities.
Methods
Subjects and databases
For our study, we analyzed a 5% national sample of Medicare beneficiaries (1998 – 2006). Using HCPCS codes, we identified patients 65 years and older with continuous enrollment in Medicare parts A and B who underwent endoscopic bladder, urethral, or ureteral surgery; microwave therapy for prostate enlargement; prostate biopsy; shockwave lithotripsy; urethral dilation; or urodynamic procedures (Appendix Table 1). We focused on these procedures for two reasons. Our preliminary analysis indicates that these procedures account for nearly 95% of all ambulatory urologic procedures. Furthermore, these procedures can be performed in each of the surgical locations of interest (i.e., hospitals, ASCs, and the office. Because services provided to Medicare Advantage patients are not consistently captured in claims files, we excluded these patients from our study.
Next, we developed a three-level categorical variable, specifying the location of care: (i.e., hospital, ASC, or office), using appropriate Place of Service codes from Medicare’s carrier file. We then determined the date of surgery from the surgeon’s claim. We considered each procedure as an independent event such that it was possible for some patients to have more than one procedure in our analysis.
Measures of surgical quality
From the Medicare files, we assessed rates of operative mortality, hospital admission, and postoperative complications. We defined operative mortality as death occurring within 30 days of surgery or death during hospitalization that began within 30 days of surgery.9 To determine rates of unexpected hospitalization, we considered both same-day admission (i.e., on the day of the procedure) and subsequent admission within 30 days of the procedure.10 We identified 30-day postoperative complications using ICD-9 CM diagnosis codes (Appendix Table 2).11
Statistical Analysis
For our initial analytic step, we examined the distribution of procedures across outpatient surgery settings and how it changed over the study period. We then made comparisons between patients based on the location where their procedure was performed. In particular, we examined differences between patients with respect to their age, gender, race (white, black, or other), comorbid status (assessed using an adaptation of the Charlson index12), and area of residence (Northeast, Midwest, South, or West), using appropriate parametric and nonparametric statistics.
Finally, we evaluated the relationship between surgical quality and the location where care was delivered. With the patient serving as our unit of analysis, we fit a series of multivariable logistic regression models for each of our binary outcomes. We accounted for case- mix differences, adjusting our models for those patient characteristics described above. Given the potential correlation of observations (i.e., multiple observations on the same subject and patients clustered within facilities), we used robust variance estimators.13 We used our full models to produce predicted probabilities of each adverse event.
We carried out all analyses using the SAS statistical package (SAS, version 9.1; SAS Institute, Cary, NC). All tests were two-tailed, and we set the probability of Type 1 error at 0.05. The Institutional Review Boards of the University of California at Los Angeles and the University of Michigan approved our study.
Results
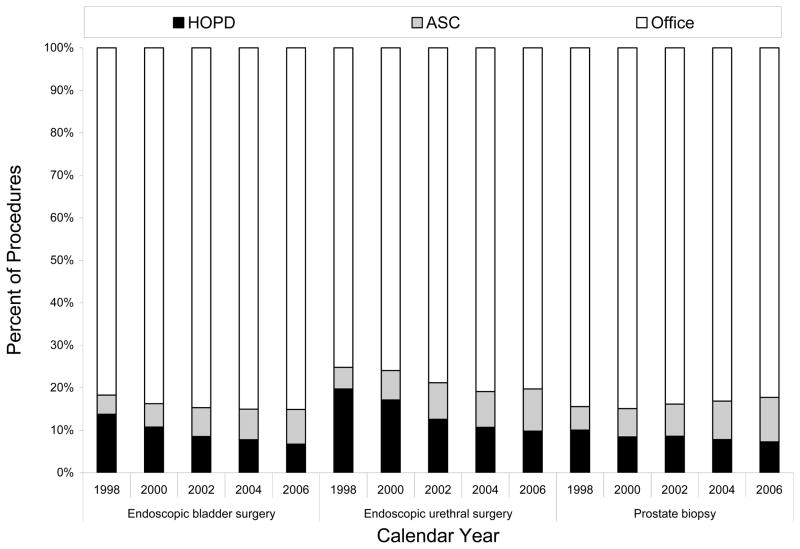
Over the study period, surgical activity at ASCs and the office increased for nearly every procedure with a concomitant decline in hospital utilization. For instance, the frequency of ASC- and office-based endoscopic bladder surgery rose from 4.5% and 81.7% in 1998 to 8.2% and 85.1%, respectively, in 2006 (Figure 1). The one exception to this trend was shockwave lithotripsy, where procedure rates at each care setting remained relatively flat.
