Abstract
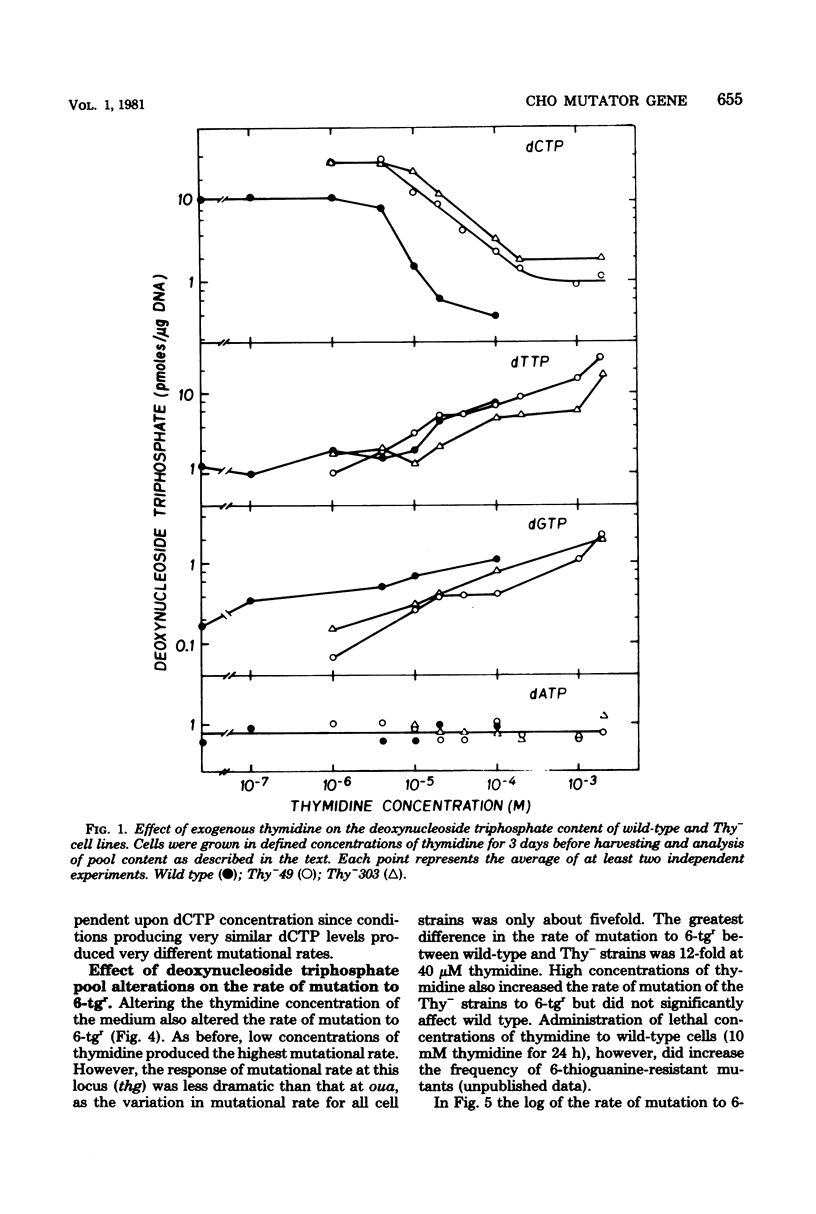
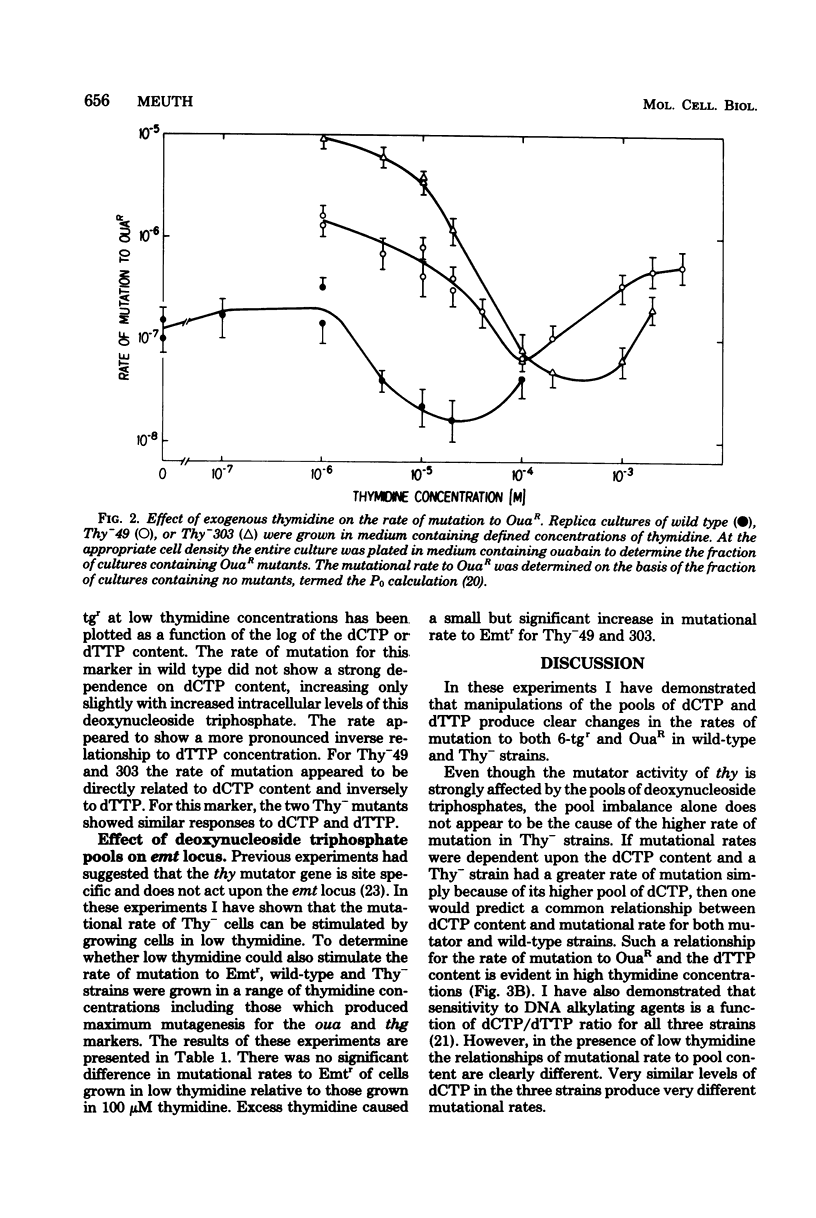
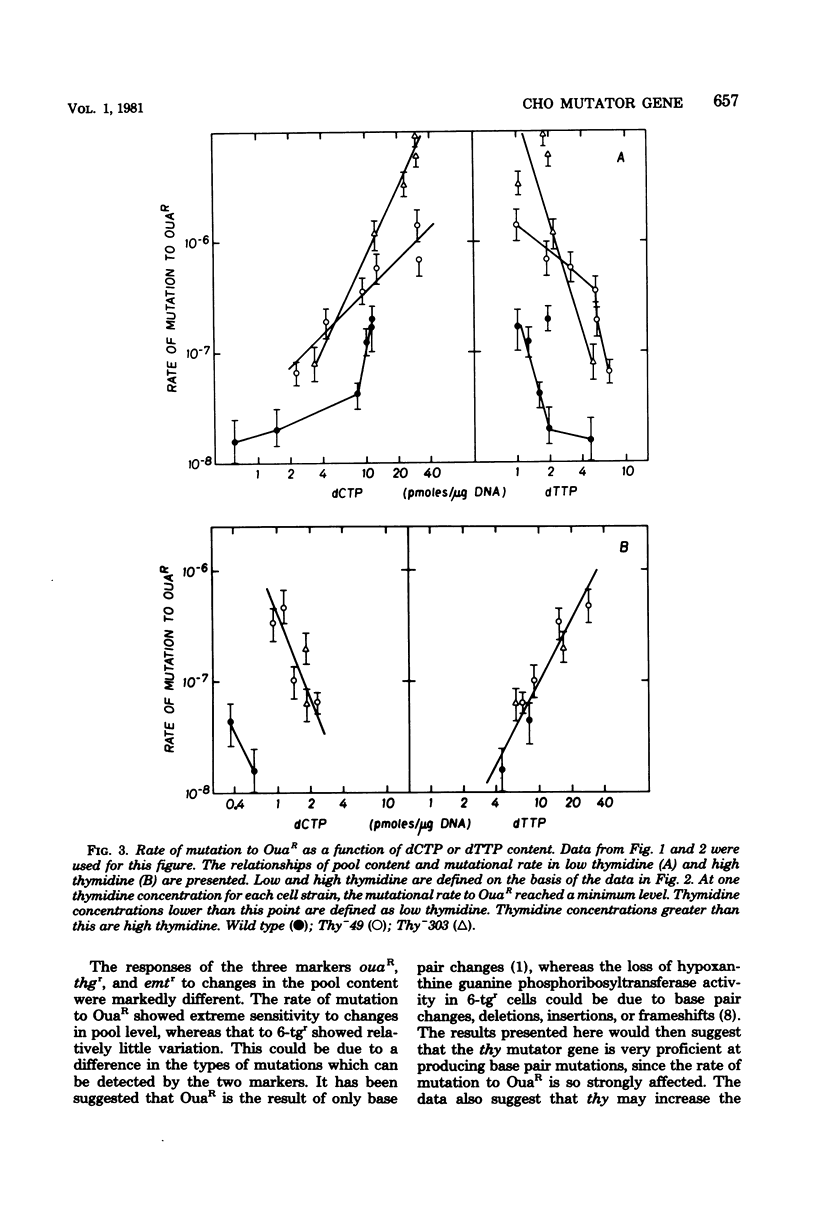
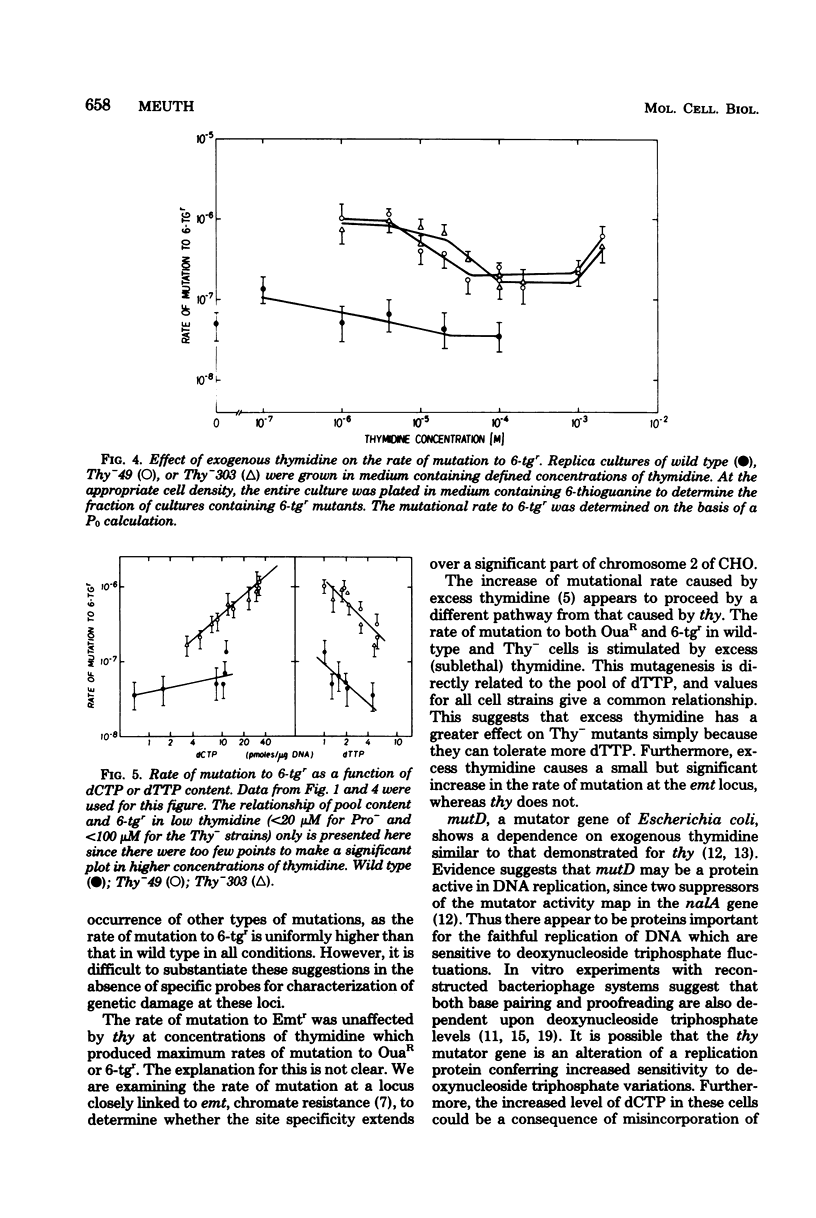
The Thy- mutants of Chinese hamster ovary cells have a 5- to 10-fold elevated pool of deoxycytidine 5'-triphosphate (dCTP) and are auxotrophic for thymidine as an apparent consequence of a single mutation. thy is also a mutator gene, elevating the spontaneous rate of mutation 5- to 200-fold for at least two genetic markers. Previous experiments suggested that this mutator activity was caused by the elevated pool of dCTP in Thy- cells. To test this, the dCTP and deoxythymidine 5'-triphosphate (dTTP) pools were manipulated by altering the external concentration of thymidine in the growth medium. The rate of mutation at one genetic locus, ouabain resistance, was directly related to cellular dCTP content. At the highest level of dCTP the rate in one Thy- strain was approximately 200 times that of wild-type cells. However, the relationship between dCTP content and the rate of mutation at the ouabain locus was different for two mutator strains and wild-type cells. The rate of mutation at a second locus, thioguanine resistance, was increased approximately 10-fold over wild type regardless of the dCTP-dTTP pools. These experiments suggest that the mutator activity of thy is clearly related to dCTP content, but the dCTP level alone does not appear to be the cause of the mutator.