Abstract
Tetracyclines moderate inflammatory responses of various etiologies. We hypothesized that tetracyclines exerted, in addition to antimicrobial function, control over inflammation elicited by Borrelia burgdorferi. To model systemic effects we used the human monocytic cell line THP-1, and for effects in the central nervous system we employed rhesus monkey brain astrocytes and microglia. Cells were stimulated with live or sonicated B. burgdorferi, or the OspA lipoprotein, in the presence of increasing concentrations of doxycycline or minocycline. Both antibiotics significantly reduced the production of TNF-alpha, IL-6, and IL-8 in a dose-dependent manner in all cell types. Microarray analyses of the effect of doxycycline on gene transcription in spirochete-stimulated monocytes revealed that the NFκB and IKKα genes were down-regulated. Functionally, phosphorylation of IκBα and binding of NFκB to target DNA were both reduced in these cells. Our results prove the principle that tetracyclines may have a dual therapeutic effect in Lyme disease.
Keywords: Lyme disease, Lyme neuroborreliosis, Borrelia burgdorferi, tetracycline, doxycycline, minocycline, inflammation
INTRODUCTION
Lyme borreliosis is a tick-transmitted disease caused by the spirochete Borrelia burgdorferi. The spirochete can invade, and persist in, a variety of tissues. This persistence has been correlated with severe pathology and may be responsible for localized inflammation [1,2]. The association between tissue invasion and localized inflammation may be explained by the fact that the spirochete possesses potent cytokine-stimulatory properties. It has been reported that B. burgdorferi can induce in vitro the production of pro-inflammatory cytokines [3-7], and other inflammatory mediators such as chemokines [8,9] and nitric oxide [10]. Thus, inflammation is thought to play an important role in Lyme disease pathogenesis.
Antibiotics, doxycycline in particular, are used for the treatment of early and early-disseminated Lyme borreliosis [11]. Doxycycline also may be used to treat late Lyme arthritis, although for late neurologic manifestations of Lyme borreliosis ceftriaxone is recommended [11]. Doxycycline is a semi-synthetic antibiotic with a broad-spectrum of antimicrobial activity; it belongs to the tetracycline family [12]. All tetracyclines are bacteriostatic and exert this effect by inhibiting bacterial protein synthesis. Protein synthesis inhibition takes place at the ribosome, by preventing the amino-acyl tRNA from binding to the acceptor site on the mRNA-ribosome complex [13].
In recent years, tetracyclines have been shown to moderate host-cell inflammatory responses in a variety of scenarios [14]. Doxycycline and another tetracycline, minocycline, were reported to induce neuroprotective effects in animal models of cerebral ischemia [15-18]. In addition, minocycline was shown to have beneficial effects in animal models of Huntington's disease [19,20], Parkinson's diseases [21], Alzheimer's disease [22], amyotrophic lateral sclerosis [23], sclerosis multiple [24], and spinal cord injury [25]. The beneficial effects of the tetracyclines were shown to be related to a reduction of microglial activation and proliferation [16-17], with inhibition of inducible nitric oxide synthase (iNOS) and IL-1β expression [26,27]. Tetracyclines were shown to inhibit the p38 MAPK and NFκB pathways [22,26,28], which play an important role in controlling the expression of pro-inflammatory mediators. Moreover, these antibiotics may promote central nervous system (CNS) cell survival through inhibition of caspase-1 and caspase-3 activity [29,23,25]. Thus, tetracyclines may attenuate multiple processes involved in mediating inflammation and cell death.
In this study we hypothesized that tetracyclines could exert, in addition to their antimicrobial function, control over inflammatory effects elicited by B. burgdorferi spirochetes, their lipoproteins, or bacterial debris left in the tissues after bacterial death. We evaluated this hypothesis with experiments performed in vitro, using cells involved in the innate immune response both in the periphery and in the CNS. To model potential effects in the periphery we used the human monocytic cell line THP-1, and for effects in the CNS we employed primary cultures of rhesus monkey brain astrocytes and microglia. Here we present the results of this study.
MATERIALS AND METHODS
THP-1 cell culture
The human monocytic cell line THP-1 was obtained from the American Type Culture Collection (ATCC, product number). Cells were cultured in RPMI 1640 medium modified (RPMI-M) so as to contain 2 mM L-glutamine, 10 mM HEPES, 1 mM sodium pyruvate, 4.5 g/L glucose, and 1.5 g/L sodium bicarbonate (ATCC), and supplemented with 0.05 mM 2-mercaptoethanol (Sigma-Aldrich), 10% fetal bovine serum (Hyclone), 100 U/mL penicillin/100 μg/mL streptomycin (Gibco) at 37° in a humidified 5% CO2 atmosphere.
Primary cultures of glial cells
Brain tissues used in this study were collected from adult rhesus macaques (Macaca mulatta) of either Chinese or Indian origin. These animals were not infected with B. burgdorferi and were euthanized for purposes unrelated to this project. The procedure used for euthanasia was consistent with the recommendations of the American Veterinary Medical Association's Panel on Euthanasia. Tissue was removed from the cortical region of the brain, and immediately processed. Glial cells were isolated using a protocol previously described [29], and maintained in DMEM/F12 with L-glutamine and HEPES buffer, 10% fetal bovine serum (Hyclone), 0.5 ng/mL of granulocyte-macrophage colony-stimulating factor (GM-CSF) (Sigma-Aldrich), 100 U/mL penicillin/100 μg/mL streptomycin (DMEM-G) at 37°C in a humidified atmosphere of 5% CO2. After 14–21 days in culture, microglia were isolated by vigorously tapping the culture flasks. To obtain purified astrocytes, glial cells were incubated for 90 min in 10 mM L-leucine methyl ester (LME) (Sigma–Aldrich). Purity of astrocytes and microglial cultures was assessed by staining with specific microglia marker (anti-IBA), and was routinely of 99%.
Bacterial Culture
B. burgdorferi strain B31 (clone 5A19, possessing all plasmids) was cultured in BSK-H medium (Sigma-Aldrich) supplemented with 10% heat-inactivated (56°C for 30 min) rabbit serum (Sigma) and antimicrobial agents (rifampin, 50 mg/ml; phosphomycin, 200 mg/ml; amphotericin B, 8 mg/ml) at 34°C in a humidified atmosphere of 5% CO2–95% N2. For subsequent experiments, B. burgdorferi were washed with phosphate-buffered saline (PBS) and resuspended in DMEM-G or RPMI-M. No antibiotics were added in the RPMI-M or DMEM-G media. Spirochetal viability was confirmed after 24-h culture in these media using the LIVE/DEAD BacLight bacterial viability kit (Molecular Probes) according to the manufacturer's instructions. We observed a spirochetal viability of 80-85%.
