Abstract
Background
Classical eyeblink conditioning (EBC) involves contingent temporal pairing of a conditioned stimulus (e.g., tone) with an unconditioned stimulus (e.g., air puff). Impairment of EBC has been demonstrated in studies of alcohol-exposed animals and in children exposed prenatally at heavy levels.
Methods
Fetal alcohol syndrome (FAS) was diagnosed by expert dysmorphologists in a large sample of Cape Coloured, South African children. Delay EBC was examined in a new sample of 63 children at 11.3 years, and trace conditioning in 32 of the same children at 12.8 years. At each age, two sessions of 50 trials each were administered on the same day; two more sessions the next day, for children not meeting criterion for conditioning.
Results
6 of 34 (17.6%) children born to heavy drinkers were diagnosed with FAS, 28 were heavily exposed nonsyndromal (HE), and 29 were non-exposed controls. Only 33.3% with FAS and 42.9% of HE met criterion for delay conditioning, compared with 79.3% of controls. The more difficult trace conditioning task was also highly sensitive to fetal alcohol exposure. Only 16.7% of the FAS and 21.4% of HE met criterion for trace conditioning, compared with 66.7% of controls. The magnitude of the effect of diagnostic group on trace conditioning was not greater than the effect on short delay conditioning, findings consistent with recent rat studies. Longer latency to onset and peak eyeblink CR in exposed children indicated poor timing and failure to blink in anticipation of the puff. Extended training resulted in some but not all of the children reaching criterion.
Conclusions
These data showing alcohol-related delay and trace conditioning deficits extend our earlier findings of impaired EBC in 5-year-olds to school-age. Alcohol-related impairment in the cerebellar circuitry required for both forms of conditioning may be sufficient to account for the deficit in both tasks. Extended training was beneficial for some exposed children. EBC provides a well-characterized model system for assessment of degree of cerebellar-related learning and memory dysfunction in fetal alcohol exposed children.
Keywords: Fetal Alcohol Syndrome, Delay Eyeblink Conditioning, Trace Eyeblink Conditioning, Fetal Alcohol Spectrum Disorder, Cerebellar-Mediated Temporal Processing
Descriptive studies spanning three decades have identified a broad range of cognitive and behavioral deficits in children with fetal alcohol spectrum disorder (FASD). FASD ranges from fetal alcohol syndrome (FAS), which is the most severe impairment characterized by a distinctive craniofacial dysmorphology, small head circumference, and pre- and/or postnatal growth retardation, to alcohol-related neurodevelopmental disorder (ARND), in which children exhibit significant cognitive and behavioral impairment but lack the distinctive facial anomalies (Hoyme et al., 2005). The long-term adverse effects associated with fetal alcohol exposure are increasingly well known, but many women continue to drink heavily during pregnancy in the U.S. (CDC, 2002) and throughout the world (e.g., Croxford & Viljoen, 1999; Riley et al., 2003; Jacobson et al., 2006). As many as 13% of infants born in the U.S. are exposed to varying levels of alcohol during pregnancy, with a higher percentage found among disadvantaged populations (CDC, 2002). Identification of alcohol affected children continues to be difficult due to the lack of specificity in behavioral diagnostic criteria and limited understanding of the pathophysiology of the disorder.
We have recently identified impaired eyeblink conditioning (EBC) to be a remarkably consistent deficit associated with fetal alcohol exposure (Jacobson et al., 2008). In the 5-year follow-up assessment of a cohort of heavily alcohol-exposed children recruited prenatally, not a single child with full FAS met criterion for conditioning, as contrasted with 75.0% of controls. A large proportion (63.8%) of the heavily alcohol-exposed nondysmorphic children also failed to meet criterion for conditioning at this age. These findings corroborate a report of poorer EBC in a U.S. sample of school-aged, alcohol-exposed children with dyslexia (Coffin et al., 2005). Our study was conducted in Cape Town, South Africa, where there is a very high prevalence of heavy alcohol use during pregnancy in the Cape Coloured (mixed ancestry) community (Croxford & Viljoen, 1999; Jacobson et al., 2008). The estimated incidence of FAS in this population is 18 to 141 times greater than in the U.S. and among the highest in the world (May et al., 2000). This population, composed mainly of descendants of white European settlers, Malaysian slaves, Khoi-San aboriginals, and black African ancestors, has historically comprised the large majority of workers in the wine-producing and fruit-growing region of the Western Cape. The high prevalence of FAS is a consequence of the very heavy maternal drinking during pregnancy commonly found in this community, due to poor psychosocial circumstances and the traditional dop system, in which farm laborers were paid, in part, with wine. Although the dop system has been outlawed, heavy alcohol consumption persists in certain sectors in urban and rural Cape Coloured communities (Carter et al., 2005; Jacobson et al., 2006), and weekend binge drinking is a major source of recreation for many in the community.