Figure 1.
Distribution of selected urologic procedures across ambulatory surgery settings over the study period.
Abbreviations: ASC, ambulatory surgery center; HOPD, hospital outpatient department.
Note: In the bar chart, black, grey, and white shading indicate the HOPD, ASC, and office, respectively.
There were significant differences between patients with respect to their gender, race, level of comorbidity, and area of residence when stratified by outpatient surgery setting (Table 1). Specifically, women and black patients were less likely than men and white patients, respectively, to be treated at an ASC or in the office (P<0.001 for each comparison). In addition, lower acuity cases were concentrated at nonhospital facilities. For instance, the proportion of patients with a Charlson score of “0” was 59.1% at hospitals versus 82.6% and 62.9% at ASCs and in the office, respectively (P<0.001). There were also significantly more ambulatory surgery visits to ASCs and the office among patients who resided in the South (P<0.001).
Table 1.
Differences in case mix across ambulatory care settings.
| Patient Characteristic | Ambulatory Care Setting
|
P-Value | ||
|---|---|---|---|---|
| HOPD (n=33,802) | ASC (16,798) | Office (233,971) | ||
|
| ||||
| Patient age (SD) | 75.4 (6.8) | 74.9 (6.5) | 75.4 (6.7) | <0.001 |
|
| ||||
| Female, % | 38.2 | 31.5 | 33.8 | <0.001 |
|
| ||||
| Race, % | <0.001 | |||
|
| ||||
| White | 89.6 | 91.0 | 90.2 | |
| Black | 7.7 | 7.2 | 5.6 | |
| Other | 2.7 | 1.8 | 4.2 | |
|
| ||||
| Charlson, % | <0.001 | |||
| 0 | 59.1 | 82.6 | 62.9 | |
| 1 | 23.1 | 9.4 | 22.3 | |
| ≥2 | 17.8 | 8.0 | 14.8 | |
|
| ||||
| Area of residence, % | <0.001 | |||
| West | 8.3 | 12.5 | 16.7 | |
| South | 34.2 | 59.1 | 38.5 | |
| Midwest | 37.8 | 17.0 | 24.4 | |
| Northeast | 19.7 | 11.4 | 20.4 | |
Abbreviations: ASC, ambulatory surgery center; HOPD, hospital outpatient department; SD, standard deviation.
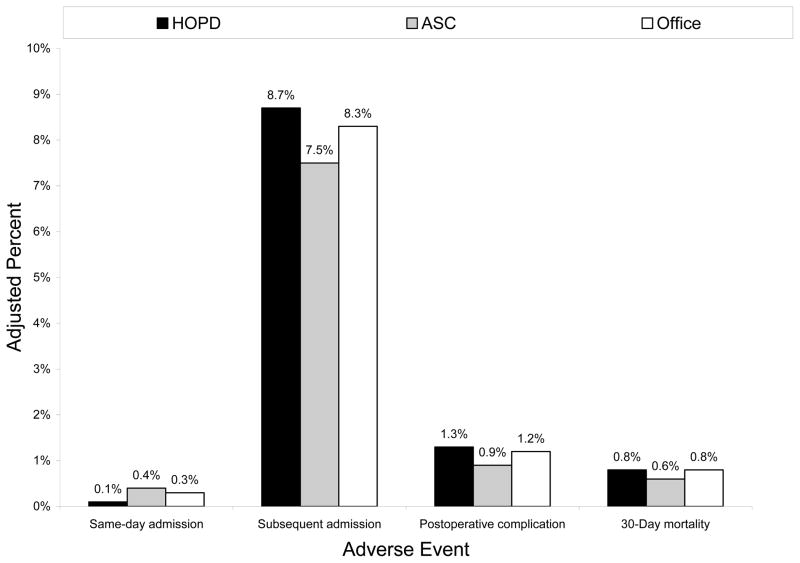
On multivariable regression, male gender, increasing age, and level of comorbidity were associated with higher odds of an adverse event (Table 2). Compared with hospitals, rates of postoperative complication were significantly lower at ASCs (Table 2). However, procedures performed outside the hospital were associated with a higher likelihood of a same-day admission [ASC: OR, 6.96 (95% CI, 4.44 – 10.90); office: OR, 3.64 (95% CI, 2.48 – 5.36)]. After adjusting for case mix differences, the probability of an adverse event was low across all ambulatory surgery settings (Figure 2).