Measurement of cytokine and chemokine concentrations
THP-1 cells (5 × 105/mL), microglia (2 × 104/mL) or astrocytes (5 × 104/mL) were pre-incubated for 24 in RPMI-M or DMEM-G (as appropriate, with no added penicillin/streptomycin) medium alone, or with added doxycycline hyclate or minocycline hydrochloride (Sigma-Aldrich) at final concentrations of 0.0025 mM, 0.005 mM, or 0.01 mM. The cells were washed in culture medium and incubated for 24 h with fresh doxycycline hyclate or minocycline hydrochloride in the appropriate culture medium alone or with added live B. burgdorferi (10 MOI), sonicated B. burgdorferi (quantity equivalent to 10 MOI), or 0.25 μg/mL recombinant lipidated outer surface protein A (L-OspA) (GlaxoSmithKline) for a period of 24 h. Cell-free culture supernatants were collected and assayed for the presence of TNF-alpha , IL-6, and IL-8 by sandwich ELISA (BD Bioscience), according to the manufacturer's instructions.
Cell viability
Cell suspensions were mixed with an equal volume of 0.4 % isotonic Trypan blue solution (Invitrogen). Total cell number and dye-accumulating cells (non-viable), were counted using a hemocytometer under a light microscope.
RNA isolation
RNA was isolated from 2 × 106 THP-1 cells. Cells were incubated in medium alone or were pretreated for 24 h with doxycycline hyclate at 0.01 mM, followed by incubation with fresh doxycycline hyclate at the stated concentration, and sonicated B. burgdorferi (10 MOI-equivalent) for 2, 8, or 12 h, after which RNA was collected using the RNeasy® kit (Qiagen) following the protocol supplied by the manufacturer. The DNA-free kit (Ambion) was used to remove DNA contamination. The RNA concentration was determined spectrophotometrically (absorbance at 260 nm).
Human DNA Microarray
Microarray experiments and analyses of data were performed according to previously described protocols [30]. We utilized the 44,544-element 70-mer Human Exonic Evidence-Based Oligonucleotide (HEEBO) microarray, supplied by the Stanford Function Genomics Facility (http://www.microarray.org/sfgf/heebo.doc). Five micrograms of mRNA from THP-1 cells was used to incorporate Cy3 (samples with no doxycycline) or Cy5 (samples with added doxycycline). Labeling, hybridization and scanning were performed as per previously described protocols [30].
Microarray analysis
Human microarrays were scanned in a dual-confocal continuous microarray scanner (GenePix 4000B), using GenePix Pro version 6.1 as the image-acquisition and extraction software. All of the microarray data were based on duplicate measurements, i.e. using RNA derived from two independent experiments with THP-1 cells in each of the experimental conditions described. The resulting text data were imported into Spotfire DecisionSite for Functional Genomics (Spotfire, Inc.) and filtered to remove unreliable data [31]. Data were then subjected to normalization (Locally Weighted Scatterplot Smoothing – LOWESS) using R-Bioconductor and subjected to statistical analysis [32]. Genes whose expression changed by 2.0 fold (with a corrected t-test P < 0.05) were considered to be differentially expressed in a statistically significant manner. Pathway analysis was performed by uploading significant dataset(s) into the Ingenuity Pathways Analysis (IPA) algorithm. Pathways that were perturbed in a statistically significant manner (P < 0.05) were included in the analysis.
Extraction of nuclear proteins
THP-1 cells at a density of 5 × 106 cells/mL were pretreated for 24 h with doxycycline hyclate or minocycline hydrochloride, each at a concentration of 0.005 mM, or 0.01 mM, or left in medium alone. Cells were then washed and incubated with fresh doxycycline hyclate at the same respective concentrations as above, and stimulated with sonicated B. burgdorferi (10 MOI) for a period of 2 or 4 h. Nuclear extracts were isolated using the Nuclear Extract Kit (Active Motif) according to the manufacturer's instructions. Protein concentrations in the nuclear extracts were determined with the Bradford assay (BioRad).
NFκB activation assay
Nuclear extracts (2 μg) were assayed for NF-κB DNA-binding activity using the TransAM™ ELISA kit following the manufacturer's protocol (Active Motif). The NFκB TransAM kit includes a 96-well plate with immobilized double-stranded oligonucleotides (5’ -GGGACTTTCC -3’), which specifically bind to the active form of NFκB contained in the nuclear extract. The primary antibody directed against the NFκB p65 subunit recognizes the transcriptionally active NFκB complex bound to the oligonucleotide. A secondary antibody conjugated to horseradish peroxidase (HRP) provides the colorimetric readout (after adding a proprietary color development reagent and stop solution), which is quantified spectrophotometrically at 450 nm, with a reference wavelength of 655 nm.
Western blot analysis
THP-1 cells (1 × 106/mL), pretreated for 24 h with doxycycline hyclate at a concentration of 0.01 mM or medium alone, were washed and incubated with fresh doxycycline hyclate (0.01 mM) and sonicated B. burgdorferi (10 MOI) for a period of 15, 30, or 60 min. Cells were lysed in RIPA buffer with Protease Inhibitor Cocktail (Pierce Biotechnology). Protein concentration was determined with the Bradford assay (BioRad). Equal amounts of sample (50 μg/lane) were separated in 12% acrylamide Tris-HCl pre-cast gels (BioRad), transferred to nitrocellulose Protran membrane (Schleicher and Schuell BioScience), and blocked in TPBS (PBS with 0.05% Tween 20) with 3% BSA fraction V (Sigma-Aldrich). Membranes were probed with primary antibody against Phospho-IκB-α (Cell Signalling Technology) (1:1000), and antibody to β-tubulin (ABCAM) (1:6000), followed by incubation with the appropriate secondary antibody (Santa Cruz Biotechnology), conjugated with horseradish peroxidase. Immunoreactive proteins were visualized by using 3,3’-diaminobenzidine as chromogen.
Statistical analysis
Results are presented as means ± SD of the number of determinations specified in each case. Cytokine concentrations and NFκB activation assay OD values were examined by one-way ANOVA, using GraphPad PRISM 3.0 (GraphPad Software). Differences were considered significant at P< 0.05.