The EBC deficits we reported in alcohol-exposed children are consistent with animal studies showing that heavy exposure to alcohol during the equivalent of the third trimester of pregnancy in humans disrupts EBC in weanling and adult rats, a deficit that is mediated by a dose-dependent cell loss and altered neural activity in the deep cerebellar nuclei (Green et al., 2002a,b). Binge exposure during this period in rodents is also associated with loss of Purkinje and granule cells in the cerebellum (Dunty et al., 2001; Hamre & West, 1993), even after only 2 days of exposure (Thomas et al., 1998). Heavy alcohol exposure in rats and mice on even a single occasion during synaptogenesis has been found to trigger acute neurodegeneration via enhanced apoptosis of Purkinje cells and other neurons in key components of the neural circuit that mediates EBC, including the deep cerebellar nuclei, cerebellar cortex, pontine nuclei, and inferior olive (Dikranian et al., 2005; Green, 2004). The earliest autopsy studies in humans reporting damaging effects of heavy prenatal alcohol exposure identified errors in cell migration, agenesis or thinning of the corpus callosum, and anomalies in the cerebellum and brain stem (Jones & Smith, 1973; Clarren, 1977; Clarren & Smith, 1978). In the only study to perform a comprehensive morphometric analysis of the four major cortical lobes, cerebellum, and principal subcortical regions, Archibald et al. (2001) found a significant deficit in total brain volume, with proportionately greater reductions particularly in the cerebellum, parietal lobe, and caudate nucleus, including a 15% reduction in cerebellar volume in individuals with FAS. By contrast, hippocampal volume was not affected.
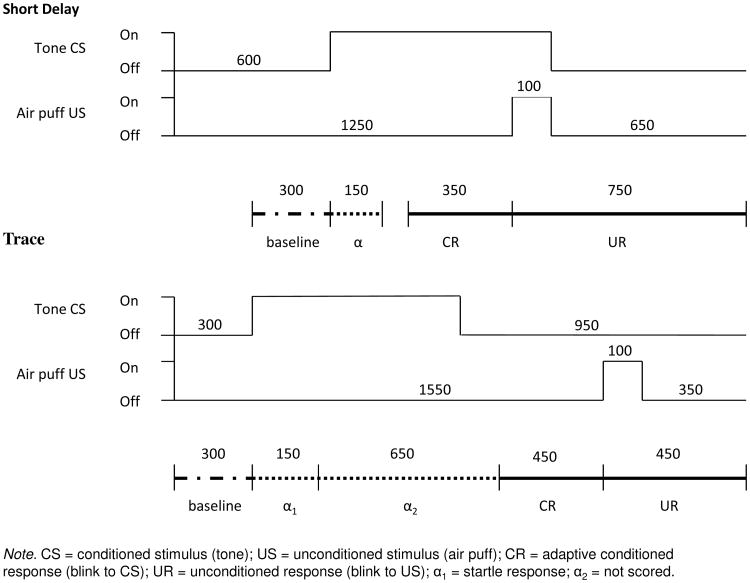
Pavlovian conditioning is a culturally neutral, non-verbal form of associative learning, in which the onset of a conditioned stimulus (CS), usually a pure tone, precedes an unconditioned stimulus (US), usually a mild air puff to the eye, which elicits a reflexive eyeblink unconditioned response (UR). With repeated pairings of the tone and air puff, the tone CS comes to elicit an eyeblink response on a large percentage of trials. This eyeblink conditioned response (CR) represents the learned association between tone and air puff. The operational simplicity and minimal sensory, motor, and motivational demands of the procedure make it applicable with little or no modification across a range of animal species—rodents, rabbits, monkeys, sheep, humans—and across the life-span, beginning in early infancy (Stanton, Claflin & Herbert, 2010). In delay conditioning, the tone CS precedes, overlaps, and co-terminates with the onset of the air puff, whereas in trace conditioning, there is a brief stimulus-free “trace interval” between the offset of the tone and the onset of the air puff (Fig. 1).