Table 2.
Adjusted odds of an adverse event following outpatient surgery.
| Covariate | Odds Ratio (95% Confidence Interval)
|
30-Day Mortality | ||
|---|---|---|---|---|
| Same-Day Admission | Subsequent Admission | Postoperative Complication | ||
|
| ||||
| Location of care | ||||
| HOPD* | 1.00 (--) | 1.00 (--) | 1.00 (--) | 1.00 (--) |
| ASC | 6.96 (4.44 – 10.90) | 0.94 (0.88 – 1.01) | 0.69 (0.57 – 0.83) | 0.92 (0.61 – 1.39) |
| Office | 3.64 (2.48 – 5.36) | 0.97 (0.94 – 1.02) | 0.91 (0.82 – 1.00) | 0.98 (0.79 – 1.21) |
|
| ||||
| Age | ||||
| 65 – 74* | 1.00 (--) | 1.00 (--) | 1.00 (--) | 1.00 (--) |
| 75 – 84 | 1.51 (1.28 – 1.78) | 1.30 (1.26 – 1.34) | 1.22 (1.13 – 1.31) | 1.18 (0.98 – 1.43) |
| ≥85 | 2.75 (2.25 – 3.36) | 1.70 (1.63 – 1.77) | 1.23 (1.10 – 1.38) | 1.91 (1.55 – 2.34) |
|
| ||||
| Gender | ||||
| Male* | 1.00 (--) | 1.00 (--) | 1.00 (--) | 1.00 (--) |
| Female | 0.66 (0.56 – 0.78) | 0.83 (0.80 – 0.85) | 0.70 (0.64 – 0.75) | 0.95 (0.80 – 1.12) |
|
| ||||
| Race | ||||
| White* | 1.00 (--) | 1.00 (--) | 1.00 (--) | 1.00 (--) |
| Black | 1.13 (0.85 – 1.50) | 0.97 (0.91 – 1.02) | 0.97 (0.84 – 1.12) | 0.82 (0.59 – 1.15) |
| Other | 0.53 (0.33 – 0.86) | 0.86 (0.80 – 0.93) | 0.91 (0.76 – 1.09) | 0.83 (0.53 – 1.32) |
|
| ||||
| Charlson score | ||||
| 0* | 1.00 (--) | 1.00 (--) | 1.00 (--) | 1.00 (--) |
| 1 | 2.33 (1.98 – 2.73) | 1.35 (1.31 – 1.39) | 1.26 (1.17 – 1.36) | 1.67 (1.41 – 1.99) |
| ≥2 | 4.18 (3.38 – 5.18) | 2.38 (2.28 – 2.49) | 1.81 (1.61 – 2.03) | 3.21 (2.62 – 3.92) |
|
| ||||
| Area of residence | ||||
| West* | 1.00 (--) | 1.00 (--) | 1.00 (--) | 1.00 (--) |
| South | 0.99 (0.80 – 1.24) | 1.06 (1.02 – 1.11) | 0.86 (0.78 – 0.95) | 0.97 (0.77 – 1.22) |
| Midwest | 1.11 (0.87 – 1.40) | 1.09 (1.04 – 1.14) | 0.94 (0.84 – 1.04) | 0.97 (0.76 – 1.25) |
| Northeast | 0.89 (0.69 – 1.15) | 0.86 (0.82 – 0.91) | 0.73 (0.65 – 0.82) | 0.97 (0.75 – 1.26) |
Abbreviations: ASC, ambulatory surgery center; CI, confidence interval; HOPD, hospital outpatient department; OR, odds ratio.
Indicates referent group.
Shaded table cells denote statistically significant associations.
Figure 2.
Predicted probability of an adverse event following surgery, stratified by ambulatory setting.
Abbreviations: ASC, ambulatory surgery center; HOPD, hospital outpatient department.
Note: In the bar chart, black, grey, and white shading indicate the HOPD, ASC, and office, respectively. Models adjusted for case mix (i.e., patient age, gender, race, comorbid status, area of residence) and calendar year.
Discussion
Our findings highlight a national trend toward increased use of lower-intensity ambulatory settings for several common outpatient urologic procedures. Outcomes varied significantly by location of care with ASCs outperforming hospitals on one dimension of surgical quality—a difference likely related to favorable patient selection. While hospital-based surgery was associated with a lower rate of same-day admission, it is important to note that the probability of any adverse event was exceedingly low, regardless of location of care.