RESULTS
Effects of doxycycline and minocycline treatment on the production of pro-inflammatory mediators by cells stimulated with L-OspA, live, and sonicated B. burgdorferi
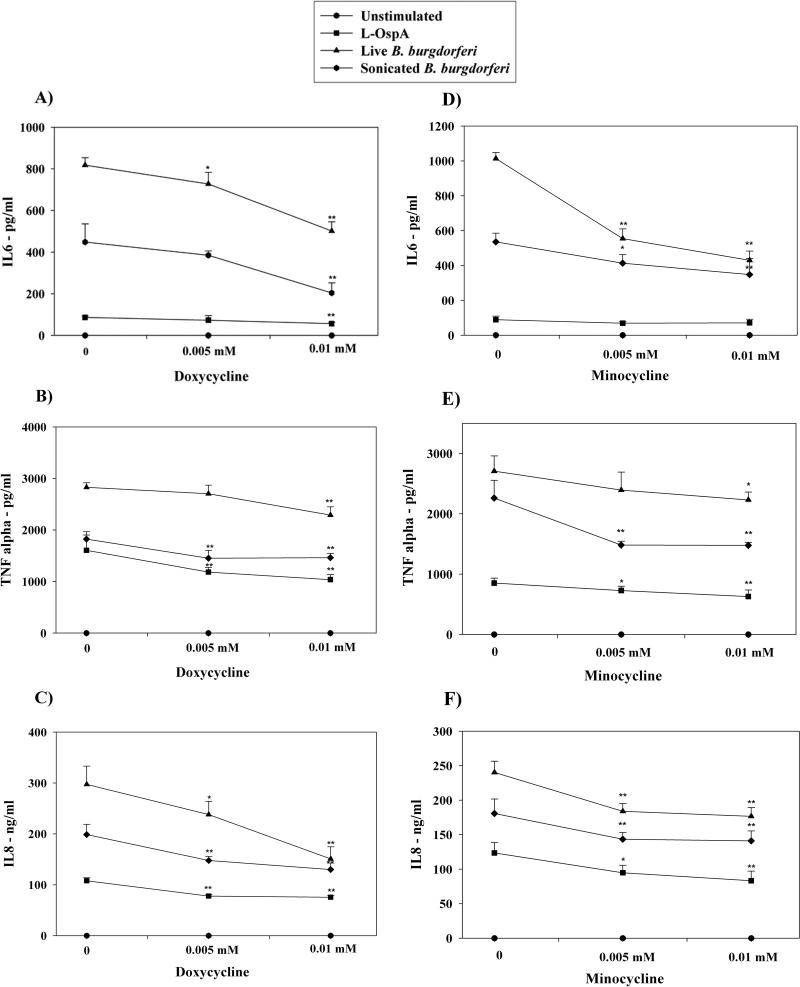
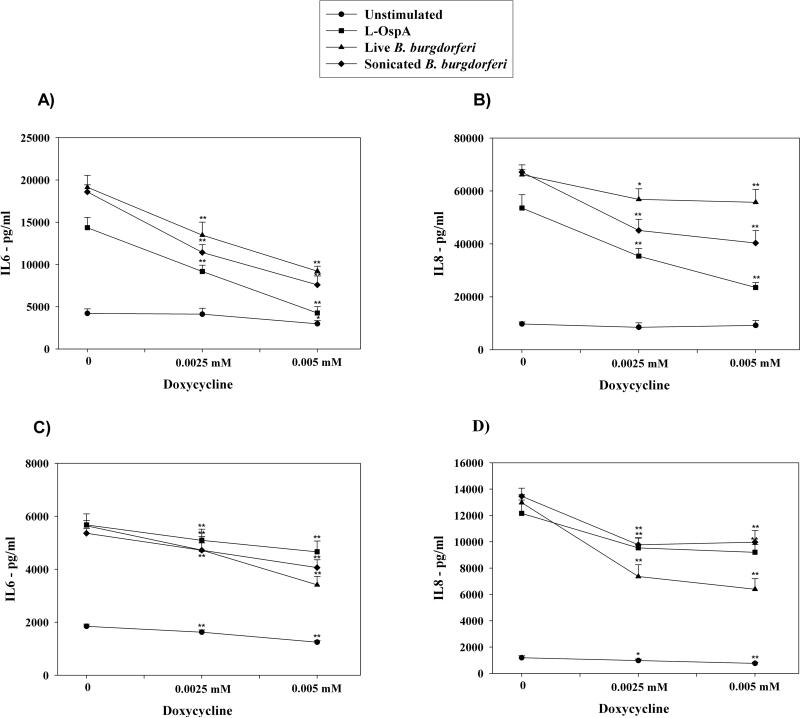
In order to explore the effects of doxycycline and minocycline on inflammation elicited by B. burgdorferi or by spirochetal antigens we quantified the differential production of three pro-inflammatory mediators, IL-6, IL-8, and TNF-alpha in three different cell types, THP-1 monocytes, astrocytes, and microglia. Following pre-treatment with medium alone, or increasing concentrations of doxycycline or minocycline, cells were stimulated for 24 h with L-OspA, live, or sonicated B. burgdorferi, in the presence of fresh antibiotics at the same concentrations as above. The production of TNF-alpha, IL-6, and IL-8 was significantly reduced by doxycycline and minocycline in a dose-dependent manner both in stimulated THP-1 cells (Fig. 1), and in glial cells (Fig. 2). The most marked anti-inflammatory effect was that of doxycycline on microglia. At a doxycycline concentration of 0.005 mM the production of IL-6 by these cells after stimulation with L-OspA, live, or sonicated B. burgdorferi was diminished by 70.4%, 51.9%, and 59.2%, respectively, and that of IL-8 by 56.1%, 15.8%, and 39.9% (Fig 2 A and B). The concentrations of doxycycline or minocycline up to 0.005 mM for glial cells and 0.01 mM for THP-1 cells were found to be nontoxic. Thus, based on viability experiments, greater than 95% of glial cells and 92% of THP-1 cells were viable at 0.005 mM and 0.01 mM doxycycline, respectively.
Figure 1. Doxycycline and minocycline affect the secretion of pro-inflammatory mediators by stimulated THP-1 cells.
Concentrations of IL-6 (A, D), TNF-alpha (B, E), and IL-8 (C, F) in THP-1 cells were determined after incubation in medium alone or with 0.005 mM, or 0.01 mM of doxycycline (1) or minocycline (2) for 24 h, followed by incubations with fresh doxycycline at the above concentrations, and with 0.25 μg/ml L-OspA, live, sonicated B. burgdorferi (both at 10 MOI), or no stimulant. Shown are the mean values ± SD obtained from triplicate specimens. * (P<0.05) and ** (P<0.005) indicates significant differences between untreated and doxycycline/minocycline-treated cells in a dose-dependent manner. Similar results were obtained with supernatants from cells of 3 additional independent experiments.