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bjursell G., Reichard P. Effects of thymidine on deoxyribonucleoside triphosphate pools and deoxyribonucleic acid synthesis in Chinese hamster ovary cells. J Biol Chem. 1973 Jun 10;248(11):3904–3909. [PubMed] [Google Scholar]
- Boersma D., McGill S. M., Mollenkamp J. W., Roufa D. J. Emetine resistance in Chinese hamster cells is linked genetically with an altered 40S ribosomal subunit protein, S20. Proc Natl Acad Sci U S A. 1979 Jan;76(1):415–419. doi: 10.1073/pnas.76.1.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley M. O., Sharkey N. A. Mutagenicity of thymidine to cultured Chinese hamster cells. Nature. 1978 Aug 10;274(5671):607–608. doi: 10.1038/274607a0. [DOI] [PubMed] [Google Scholar]
- Brennand J., Fox M. Excess thymidine is not mutagenic in Chinese hamster V79 fibroblasts. Cell Biol Int Rep. 1980 Oct;4(10):923–932. doi: 10.1016/0309-1651(80)90194-0. [DOI] [PubMed] [Google Scholar]
- Campbell C. E., Worton R. G. Linkage of genetic markers emt and chr in Chinese hamster cells. Somatic Cell Genet. 1980 Mar;6(2):215–224. doi: 10.1007/BF01538797. [DOI] [PubMed] [Google Scholar]
- Caskey C. T., Kruh G. D. The HPRT locus. Cell. 1979 Jan;16(1):1–9. doi: 10.1016/0092-8674(79)90182-x. [DOI] [PubMed] [Google Scholar]