To date, much of the literature on ambulatory surgical care in nonhospital-based facilities has focused on the issue of physician ownership and overuse.14, 15 Little empirical work has assessed the relationship between surgical quality and the setting for ambulatory surgery. Our results are concordant with prior research, showing ASCs and hospitals to have similar risk-adjusted postoperative mortality.5 However, unlike Vila and colleagues,16 we found death rates at ASCs and the office to be both low and comparable. This finding may relate, in part, to increased state-level regulation of office-based surgery.17
We also observed more frequent same-day admissions following outpatient surgery in ASCs versus hospitals. This observation contrasts with that from a previous Medicare analysis.18 It may be a reflection of the limited organizational resources at ASCs. For instance, a cautious urologist could exercise clinical judgment and admit a less healthy patient following an ASC-based surgery because the hospital would be better equipped to handle problems if they occurred. Alternatively, it may reflect decreased staffing hours at ASCs or efficiency priorities, whereby urologists at these facilities have a lower threshold to admit patients in order to maximize throughput rather than holding on to them in the post-anesthesia care unit.
Our study has several limitations that merit further discussion. First, there is clear selection bias in our study population. While we attempted to adjust for differences in case mixes between hospitals, ASCs, and the office, it is entirely possible that urologists recommend certain settings based on information that is not readily available in billing claims. Second, we used HCPCS codes to identify procedures of interest. Medical coding, which is intended for billing and reimbursement, may not accurately reflect how complex a surgery was. To the extent that significant heterogeneity in procedure complexity exists within a given procedure code, this could also account for quality differences across locations of care. Third, we used Medicare claims exclusively for our analysis, and our findings may not be generalizable to nonelderly Americans.
Limitations notwithstanding, our study has both clinical and policy implications. From a clinical perspective, these data highlight the need to consider a patient’s age and level of comorbidity when planning an outpatient procedure. As expected, we observed that older patients and those with more comorbid illnesses had higher odds of an adverse event. Being able to identify subgroups of patients at risk for an adverse event following ambulatory surgery will allow for more effective targeting of care delivery. From a policy perspective, large payers, like Medicare, may use our data to inform their payment systems for providers. For instance, they might consider directing patients to certain nonhospital-based facilities given the comparable quality that they provide.
Conclusions
In summary, we observed variation in surgical outcomes by location of care. However, adverse events following outpatient urologic surgery are exceedingly rare, regardless of location of care. Additional studies are needed to determine how episode costs vary across hospitals, ASCs, and the office. Insofar as ASCs and the office are more convenient for patients and/or increase surgeon productivity, these facilities may provide higher value care.
Acknowledgments
Funding: This project was supported, in part, by grant number R01HS018726 from the Agency for Healthcare Research and Quality. The content is solely the responsibility of the authors and does not necessarily represent the official views of the Agency for Healthcare Research and Quality.
Appendix Table 1.
Identifying the study population.
| Procedure Type | HCPCS | Description |
|---|---|---|
|
| ||
| Endoscopic bladder surgery | 51700 | Bladder irrigation, simple, lavage and/or instillation |
| 52000 | Cystourethroscopy (separate procedure) | |
| 52310 | Cystourethroscopy, with removal of foreign body, calculus, or ureteral stent from urethra or bladder (separate procedure); simple | |
| 52204 | Cystourethroscopy, with biopsy | |
| 52214 | Cystourethroscopy, with fulguration (including cryosurgery or laser surgery) of trigone, bladder neck, prostatic fossa, urethra, or periurethral glands | |
| 52224 | Cystourethroscopy, with fulguration (including cryosurgery or laser surgery) or treatment of MINOR (less than 0.5 cm) lesion(s) with or without biopsy | |
| 52234 | Cystourethroscopy, with fulguration (including cryosurgery or laser surgery) and/or resection of; SMALL bladder tumor(s) (0.5 to 2.0 cm) | |
|
| ||
| Endoscopic urethral surgery | 51715 | Endoscopic injection of implant material into the submucosal tissues of the urethra and/or bladder neck |
| 52281 | Cystourethroscopy, with calibration and/or dilation of urethral stricture or stenosis, with or without meatotomy, with or without injection procedure for cystography, male or female | |
|
| ||
| Endoscopic ureteral surgery | 52005 | Cystourethroscopy, with ureteral catheterization, with or without irrigation, instillation, or ureteropyelography, exclusive of radiologic service |
| 52332 | Cystourethroscopy, with insertion of indwelling ureteral stent | |
|
| ||
| Microwave therapy | 53850 | Transurethral destruction of prostate tissue; by microwave therapy |
|
| ||
| Prostate biopsy | 55700 | Biopsy, prostate; needle or punch, single or multiple, any approach |
| 55859 | Transperineal placement of needles or catheters into prostate for interstitial radioelement application, with or without cystoscopy | |
|
| ||
| Shockwave lithotripsy | 50590 | Lithotripsy, extracorporeal shock wave |
|
| ||
| Urethral dilation | 53600 | Dilation of urethral stricture by passage of sound or urethral dilator, male; initial |
| 53660 | Dilation of female urethra including suppository and/or instillation; initial | |
|
| ||
| Urodynamics procedures | 51725 | Simple cystometrogram |
| 51726 | Complex cystometrogram | |
| 51741 | Complex uroflowmetry | |
| 51784 | Electromyography studies of anal or urethral sphincter, other than needle, any technique | |
| 51795 | Voiding pressure studies; bladder voiding pressure, any technique | |
Abbreviation: HCPCS, Healthcare Common Procedure Coding System.