Figure 2. Doxycycline markedly reduces the secretion of pro-inflammatory mediators in stimulated primary glial cells.
IL-6 (A, C) and IL-8 (B, D) production by microglia (1) and astrocytes (2) was measured after pre-incubation of the cells in medium alone or pre-treatment with 0.0025 mM, or 0.005 mM of doxycycline for 24 h, followed by 24 h incubation with fresh doxycycline at the above concentrations and 0.25 μg/ml L-OspA, live, sonicated B. burgdorferi (both at 10 MOI), or not stimulant. Shown are the mean values ± SD obtained from triplicate specimens. * (P<0.05) and ** (P<0.005) indicates significant differences between untreated and doxycycline-treated cells in a dose-dependent manner. Similar results were obtained with supernatants obtained from cells purified from 3 additional rhesus macaques.
Microarray analysis of gene expression in stimulated THP-1 cells treated with doxycycline
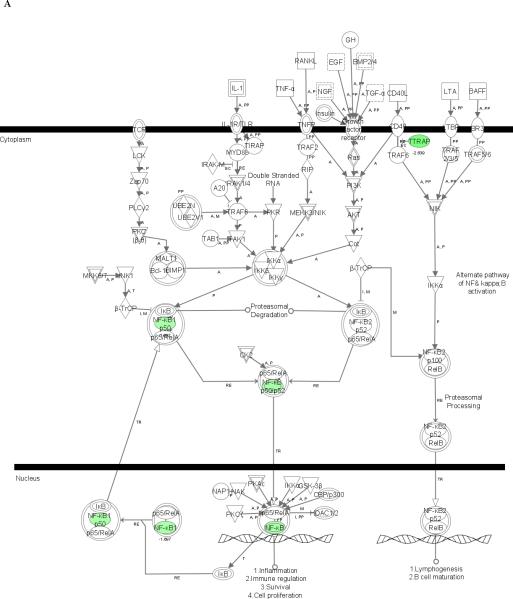
To further evaluate the effects of doxycycline on control of inflammation, we employed microarray analysis to detect genes in THP-1 cells whose expression upon stimulation with sonicated B. burgdorferi was affected by doxycycline. We observed that the expression of 326 genes (148 up-regulated and 178 down-regulated) was altered in THP-1 cells upon treatment with 0.01 mM doxycycline and 2-h stimulation with sonicated B. burgdorferi (Table 1, supplementary data). Using the Ingenuity Pathways Analysis (IPA) algorithm to examine pertinent regulatory pathways affected by doxycycline, we identified one such pathway in which the NFκB gene, which encodes a transcription factor that regulates the expression of a large number of genes involved in inflammation, was among those found to be down-regulated (Fig. 3A).
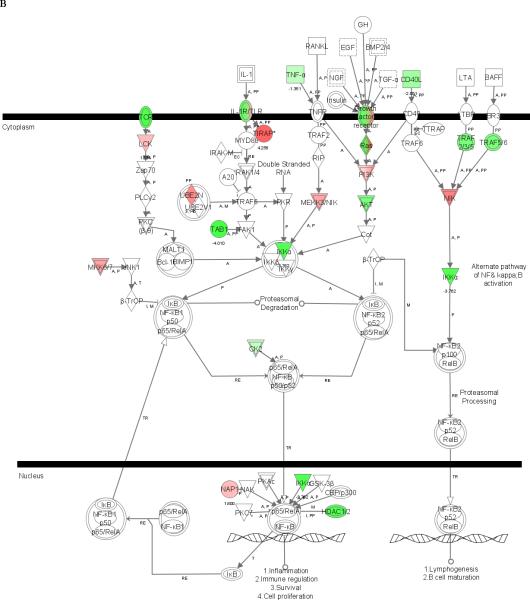
Figure 3. Microarray analysis showing the NFκB signaling pathway affected by doxycycline in stimulated THP-1 cells.
Cells were incubated in medium alone or pretreated with 0.01 mM of doxycycline for 24 h, followed by incubation with fresh 0.01 mM doxycycline and sonicated B. burgdorferi (10 MOI-equivalent) for 2 or 12 h. Pathway analysis was performed using Ingenuity Pathways Analysis (IPA) software, version 5.0. Two pathways with high significance of genes regulated by doxycycline in THP-1 cells at 2 h (A) and 12 h (B) were identified and merged. Color indicates the degree of up- (red) or down-regulation (green) of the genes identified by microarray analysis and others are those associated with the regulated genes based on the pathway analysis.
To expand our evaluation of the doxycycline effect, we performed microarray analysis of doxycycline-treated THP-1 cells incubated with sonicated B. burgdorferi for 12 h. Upon expanding the stimulation time, the number of genes affected by doxycycline increased to 3567, with 1890 up-regulated and 1677 down-regulated (Table 1, supplementary data). IPA revealed a significantly perturbed pathway that involved the IKKα (CHUK) gene, which encodes a catalytic subunit of the IKK kinase complex, a critical mediator for NFκB activation (Fig. 3B). We also examined by IPA whether doxycycline had an effect on MAPK pathways that are known to be affected by tetracyclines in microglia stimulated with LPS, namely, p38, c-Jun-N-terminal activated protein kinase (JNK) 1/2 and extracellular signal regulated kinase (ERK) 1/2 [28]. Of these, only the JNK pathway was significantly perturbed (not shown).