- Chan T. S. Deoxyguanosine toxicity on lymphoid cells as a cause for immunosuppression in purine nucleoside phosphorylase deficiency. Cell. 1978 Jul;14(3):523–530. doi: 10.1016/0092-8674(78)90238-6. [DOI] [PubMed] [Google Scholar]
- Chen T. R. In situ detection of mycoplasma contamination in cell cultures by fluorescent Hoechst 33258 stain. Exp Cell Res. 1977 Feb;104(2):255–262. doi: 10.1016/0014-4827(77)90089-1. [DOI] [PubMed] [Google Scholar]
- Clayton L. K., Goodman M. F., Branscomb E. W., Galas D. J. Error induction and correction by mutant and wild type T4 DNA polymerases. Kinetic error discrimination mechanisms. J Biol Chem. 1979 Mar 25;254(6):1902–1912. [PubMed] [Google Scholar]
- Cox E. C. Bacterial mutator genes and the control of spontaneous mutation. Annu Rev Genet. 1976;10:135–156. doi: 10.1146/annurev.ge.10.120176.001031. [DOI] [PubMed] [Google Scholar]
- Degnen G. E., Cox E. C. Conditional mutator gene in Escherichia coli: isolation, mapping, and effector studies. J Bacteriol. 1974 Feb;117(2):477–487. doi: 10.1128/jb.117.2.477-487.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksson S., Thelander L., Akerman M. Allosteric regulation of calf thymus ribonucleoside diphosphate reductase. Biochemistry. 1979 Jul 10;18(14):2948–2952. doi: 10.1021/bi00581a005. [DOI] [PubMed] [Google Scholar]