Figure. 1.
Schematic diagram of trial epochs used in short delay and trace conditioning.
The neural circuitry involved in eyeblink conditioning has been documented in considerable detail (Woodruff-Pak & Steinmetz, 2000a, b; Woodruff-Pak & Disterhoft, 2008). A brain stem-cerebellar circuit has been identified that is both necessary and sufficient for delay conditioning (McCormick & Thompson, 1984; Thompson, 1986; Lavond et al., 1993; Logan & Grafton, 1995; Kim & Thompson, 1997; Thompson, 2005). In delay conditioning, neural activity representing the tone CS is projected via the colliculus to discrete portions of the pontine nuclei, which convey this information to the cerebellum via mossy fibers in the middle cerebellar peduncle. Neural activity representing the air-puff US is projected via the inferior olive to the cerebellum via climbing fiber projections in the inferior peduncle. Both pontine and olivary inputs reach Purkinje cells in cerebellar cortex and send collateral inputs directly to the cerebellar deep nuclei (Christian & Thompson, 2003; Lavond & Steinmetz 1989). Neural plasticity in cerebellar cortex and deep nuclei produced by appropriately timed activation of climbing- and mossy-fiber inputs underlies short-delay conditioning (Thompson, 1986, 2005; Krupa et al., 1993; Kim & Thompson, 1997; Ohyama et al., 2003). The essential efferent CR pathway consists of fibers that project from the deep nuclei via the superior cerebellar peduncle to the red nucleus. The CR-related neural activity is then projected to the motor neurons that generate conditioned eyeblinks. Although this brain stem-cerebellar circuitry is sufficient for delay conditioning, trace conditioning with a 500-ms stimulus-free interval has been shown to engage both the cerebellum and the hippocampus in experiments with laboratory animals (Moyer et al. 1990; Stanton et al., 2010; Steinmetz, 2000; Woodruff-Pak & Disterhoft, 2008). Findings from human studies suggest that a trace interval of 500 ms impairs conditioning in medial temporal lobe amnesiacs (McGlinchey-Berroth et al., 1997) and activates the hippocampus more than delay conditioning in normal adults (Cheng et al., 2008). However, temporal lobe damage does not always impair conditioning with a 500 ms trace interval (Woodruff-Pak, 1993); whereas this damage appears to entirely prevent conditioning involving a 1000 ms trace interval (e.g., Clark & Squire, 1998). Eyeblink conditioning emerges gradually over the course of development in both rodents (Stanton & Freeman, 2000) and humans (Ivkovich et al., 2000; Stanton et al., 2010). By 5 months post-term, normal human infants reach the same terminal level of conditioning as adults in the short delay procedure (Herbert et al., 2003).
The aims of this study were (1) to replicate our previous findings of a fetal alcohol-related deficit on delay EBC seen in children at 5 years in a new sample of school-age, heavily alcohol exposed children; (2) to determine whether increased maturation between 5 and 9 years of age attenuates the alcohol effect of prenatal alcohol exposure on delay EBC; (3) to determine if trace conditioning is also impaired in children with fetal alcohol exposure and, if so, whether this task is more or less sensitive to this exposure than delay conditioning; (4) to examine whether retention of the delay CR after a 1.5-year period is altered by fetal alcohol exposure and whether alcohol exposure affects the rate of extinction of this response; (5) to determine if the effects of prenatal alcohol exposure on delay and trace conditioning are independent of the effects of this exposure on IQ; (6) to examine effects of prenatal alcohol exposure on precision of timing of the eyeblink CR; and (7) to determine whether extended training can result in conditioning in exposed children who do not initially meet learning criterion.
Methods
Participants
Participants were 63 8- to 12-year-old children (23 males, 40 females) from the Cape Coloured community in Cape Town, South Africa—34 heavily exposed to alcohol and 29 born to women who abstained or drank at low levels during pregnancy. All of the children in this study were right-handed because they were originally recruited to participate in neuroimaging studies (Dodge et al., 2009; Meintjes et al., 2010). Thirty were the older siblings of participants in our Cape Town longitudinal cohort who had been recruited during gestation and whose EBC performance at 5 years was previously reported (Jacobson et al., 2008). The other 33 heavily alcohol exposed children and matched non-exposed controls were identified by screening all 8- to 12-year-old children from an elementary school in a rural section of Cape Town, where there is a very high incidence of alcohol abuse among local farm workers. EBC data from the 81 Cape Coloured children from the Cape Town longitudinal cohort assessed at 5 years are included in analyses comparing EBC performance across age. Characteristics of that sample are presented in detail in our previous report.