Effect of Doxycycline on the DNA binding activity of NFκB in stimulated THP-1 cells
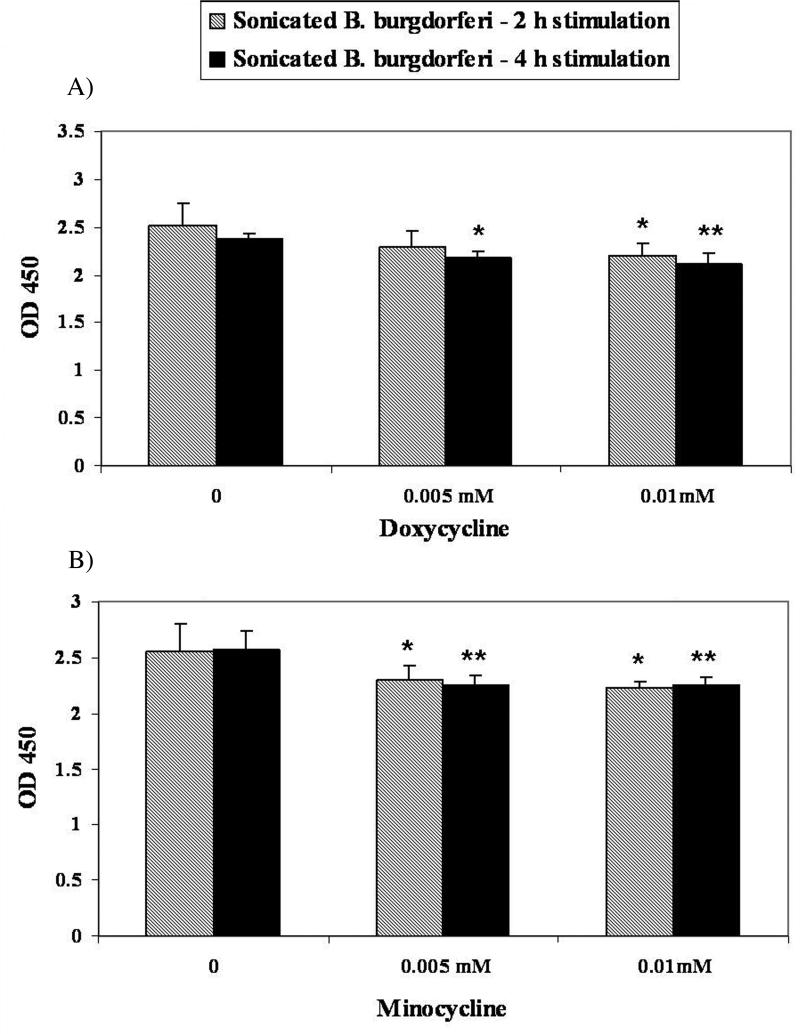
To investigate if the effects of doxycycline on NFκB expression observed at the mRNA level were functionally manifest we quantified functional NFκB in nuclear extracts of THP-1 cells that had been incubated with sonicated B. burgdorferi for 2 h and 4h in the presence of increasing concentrations of doxycycline or minocycline. The NFκB binding activity was significantly reduced by incubation with both of the tetracyclines in a dose-dependent manner (Fig. 4).
Figure 4. DNA-binding activity of NFκB in untreated and doxycycline/minocycline-treated stimulated THP-1 cells.
The activity of NFκB subunit p65 in THP-1 cells was measured using the TransAM ELISA kit. Cells were incubated in medium alone or treated with doxycycline or minocycline at a concentration of 0.005 mM, or 0.01 mM, followed by incubation with sonicated B. burgdorferi (10 MOI-equivalent) for 2h or 4h. Shown are the mean values obtained from triplicate specimens ± SD. *(P<0.05) and **(P<0.005) indicate a significant difference between untreated and doxycycline/minocycline-treated cells in a dose-dependent manner.
Effect of Doxycycline on the phosphorylation of IκBα in stimulated THP-1 cells
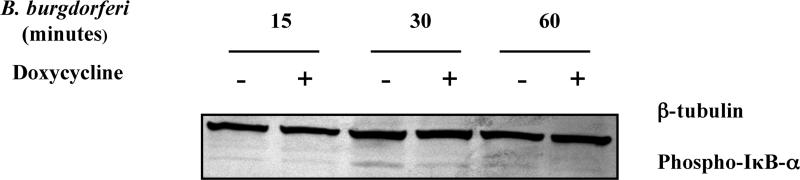
Western blot analysis was performed to test the hypothesis that the phosphorylation of IκBα is affected by the down-regulation of IKKα (CHUK) gene. IKKα and IKKβ kinase complex mediates the phosphorylation of IκB proteins, freeing NFκB for translocation into the nucleus. We demonstrated that the phosphorylation of IκBα is reduced in doxycycline pre-treated THP-1 cells after 30 min stimulation with sonicated B. burgdorferi (Fig. 5). The concentration of doxycycline was 0.01 mM.
Figure 5. Western blot analysis showing the effects of doxycycline on IκBα phosphorylation.
THP-1 cells were pre-treated for 24 hours with doxycycline at 0.01 mM, washed, and then incubated with fresh doxycycline followed by stimulation with sonicated B. burgdorferi for 15, 30, or 60 min. IκBα phosphorylation was determined by Western blot (A) using anti-phospho-IκBα antibody; β-tubulin was the control for equal protein loading. Similar results were obtained in three independent experiments.
DISCUSSION
Both doxycycline and minocycline exhibit an immune modulatory role in several neurodegenerative disease models [15-25,27]. Minocycline has been shown to exert an anti-inflammatory effect in CNS bacterial infection independently of its antimicrobial properties. Thus, a previous study demonstrated that minocycline modulates the acute host response to Staphylococcus aureus (a minocycline-resistant strain) in the mouse CNS parenchyma, reducing abscess-associated mortality and improving brain survival [33].
In Lyme borreliosis, the pathological processes caused by the spirochete B. burgdorferi are mainly associated with consistent and strong inflammatory responses [3-7]. We argued that tetracyclines, beyond their antimicrobial properties, might attenuate the inflammatory effects elicited by B. burgdorferi spirochetes, their lipoproteins, or bacterial debris left in the tissues after bacterial demise.
Previous reports have demonstrated that THP-1 cells respond to B. burgdorferi and their lipoproteins eliciting pro-inflammatory cytokines, such as TNF-alpha, IL1-beta and IL-6 [34,35]. Based on these studies, we used the THP-1 monocytic cell line to examine the anti-inflammatory properties of tetracyclines. Both doxycycline and minocycline were able to significantly reduce the release of TNF-alpha, IL-6, and IL-8 in stimulated THP-1 cells. Microarray analysis verified that this down-regulation of inflammatory mediators was not part of a general shut down of protein synthesis, as up to 1890 genes were significantly upregulated upon stimulation with sonicated B. burgdorferi for 12 h in the presence of doxycycline. Gene expression analysis also revealed the down-regulation of NFκB and IKKα, after 2 h and 12 h stimulation, respectively. NFκB is a transcription factor that regulates expression of a large number of genes that are critical for the regulation of cellular process, such as inflammatory responses, apoptosis, and cell proliferation [36,37]. NFκB activity is regulated by the IKKα and IKKβ kinase complex [38], which mediates the phosphorylation of IκB proteins, freeing NFκB for translocation into the nucleus to function as a transcription factor. Previous studies have shown that B. burgdorferi and spirochetal lipoprotein are able to induce the production of NFκB-dependent cytokines in monocytes [39,40]. Therefore, interfering with the NFκB activity could have critical effects on pro-inflammatory gene expression induced by B. burgdorferi. Recently, evidence has been presented [28] that minocycline inhibits IκB degradation preventing the translocation of NFκB to the nucleus. Considering that most of the stimuli that activate NFκB also activate IKK [41], and in view of our microarray results, we hypothesized that doxycycline moderates the production of pro-inflammatory mediators through the IKK/NFκB signaling pathway. We tested this hypothesis by determining the phosphorylation status of IκBα and quantifying functional NFκB in cell or nuclear extracts of stimulated THP-1 cells after pretreatment with doxycycline or minocycline. IκBα, the best characterized member of the IκB family, is a protein mainly regulated by phophorylation [41]. Our results showed that doxycycline may interfere in phophorylation processes and consequently in the degradation of the IκBα protein in THP-1 cells stimulated for 30 min with sonicated B. burgdorferi. We also demonstrated that NFκB DNA-binding activity was reduced by both doxycycline and minocycline.