Appendix Table 2.
Identifying postoperative complications.
| ICD-9 Code | Description of Complication |
|---|---|
| 415.11 | Iatrogenic pulmonary embolism and infarction |
| 458.2 | Iatrogenic hypotension |
| 512.1 | Iatrogenic pneumothorax |
| 518.5 | Pulmonary insufficiency following trauma and surgery |
| 598.2 | Postoperative urethral stricture |
| 995.0 | Other anaphylactic shock |
| 995.4 | Shock due to anesthesia |
| 997.00 | Nervous system complications |
| 997.01 | Central nervous system complication |
| 997.02 | Iatrogenic cerebrovascular infarction or hemorrhage |
| 997.09 | Other nervous system complications |
| 997.1x | Cardiac complications |
| 997.2x | Peripheral vascular complications |
| 997.3x | Respiratory complications |
| 997.4x | Digestive complications |
| 997.5x | Urinary complications |
| 997.61 | Neuroma of amputation stump |
| 997.62 | Infection (chronic) |
| 997.69 | Other |
| 997.79 | Vascular complications of other vessels |
| 997.9x | Complications affecting other specified body systems, not elsewhere classified |
| 997.91 | Hypertension |
| 997.99 | Other |
| 998.0 | Postoperative shock |
| 998.00 | Postoperative shock |
| 998.1 | Hemorrhage or hematoma or seroma complicating a procedure |
| 998.10 | Hemorrhage or hematoma or seroma complicating a procedure |
| 998.11 | Hemorrhage complicating a procedure |
| 998.12 | Hematoma complicating a procedure |
| 998.13 | Seroma complicating a procedure |
| 998.2x | Accidental puncture or laceration during a procedure |
| 998.3x | Disruption of operative wound |
| 998.4 | Foreign body accidentally left during a procedure |
| 998.5x | Postoperative infection |
| 998.6x | Persistent postoperative fistula |
| 998.7 | Acute reaction to foreign substance accidentally left during a procedure |
| 998.80 | Other specified complications of procedures, not elsewhere classified |
| 998.81 | Emphysema (subcutaneous)(surgical) resulting from a procedure |
| 998.83 | Non-healing surgical wound |
| 998.89 | Other specified complications |
| 998.9 | Unspecified complication of procedure, not elsewhere classified |
| 999.1 | Air embolism |
| 999.2x | Other vascular complications |
| 999.3x | Other infection |
| 999.5 | Other serum reaction |
| 999.7 | Rh incompatibility reaction |
| 999.8 | Other transfusion reaction |
| 999.9 | Other and unspecified complications of medical care, not elsewhere classified |
Abbreviation: International Classification of Diseases, 9th Revision, Clinical Modification.