Each child was examined for growth and FAS dysmorphology by two expert dysmorphologists (HEH, LKR) using a standard protocol (Hoyme et al., 2005) during a 6-day clinic held in September 2005 at a neighborhood church (Jacobson et al., 2008). There was substantial agreement between the two examiners on the assessment of all dysmorphic features, including the three principal fetal alcohol-related features—philtrum and vermilion measured on the Astley and Clarren (2001) rating scales and palpebral fissure length (rs = .80, .84, and .77, respectively) and between them and a third dysmorphologist (NK), who examined a small subset who could not attend the clinic (median r = .78).
Procedure
A staff driver and research nurse transported the mother and child from their home to the University of Cape Town (UCT) Faculty of Health Sciences for two 3-hour neurobehavioral assessment visits held within 2-3 consecutive days. The examiner who conducted the neuropsychological and EBC assessments was blind with regard to maternal alcohol history and the child's diagnostic status, except in the most severe cases where FAS status was obvious. Written informed consent was obtained from the mothers and oral assent from the children. Approval for human research was obtained from the Human Investigation Committee at Wayne State University and the Ethics Committee at the UCT Faculty of Health Sciences. Mothers and children were given breakfast, lunch, and a snack during the morning at each visit. At the end of each visit, the mother received a small monetary compensation and photograph of her child, and the child was given a small gift.
Each mother was interviewed in her primary language, Afrikaans or English, regarding her alcohol and smoking and drug use during pregnancy. The alcohol interviews used a timeline follow-back approach to determine incidence and amount of drinking on a day-by-day basis during pregnancy (Jacobson et al., 2002). Volume was recorded for each type of beverage consumed and converted to oz of absolute alcohol (AA). Any child whose mother reported consuming at least 14 standard drinks per week (1.0 oz AA/day) on average or engaged in binge drinking during pregnancy (four or more drinks/occasion) was considered heavily exposed.
EBC and Neuropsychological Assessment
EBC
The EBC procedures were the same as those used in our earlier study with 81 5-year-olds (Jacobson et al., 2008). For the EBC assessment, the child sat facing a television monitor, on which s/he watched a video (Finding Nemo). The same commercially available human EBC system was used (San Diego Instruments, Model #2325-0145-W). The child wore a lightweight headgear, which supports a flexible plastic tube that delivers an air puff to the right eye, at a distance of approximately 2.5 cm. Eyelid closure was measured using a photodiode device placed at the corner of the right eye. Above the head, about 45 cm to either side, two small 7-Ohm speakers were directed to the child's ears for delivery of the auditory conditioned stimulus, a 1-kHz, 80-dB tone. The EBC system generated the auditory conditioned stimulus (CS) and air puff unconditioned stimulus (US), processed the eyeblink signal, and integrated the data from the peripheral devices on a desk top computer. The child was told that s/he would watch a video and would occasionally feel a puff of air to the eye. The air puff was then administered three times to ensure that the headgear was well placed and that the air puff was reaching the eye. The child was then told, “You'll hear a sound, which you will continue to hear every now and then” and that s/he should continue to watch the video. The child was not debriefed at the end of the session regarding the delay and trace conditions but was only told that s/he had “done a good job.”
Each delay session consisted of five 10-trial blocks. In the eight paired trials in each block, the air puff was administered simultaneously during the last 100 ms of the presentation of the 750-ms tone, yielding a delay interval (or interstimulus interval) between onset of CS and US of 650 ms (Fig. 1). The intertrial interval ranged from 8 to 16 s, with an average of one trial every 12 s. The first and sixth trial in each block were unpaired trials. The first was a tone-alone trial to test for conditioned responding in the absence of the subsequent air puff (US). Every sixth trial was an air puff-alone trial to test for somatosensory responsiveness (UR) without potential modulatory influences of CS presentation.
Two 50-trial sessions were administered on the same day, one early in the morning, the second about 2 hr later; two more sessions were administered the following day to those children who did not meet a criterion of 40% CRs in session 2. Six children were not included in the analyses presented in this paper: one child with FAS who had previously participated in an EBC study in our laboratory, three children with questionable alcohol history or diagnosis, and two controls whose EBC data were considered questionable due to technical problems.