Minocycline has been implicated in the inhibition of MAPK pathways in a stimulus-specific manner in microglia. Primary rat microglia stimulated with LPS or H2O2 showed enhanced phosphorylation of p38, ERK1/2, and JNK1/2. Minocycline (0.1 mM) had an inhibitory effect on all three of these pathways when the stimulant was LPS, but no effect at all when the stimulant was H2O2 [28]. When we examined by IPA the effect of doxycycline on these pathways in THP-1 cells stimulated with sonicated B. burgdorferi, only the JNK pathway was inhibited. This difference in outcome to that reported by Nikodemova et al. [28] admits several explanations, including response differences between human THP-1 monocytes and rat microglia, the use of a TLR2-activating stimulus (sonicated B. burgdorferi [29]) as opposed to LPS, which binds to TLR4, or the utilization of a 10-fold lower concentration of antibiotic (0.01 mM vs. 0.1 mM). The JNK pathway has recently been shown to mediate production of TNF-alpha in murine macrophages stimulated with B. burgdorferi lysates [42]. Thus, inhibition of this pathway by doxycycline, as was indicated by the IPA analysis, may have further contributed to the observed down-regulation of inflammatory mediator production.
Microglia are the primary immune-effector cells of the CNS and play an important role in initiation of inflammatory responses [43,44]. Inhibition of microglial activation and thereby of their release of pro-inflammatory and toxic molecules using tetracycline has been demonstrated in vitro [27,45] and in other models of neurodegeneration [16-19,22]. In our study, we observed that doxycycline markedly attenuated release of IL-6 and IL-8 that was elicited in primary microglia in response to L-OspA, live, or sonicated B. burgdorferi, in a dose-dependent manner. Furthermore, we demonstrated that doxycycline also influences the production of IL-6 and IL-8 elicited by the same stimuli in primary astrocytes. Astrocytes are the major glial cell in the brain. They have been shown to contribute to CNS inflammatory activity by expressing inflammatory cytokines and chemokines [46]. Our results suggest that doxycycline, an antibiotic that crosses the blood-brain barrier and which is used in Lyme disease chemotherapy [11], may affect one or more inflammatory pathways common to both cell types, thus inhibiting the ability of these cells, especially microglia, to produce pro-inflammatory mediators in response to B. burgdorferi. In fact, preliminary microarray analyses indicate that the expression of 460 genes (262 up-regulated and 198 down-regulated) was perturbed at 12 h of stimulation with sonicated B. burgdorferi upon treatment with doxycycline. Among the significantly down-regulated genes were TNF-alpha, IL-1, and IFN-alpha and beta (our unpublished data). As previous reports have shown, minocycline is associated with decrease of TNF-alpha and IL-1 beta expression in LPS-stimulated microglia [47,48]. Here, our preliminary data suggest that doxycycline affects complex transcriptional processes involving the expression of pro-inflammatory cytokines such as TNF-alpha.
About 10% of Lyme borreliosis patients that are treated for erythema migrans, the rash that in most patients heralds the infection, have subjective musculoskeletal, cognitive, or fatigue complaints at 12mo of follow-up; these symptoms correlate retrospectively with disseminated disease and a greater severity of illness at presentation [49]. The underlying mechanism for these subjective symptoms is unknown. These patients do not develop objective manifestations of late Lyme disease (e.g., Lyme arthritis), and lack evidence of persistence of infection by several different microbiological testing methods. These facts argue against the notion that their cause is a residual B. burgdorferi infection [50]. In view of our results, we submit that the beneficial effect of doxycycline in Lyme borreliosis therapy may not be confined to the antimicrobial properties of this antibiotic but may include an anti-inflammatory component. It follows that if patients with post-treatment symptoms experience such beneficial effect upon retreatment with doxycycline, this effect may be due to the anti-inflammatory properties of the antibiotic.
Supplementary Material
ACKNOWLEDGEMENTS
We thank Dorothy Scholl-Meeker, Tereance A. Myers, and Vida Dennis for advice.
This work was supported in part by grants NS048952 and RR00164 from the National Institutes of Health and by grant UO1-CI000153 from the Centers for Disease Control and Prevention.
Footnotes
The authors have no conflicts of interest in relation to this work.