- Fersht A. R. Fidelity of replication of phage phi X174 DNA by DNA polymerase III holoenzyme: spontaneous mutation by misincorporation. Proc Natl Acad Sci U S A. 1979 Oct;76(10):4946–4950. doi: 10.1073/pnas.76.10.4946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrett C., Santi D. V. A rapid and sensitive high pressure liquid chromatography assay for deoxyribonucleoside triphosphates in cell extracts. Anal Biochem. 1979 Nov 1;99(2):268–273. doi: 10.1016/s0003-2697(79)80005-6. [DOI] [PubMed] [Google Scholar]
- Gupta R. S., Siminovitch L. The isolation and preliminary characterization of somatic cell mutants resistant to the protein synthesis inhibitor-emetine. Cell. 1976 Oct;9(2):213–219. doi: 10.1016/0092-8674(76)90112-4. [DOI] [PubMed] [Google Scholar]
- Gupta R. S., Siminovitch L. The molecular basis of emetine resistance in Chinese hamster ovary cells: alteration in the 40S ribosomal subunit. Cell. 1977 Jan;10(1):61–66. doi: 10.1016/0092-8674(77)90140-4. [DOI] [PubMed] [Google Scholar]
- Hibner U., Alberts B. M. Fidelity of DNA replication catalysed in vitro on a natural DNA template by the T4 bacteriophage multi-enzyme complex. Nature. 1980 May 29;285(5763):300–305. doi: 10.1038/285300a0. [DOI] [PubMed] [Google Scholar]
- Luria S. E., Delbrück M. Mutations of Bacteria from Virus Sensitivity to Virus Resistance. Genetics. 1943 Nov;28(6):491–511. doi: 10.1093/genetics/28.6.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meuth M., Aufreiter E., Reichard P. Deoxyribonucleotide pools in mouse-fibroblast cell lines with altered ribonucleotide reductase. Eur J Biochem. 1976 Dec;71(1):39–43. doi: 10.1111/j.1432-1033.1976.tb11087.x. [DOI] [PubMed] [Google Scholar]