The principal analyses of URs, alphas, and CRs focus on sessions 1 and 2 because most children who met criterion for conditioning did so within two sessions. The data from the third and fourth sessions were used to examine the effectiveness of additional training for those who did not meet criterion within two sessions.
Thirty-two of these children were administered trace conditioning 0.4-1.8 years later (Mean = 1.4 years; SD = 0.3). This conditioning procedure is the same as delay conditioning except that a 500-ms stimulus-free interval occurred between the offset of the 750-ms tone and the onset of the air puff (Herbert et al., 2003; see Fig. 1).
In the delay procedure the child must learn to adjust the timing of the anticipatory blink, which occurred optimally between 300 and 650 ms after the onset of the tone. In trace conditioning the child must adjust the blink to occur between 800 and 1250 ms after the onset of the tone. Eyeblinks within 350 ms prior to the air puff onset are considered CRs (see Herbert et al., 2003). This definition eliminates “voluntary” responses that may occur earlier in the CR period (Spence & Ross, 1959). Eyeblinks during the tone alone trials are considered CRs only if they occur between 350 ms prior to when the air puff would have occurred and the end of the trial epoch (Claflin et al., 2002; Ivkovich et al., 2000). Responses that peak within 150 ms after the onset of the tone are considered alpha or startle responses (SR; Kadlac JA, Grant, 1977). Because the children who were assessed on trace conditioning had been administered delay conditioning 1.5 years earlier, we also examined the degree to which a conditioned eyeblink response during 300-650 ms post-tone onset (the optimal period for a delay CR) was retained during trace conditioning and, if so, how long it took to extinguish. In the trace conditioning paradigm, eyeblinks within the 300-650 ms post-tone onset period are considered “non-adaptive” CRs, reflecting carryover effects from earlier delay conditioning.
The principal dependent measure was whether the child met the criterion of 40% CRs during an EBC session. Sensitivity to the air puff US was assessed by measuring % URs, defined as unconditioned responses to the air puff for the paired trials in which there were no CRs. Sensitivity to the tone CS was assessed by measuring the percentage of paired trials with alpha (SR) responses, i.e., blinks that peaked within 150 ms after onset of the tone. Precision of CR timing was assessed by examining latency to CR onset and to peak CR during the tone alone trials to determine how well timed the conditioned blink was in relation to when the air puff occurred during the paired trials (i.e., 650 ms post-tone onset for delay conditioning; 1250 ms post-tone onset for trace). Because only two sessions were administered to many of the children, the analyses of the CR, UR, and startle measures were limited to the data in Sessions 1 and 2. However, given that only five tone alone trials occurred in each session, the data for the latency measures were averaged across all the sessions to insure sufficient data to provide a reliable assessment. To increase the likelihood that only true CRs were counted, these analyses were based only on data from children who blinked on 20% or more of the tone only trials for delay EBC, that is, above the base rate for nonassociative CRs.
IQ assessment
All of the children were administered 7 of the 10 subtests from the Wechsler Intelligence Scale for Children, third edition (WISC-III)—Similarities, Arithmetic, Digit Span, Symbol Search, Coding, Block Design, and Picture Completion—and Matrix Reasoning from the WISC-IV at the first 11-year EBC visit. These WISC subtests were translated into Afrikaans by a clinical psychologist, whose first language is Afrikaans. IQ was estimated from these subtests using Sattler's (1992) formula for computing Short Form IQ; validity coefficients for Sattler Short Form IQ based on five or more subtests consistently exceed r = .90. In the 5-year assessment of the children from our longitudinal cohort, we administered the Junior South African Intelligence Scale (JSAIS; Madge et al., 1981), which is available in Afrikaans and English and has been normed for South African children. Sixty-two of those children have been administered the WISC IQ test at 9 years. IQ scores obtained using the JSAIS at 5 years were strongly correlated with the 9-year WISC scores, r = .77, p < 001.