REFERENCES
- 1.Barthold SW, Persing DH, Armstrong AL, Peeples RA. Kinetics of Borrelia burgdorferi dissemination and evolution of disease after intradermal inoculation of mice. Am J Pathol. 1991;139:263–73. [PMC free article] [PubMed] [Google Scholar]
- 2.Yang L, Weis JH, Eichwald E, Kolbert CP, Persing DH, Weis JJ. Heritable susceptibility to severe Borrelia burgdorferi-induced arthritis is dominant and is associated with persistence of large numbers of spirochetes in tissues. Infect Immun. 1994;62:492–500. doi: 10.1128/iai.62.2.492-500.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Defosse DL, Johnson RC. In vitro and in vivo induction of tumor necrosis factor alpha by Borrelia burgdorferi. Infect Immun. 1992;60:1109–13. doi: 10.1128/iai.60.3.1109-1113.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kenefick KB, Lim LC, Alder JD, Schmitz JL, Czuprynski CJ, Schell RF. Induction of interleukin-1 release by high- and low-passage isolates of Borrelia burgdorferi. J Infect Dis. 1993;167:1086–92. doi: 10.1093/infdis/167.5.1086. [DOI] [PubMed] [Google Scholar]
- 5.Tai KF, Ma Y, Weis JJ. Normal human B lymphocytes and mononuclear cells respond to the mitogenic and cytokine-stimulatory activities of Borrelia burgdorferi and its lipoprotein OspA. Infect Immun. 1994;62:520–8. doi: 10.1128/iai.62.2.520-528.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ganapamo F, Dennis V, Phillip MT. CD19(+) cells produce IFN-gamma in mice infected with Borrelia burgdorferi. Eur J Immunol. 2001;31:3460–8. doi: 10.1002/1521-4141(200112)31:12<3460::aid-immu3460>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 7.Knauer J, Siegemund S, Müller U, et al. Borrelia burgdorferi potently activates bone marrow-derived conventional dendritic cells for production of IL-23 required for IL-17 release by T cells. FEMS Immunol Med Microbiol. 2007;49:353–63. doi: 10.1111/j.1574-695X.2006.00210.x. [DOI] [PubMed] [Google Scholar]
- 8.Grygorczuk S, Pancewicz S, Zajkowska J, Kondrusik M, Rwierzbinska R, Hermanowska-Szpakowicz T. Concentrations of macrophage inflammatory proteins MIP-1alpha and MIP-1beta and interleukin 8 (IL-8) in Lyme borreliosis. Infection. 2004;32:350–5. doi: 10.1007/s15010-004-3110-4. [DOI] [PubMed] [Google Scholar]
- 9.Zhao Z, McCloud B, Fleming R, Klempner MS. Borrelia burgdorferi-induced monocyte chemoattractant protein-1 production in vivo and in vitro. Biochem Biophys Res Commun. 2007;358:528–33. doi: 10.1016/j.bbrc.2007.04.150. [DOI] [PubMed] [Google Scholar]
- 10.Seiler KP, Vavrin Z, Eichwald E, Hibbs JB, Jr, Weis JJ. Nitric oxide production during murine Lyme disease: lack of involvement in host resistance or pathology. Infect Immun. 1995;63:3886–95. doi: 10.1128/iai.63.10.3886-3895.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wormser GP, Dattwyler RJ, Shapiro ED, et al. The clinical assessment, treatment, and prevention of Lyme disease, human granulocytic anaplasmosis, and babesiosis: clinical practice guidelines by the Infectious Diseases Society of America. Clin Infect Dis. 2006;43:1089–134. doi: 10.1086/508667. [DOI] [PubMed] [Google Scholar]
- 12.Klein NC, Cunha BA. Tetracycline. Med Clin North Am. 1995;79:789–801. doi: 10.1016/s0025-7125(16)30039-6. [DOI] [PubMed] [Google Scholar]
- 13.Franklin TJ. The inhibition of incorporation of leucine into protein of cell-free systems from rat liver and Escherichia coli by chlortetracycline. Biochem J. 1963;87:449–53. doi: 10.1042/bj0870449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sapadin AN, Fleischmajer R. Tetracycline: nonantibiotic properties and their clinical implication. J Am Acad Dermatol. 2006;54:258–65. doi: 10.1016/j.jaad.2005.10.004. [DOI] [PubMed] [Google Scholar]
- 15.Clark WM, Lessov N, Lauten JD, Hazel K. Doxycycline treatment reduces ischemic brain damage in transient middle cerebral artery occlusion in the rat. J Mol Neurosci. 1997;9:103–08. doi: 10.1007/BF02736854. [DOI] [PubMed] [Google Scholar]
- 16.Yrjanheikki J, Keinanen R, Pellikka M, Hokfelt T, Koistinaho J. Tetracyclines inhibit microglial activation and are neuroprotective in global brain ischemia. Proc Natl Acad Sci. 1998;95:15769–74. doi: 10.1073/pnas.95.26.15769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yrjanheikki J, Tikka T, Keinanen R, Goldsteins G, Chain PH, Koistinaho J. A tetracycline derivative, minocycline, reduces inflammation and protects against focal cerebral ischemia with a wide therapeutic window. Proc Natl Acad Sci. 1999;96:13496–500. doi: 10.1073/pnas.96.23.13496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jantzie LL, Cheung PY, Todd KG. Doxycycline reduces cleaved caspase-3 and microglial activation in an animal model of neonatal hypoxia-ischemia. J Cereb Blood Flow Metab. 2005;25:314–24. doi: 10.1038/sj.jcbfm.9600025. [DOI] [PubMed] [Google Scholar]
- 19.Chen M, Ona VO, Li M, et al. Minocycline inhibits caspase-1 and caspase-3 expression and delays mortality in a transgenic mouse model of Huntington's disease. Nat Med. 2000;6:797–801. doi: 10.1038/77528. [DOI] [PubMed] [Google Scholar]
- 20.Wang X, Zhu S, Drozda M, et al. Minocycline inhibits caspase-independent and –dependent mitochondrial cell death pathways in models of Huntington's disease. Proc Natl Acad Sci. 2003;100:10483–7. doi: 10.1073/pnas.1832501100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Du Y, Ma Z, Lin S, et al. Minocycline prevents nigrostriatal dopaminergic neurodegeneration in the MPTP model of Parkinson's disease. Proc Natl Acad Sci USA. 2001;98:14669–74. doi: 10.1073/pnas.251341998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Choi Y, Kim HS, Shin KY, et al. Minocycline attenuates neuronal cell death and improves cognitive impairment in Alzheimer's disease models. Neuropsychopharmacology. 2007;32:2393–404. doi: 10.1038/sj.npp.1301377. [DOI] [PubMed] [Google Scholar]
- 23.Zhu S, Stavrovskaya IG, Drozda M, et al. Minocycline inhibits cytochrome c release and delays progression of amyotrophic lateral sclerosis in mice. Nature. 2002;417:74–8. doi: 10.1038/417074a. [DOI] [PubMed] [Google Scholar]
- 24.Brundula V, Rewcastle NB, Metz LM, Bernard CC, Yong VW. Targeting leukocyte MMPs and transmigration: minocycline as a potencial therapy for multiple sclerosis. Brain. 2002;125:1297–308. doi: 10.1093/brain/awf133. [DOI] [PubMed] [Google Scholar]