References
- 1.Owings MF, Kozak LJ. Ambulatory and inpatient procedures in the United States, 1996. Vital Health Stat. 1998;13(139):1. [PubMed] [Google Scholar]
- 2.Winter A. Comparing the mix of patients in various outpatient surgery settings. Health Aff (Millwood) 2003;22:68. doi: 10.1377/hlthaff.22.6.68. [DOI] [PubMed] [Google Scholar]
- 3.Cullen KA, Hall MJ, Golosinskiy A. Ambulatory surgery in the United States, 2006. Natl Health Stat Report. 2009;(11):1. [PubMed] [Google Scholar]
- 4.Rohrich RJ, White PF. Safety of outpatient surgery: is mandatory accreditation of outpatient surgery centers enough? Plast Reconstr Surg. 2001;107:189. doi: 10.1097/00006534-200101000-00031. [DOI] [PubMed] [Google Scholar]
- 5.Chukmaitov AS, Menachemi N, Brown LS, Suanders C, Brooks RG. A comparative study of quality outcomes in freestanding ambulatory surgery centers and hospital-based outpatient departments: 1997–2004. Health Serv Res. 2008;43:1485. doi: 10.1111/j.1475-6773.2007.00809.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Medicare Payment Advisory Commission. [Accessed on May 28, 2011];Report to the Congress: Medicare payment policy. 2004 Available at: http://www.medpac.gov/documents/Mar04_Entire_reportv3.pdf.
- 7.Birkmeyer JD, Finlayson SR, Tosteson AN, Sharp SM, Warshaw AL, Fisher ES. Effect of hospital volume on in-hospital mortality with pancreaticoduodenectomy. Surgery. 1999;125:250. [PubMed] [Google Scholar]
- 8.Birkmeyer JD, Warshaw AL, Finlayson SR, Grove MR, Tosteson AN. Relationship between hospital volume and late survival after pancreaticoduodenectomy. Surgery. 1999;126:178. [PubMed] [Google Scholar]
- 9.Birkmeyer JD, Stukel TA, Siewers AE, Goodney PP, Wennberg DE, Lucas FL. Surgeon volume and operative mortality in the United States. N Engl J Med. 2003;349:2117. doi: 10.1056/NEJMsa035205. [DOI] [PubMed] [Google Scholar]
- 10.Jencks SF, Williams MV, Coleman EA. Rehospitalizations among patients in the Medicare fee-for-service program. N Engl J Med. 2009;360:1418. doi: 10.1056/NEJMsa0803563. [DOI] [PubMed] [Google Scholar]
- 11.Iezzoni LI, Daley J, Heeren T, Foley SM, Fisher ES, Duncan C, et al. Identifying complications of care using administrative data. Med Care. 1994;32:700. doi: 10.1097/00005650-199407000-00004. [DOI] [PubMed] [Google Scholar]
- 12.Klabunde CN, Potosky AL, Legler JM, Warren JL. Development of a comorbidity index using physician claims data. J Clin Epidemiol. 2000;53:1258. doi: 10.1016/s0895-4356(00)00256-0. [DOI] [PubMed] [Google Scholar]
- 13.White H. A heteroskedasticity-consistent covariance matrix estimator and a direct test for heteroskedasticity. Econometrica. 1980;48:817. [Google Scholar]
- 14.Hollingsworth JM, Ye Z, Stope SA, Krein SL, Hollenbeck AT, Hollenbeck BK. Physician-ownership of ambulatory surgery centers linked to higher volume of surgeries. Health Aff (Millwood) 2010;29:683. doi: 10.1377/hlthaff.2008.0567. [DOI] [PubMed] [Google Scholar]
- 15.Hollingsworth JM, Krein SL, Ye Z, Kim HM, Hollenbeck BK. Opening of ambulatory surgery centers and procedure use in elderly patients: Data from Florida. Arch Surg. 146:187. doi: 10.1001/archsurg.2010.335. [DOI] [PubMed] [Google Scholar]
- 16.Vila H, Jr, Soto R, Cantor AB, Mackey D. Comparative outcomes analysis of procedures performed in physician offices and ambulatory surgery centers. Arch Surg. 2003;138:991. doi: 10.1001/archsurg.138.9.991. [DOI] [PubMed] [Google Scholar]
- 17.Lapetina EM, Armstrong EM. Preventing errors in the outpatient setting: a tale of three states. Health Aff (Millwood) 2002;21:26. doi: 10.1377/hlthaff.21.4.26. [DOI] [PubMed] [Google Scholar]
- 18.Fleisher LA, Pasternak LR, Herbert R, Anderson GF. Inpatient hospital admission and death after outpatient surgery in elderly patients: importance of patient and system characteristics and location of care. Arch Surg. 2004;139:67. doi: 10.1001/archsurg.139.1.67. [DOI] [PubMed] [Google Scholar]