Data analysis
Prior to analysis, all variables were checked for normality of distribution. Average AA per day across pregnancy was positively skewed (skew >3.0) and was normalized by means of log (X + 1) transformation. Eight control variables were assessed for consideration as potential confounders of the relation of prenatal alcohol exposure to EBC: maternal age at delivery, parity, years of education, marital status, and smoking during pregnancy, and child gender, age at assessment, and postnatal lead exposure based on a venous blood lead sample obtained from the child. The principal analysis examined the relation of diagnostic group to whether the child met criterion for conditioning (at least 40% CRs in session 2), which was tested using chi-square. Each control variable that related at p < 0.10 to whether the child met criterion for conditioning was considered a potential confounder of this effect. Logistic regression analyses were used to determine whether the effect of exposure on short delay or trace conditioning remained significant after adjustment for each potential confounder. Repeated measures analysis of variance (ANOVA) was used to examine the relation of diagnostic group to percent URs to the air puff, startles (SRs) to the tone, CRs, non-adaptive CRs in trace conditioning, and latency to onset and to peak CRs. Data from the 5-year delay conditioning assessments (Jacobson et al., 2008) were included to examine age effects on percent CRs, URs, and SRs and latency to onset and peak CRs.
Results
Demographic and background characteristics are summarized in Table 1. Six (17.6%) children born to the 34 heavy drinking mothers met the revised Institute of Medicine criteria (Hoyme et al., 2005) for full FAS: at least two of the principal dysmorphic features (short palpebral fissures, thin upper lip, flat or smooth philtrum), small head circumference (bottom 10th percentile), and low weight or short stature (bottom 10th percentile). Twenty-eight children, the heavily exposed (HE) group, were born to heavy drinking mothers but did not meet criteria for FAS, and 29 children, the control group, were born to women who abstained or drank at very low levels during pregnancy. There were no significant between-group differences for parity, gender, age at dysmorphology clinic or delay or trace conditioning assessment visit, or number of years between the delay and trace conditioning visits, all ps > .10 (Table 1). Mothers of children with FAS were older, and fewer were married than mothers of children in the HE and control groups. Mothers in the two alcohol consuming groups were also somewhat less educated than control mothers.
Table 1. Sample Characteristics by Diagnostic Group (N = 63).
| FAS (6) | HE (28) | Control (29) | F or χ2 | |
|---|---|---|---|---|
| Maternal characteristics | ||||
| Age at delivery | 30.8 (5.2) | 23.7 (4.7) | 25.7 (6.0) | 4.45* |
| Years of education | 6.2 (3.7) | 7.2 (2.2) | 8.8 (1.8) | 5.58** |
| Married % | 16.7 | 50.0 | 69.0 | 6.14* |
| Parity | 3.0 (0.6) | 2.0 (1.0) | 2.2 (1.3) | 2.03 |
| Alcohol during pregnancy | ||||
| oz AA/day | 2.2 (0.7) | 2.4 (2.3) | 0.002 (0.008) | 18.99*** |
| oz AA/occasion | 5.7 (1.1) | 6.3 (5.1) | 0.07 (0.5) | 25.02*** |
| Frequency (days/wk) | 2.7 (0.8) | 2.7 (1.5) | 0.01 (0.06) | 52.05*** |
| Cigarettes/day during | ||||
| pregnancy | 9.7 (6.7) | 10.8 (8.3) | 3.2 (5.2) | 9.14*** |
| Alcohol dependent (%)a | 100.0 | 85.2 | 16.7 | 27.59*** |
| Child characteristics | ||||
| Child gender (% male) | 50.0 | 39.3 | 31.0 | 0.94 |
| Age at dysmorphology | ||||
| clinic assessment | 9.6 (2.6) | 10.1 (1.0) | 10.0 (0.9) | 0.17 |
| Age at short delay visit | 11.0 (1.5) | 11.6 (1.2) | 11.0 (0.9) | 2.01 |
| Age at trace conditioning | 12.5 (1.5) | 13.0 (1.0) | 12.8 (1.1) | 0.46 |
| Years between delay and trace conditioning visits | 1.5 (0.1) | 1.4 (0.2) | 1.4 (0.5) | 0.62 |
| Weight (kg)b | 24.2 (4.1) | 31.4 (8.9) | 35.3 (10.0) | 4.01* |
| Height (cm)b | 125.9 (8.2) | 135.0 (9.5) | 137.2 (8.1) | 4.19* |
| Head circumference (cm)b | 48.3 (1.7) | 52.6 (1.6) | 53.2 (1.4) | 25.05*** |
| IQ | 56.7 (10.8) | 67.6 (10.3) | 76.7 (9.1) | 13.04*** |
Values are means (SD).