- 25.Lee SM, Yune TY, Kim SJ, et al. Minocycline reduces cell death and improves functional recovery after traumatic spinal cord injury in the rat. J Neurotrauma. 2003;20:1017–27. doi: 10.1089/089771503770195867. [DOI] [PubMed] [Google Scholar]
- 26.Tikka T, Fiebich BL, Goldsteins G, Keinanen R, Koistinaho J. Minocycline, a tetracycline derivative, is neuroprotective against excitotoxicity by inhibiting activation and proliferation of microglia. J Neurosci. 2001;21:2580–8. doi: 10.1523/JNEUROSCI.21-08-02580.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wu DC, Jackson-Lewis V, Vila M, et al. Blockade of microglial activation is neuroprotective in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine mouse model of Parkinson disease. J Neurosci. 2002;22:1763–71. doi: 10.1523/JNEUROSCI.22-05-01763.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nikodemova M, Ducan ID, Watters JJ. Minocycline exerts inhibitory effects on multiple mitogen-activated protein kinases and IκBα degradation in a stimulus-specific manner in microglia. J Neurochem. 2006;96:314–23. doi: 10.1111/j.1471-4159.2005.03520.x. [DOI] [PubMed] [Google Scholar]
- 29.Bernardino AL, Myers TA, Alvarez X, Hasegawa A, Philipp MT. Toll-like receptors: insights into their possible role in the pathogenesis of Lyme neuroborreliosis. Infect Immun. 2008 doi: 10.1128/IAI.00394-08. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tekautz TM, Zhu K, Grenet J, Kaushal D, Kidd VJ, Lahti JM. Evaluation of IFN-gamma effects on apoptosis and gene expression in neuroblastoma-preclinical studies. Biochim Biophys Acta. 2006;1763:1000–10. doi: 10.1016/j.bbamcr.2006.06.014. [DOI] [PubMed] [Google Scholar]
- 31.Kaushal D, Naeve CW. Baxevanis, et al., editors. Analyzing and Visualizing Expression Data with Spotfire. Curr Prot Bioinformatics. 2004:7.9.1–7.9.25. doi: 10.1002/0471250953.bi0709s7. Unit 7 Chapter 9. [DOI] [PubMed] [Google Scholar]
- 32.Yang YH, Dudoit S, Luu P, Lin DM, Peng V, Ngai J, Speed TP. Normalization for cDNA microarray data: a robust composite method addressing single and multiple slide systematic variation. Nucleic Acids Res. 2002;30:e15. doi: 10.1093/nar/30.4.e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kielian T, Esen N, Liu S, et al. Minocycline modulates neuroinflammation independently of its antimicrobial activity in Staphylococcus aureus-induced brain abscess. Am J Pathol. 2007;171:1199–213. doi: 10.2353/ajpath.2007.070231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Giambartolomei GH, Dennis V, Lasater BL, Philipp MT. Induction of pro- and anti-inflammatory cytokines by Borrelia burgdorferi lipoproteins in monocytes is mediated by CD14. Infect Immun. 1999;67:140–7. doi: 10.1128/iai.67.1.140-147.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Moore MW, Cruz AR, LaVake CJ, et al. Phagocytosis of Borrelia burgdorferi and Treponema pallidum potentiates innate immune activation and induces gamma interferon production. Infect Immun. 2007;75:2046–62. doi: 10.1128/IAI.01666-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang J, Ghosh S. Toll-like receptor-mediated NF-κB activation: a phylogenetically conserved paradigm in innate immunity. J Clin Invest. 2001;107:13–9. doi: 10.1172/JCI11837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Karin M, Lin A. NF-κB at the crossroad of life and death. Nat Immunol. 2002;3:221–7. doi: 10.1038/ni0302-221. [DOI] [PubMed] [Google Scholar]
- 38.Hayden MS, Ghosh S. Shared principles in NF-κB signaling. Cell. 2008;132:344–61. doi: 10.1016/j.cell.2008.01.020. [DOI] [PubMed] [Google Scholar]
- 39.Norgard MV, Arndt LL, Akins DR, Curetty LL, Harrich DA, Radolf JD. Activation of human monocytes cells by Trepanema pallidum and Borrelia burgdorferi lipoproteins and synthetic lipopropeptides proceeds via a pathway distinct from the lipopolysaccharide but involves the transcriptional activator NF-kappa B. Infect Immun. 1996;64:3845–52. doi: 10.1128/iai.64.9.3845-3852.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cruz AR, Moore MW, La Vake CJ, Eggers CH, Salazar JC, Radolf JD. Phagocytosis of Borrelia burgdorferi, the Lyme disease spirochete, potentiates innate immune activation and induces apoptosis in human monocytes. Infect Immun. 2008;76:56–70. doi: 10.1128/IAI.01039-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ghosh S, Karin M. Missing piece in the NF-κB puzzle. Cell. 2002;109:81–96. doi: 10.1016/s0092-8674(02)00703-1. [DOI] [PubMed] [Google Scholar]
- 42.Izadi H, Motameni AT, Bates TC, Olivera ER, Villar-Suarez V, Joshi I, Garg R, Osborne BA, Davis RJ, Rincón M, Anguita J. c-Jun N-terminal kinase 1 is required for Toll-like receptor 1 gene expression in macrophages. Infect Immun. 2007;75:5027–34. doi: 10.1128/IAI.00492-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gonzalez-Scarano F, Baltuch G. Microglia as mediators of inflammatory and degenerative disease. Annu Rev Neurosci. 1999;22:219–40. doi: 10.1146/annurev.neuro.22.1.219. [DOI] [PubMed] [Google Scholar]
- 44.Kreutzberg GW. Microglia: a sensor for pathological events in the CNS. Trends Neurosci. 1996;19:312–18. doi: 10.1016/0166-2236(96)10049-7. [DOI] [PubMed] [Google Scholar]
- 45.Familian A, Boshuizen RS, Eikelenboom P, Veerhuis R. Inhibitory effect of minocycline on amyloid β fibril formation and human microglial activation. Glia. 2006;53:233–40. doi: 10.1002/glia.20268. [DOI] [PubMed] [Google Scholar]
- 46.Dong Y, Benveniste EN. Immune function of astrocytes. Glia. 2001;36:180–90. doi: 10.1002/glia.1107. [DOI] [PubMed] [Google Scholar]
- 47.Lee SM, Yune TY, Kim SJ, et al. Minocycline inhibits apoptotic cell death via attenuation of TNF-α expression following iNOS/NO induction by lipopolysaccharide in neuron/glia co-cultures. J Neurochem. 2004;91:568–78. doi: 10.1111/j.1471-4159.2004.02780.x. [DOI] [PubMed] [Google Scholar]
- 48.Wang AL, Yu ACH, Lau LT, et al. Minocycline inhibits LPS-induced retinal microglia activation. Neurochem Int. 2005;47:152–8. doi: 10.1016/j.neuint.2005.04.018. [DOI] [PubMed] [Google Scholar]
- 49.Nowakowski J, Nadelman RB, Sell R, et al. Long-term follow-up of patients with culture-confirmed Lyme disease. Am J Med. 2003;115:91–6. doi: 10.1016/s0002-9343(03)00308-5. [DOI] [PubMed] [Google Scholar]
- 50.Klempner MS, Hu LT, Evans J, et al. Two controlled trials of antibiotic treatment in patients with persistent symptoms and a history of Lyme disease. N Engl J Med. 2001;345:85–92. doi: 10.1056/NEJM200107123450202. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.