- Meuth M., L'Heureux-Huard N., Trudel M. Characterization of a mutator gene in Chinese hamster ovary cells. Proc Natl Acad Sci U S A. 1979 Dec;76(12):6505–6509. doi: 10.1073/pnas.76.12.6505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meuth M. Role of deoxynucleoside triphosphate pools in the cytotoxic and mutagenic effects of DNA alkylating agents. Somatic Cell Genet. 1981 Jan;7(1):89–102. doi: 10.1007/BF01544750. [DOI] [PubMed] [Google Scholar]
- Moore E. C., Hurlbert R. B. Regulation of mammalian deoxyribonucleotide biosynthesis by nucleotides as activators and inhibitors. J Biol Chem. 1966 Oct 25;241(20):4802–4809. [PubMed] [Google Scholar]
- Prem veer Reddy G., Pardee A. B. Multienzyme complex for metabolic channeling in mammalian DNA replication. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3312–3316. doi: 10.1073/pnas.77.6.3312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichard P. From deoxynucleotides to DNA synthesis. Fed Proc. 1978 Jan;37(1):9–14. [PubMed] [Google Scholar]
- Skoog L., Nordenskjöld B. Effects of hydroxyurea and 1-beta-D-arabinofuranosyl-cytosine on deoxyribonucleotide pools in mouse embryo cells. Eur J Biochem. 1971 Mar 1;19(1):81–89. doi: 10.1111/j.1432-1033.1971.tb01290.x. [DOI] [PubMed] [Google Scholar]