Based on the NAWS; missing for 8 women.
At dysmorphology clinic.
p < .05
p < .01
p < .001
There were significant between-group differences in weight, height, and head circumference, with children with FAS showing expected growth retardation as compared with controls and heavily exposed nonsyndromal children (Table 1; for weight: FAS < HE and Controls, ps = .085 and .005, respectively; for height: FAS < HE and Controls, ps = .024 and .005; head circumference: FAS < HE and Controls, both ps = .001. No differences were found between HE vs. Controls on any of these measures.) In addition, IQ was related to diagnosis with both the FAS and HE groups scoring more poorly than controls, both ps < .001.
Total intracranial (TIV) and cerebellar volumes were assessed for 30 of the children following the procedures described in Meintjes et al. (2010). Both TIV and cerebellar volumes were reduced for children with FAS compared with those with HE and control children, Fs = 12.28 and 10.00, ps < .001, respectively. Reduced TIV and cerebellar volume were related to amount of alcohol consumed and frequency of consumption during pregnancy, rs ranged from -.37 to -.40, p < .05. TIV was smaller in children born to mothers who met criteria for alcohol abuse or dependence on the NAWs, t (23), p = .05.
Mothers who reported drinking consumed an average of 4-5 standard drinks/day (Table 1). However, virtually none drank on a daily basis, and most concentrated their drinking on the weekends, consuming an average of about 11.5 standard drinks per occasion as often as 2-3 days/week. Among the drinkers, the majority (87.1%) met DSM-IV criteria for alcohol abuse or alcohol dependence. By contrast, women recruited for the control group abstained or drank very little alcohol during pregnancy. Only one woman in the HE group reported using marijuana, and none of the women reported using cocaine or methaqualone (“mandrax”) during pregnancy. Two-thirds of the women (66.7%) smoked cigarettes, with 36.5% smoking an average of 10 or more cigarettes per day; mothers of children with FAS and in the HE group smoked more than control mothers, ps > .05 and .001, respectively.
Eyeblink Conditioning
URs and SRs
Delay conditioning
Rate of unconditioned responses (URs) to the air puff for the paired trials in which there were no CRs was assessed in a three-way repeated measures ANOVA examining percent URs in relation to one within-subjects factor (sessions 1 and 2) and two between-subjects factors—diagnosis (FAS, HE, controls) and age (5 and 11 years) (Table 2). Since there were no main, F (1,137) = 2.67, p = .105, or interaction effects for session, all ps > .15, the data were pooled across sessions. The children in all of the diagnostic groups exhibited a consistently high rate of URs to the air puff in the short delay condition, F (2,137) = 1.92, p = .150, indicating that all groups could perceive the air puff and perform the eyeblink response to a similar extent, which is consistent with our previous 5-year findings (Jacobson et al., 2008). There was a main effect for age, F (1,137) = 25.52, p < .001, with increasing URs at 11 years, but no interaction for age × diagnostic group, F (2,137) = 0.98, p = .380.
Table 2. Percent unconditioned (URs) and startle responses (SRs) by diagnostic group and age.
| FAS | Heavy exposed | Controls | Mean | |
|---|---|---|---|---|
| Short delay | ||||
| Percent URs | ||||
| 5 years | 72.4 (3.8) | 81.6 (1.7) | 77.2 (2.5) | 77.0 (1.6) |
| 11 years | 90.9 (4.8) | 90.7 (2.2) | 87.5 (2.2) | 89.7 (1.9) |
| Mean | 81.6 (3.1) | 86.2 (1.4) | 82.4 (1.7) | 83.4 (1.3) |
| Percent SRs/alphas | ||||
| 5 years | 6.4 (1.3) | 3.1 (0.6) | 4.7 (0.9) | 4.7 (0.6) |
| 11 years | 3.7 (1.7) | 3.9 (0.8) | 3.5 (0.8) | 3.7 (0.7) |
| Mean | 5.0 (1.1) | 3.5 (0.5) | 4.1 (0.6) | 4.2 (0.4) |
| Trace | ||||
| Percent URs | ||||
| 89.6 (5.6) | 85.3 (3.6) | 87.4 (3.9) | 87.4 (2.6) | |
| Percent SRs/alphas | ||||
| 3.1 (2/6) | 13.6 (1.7) | 6.7 (1.8) | 7.8 (1.2) |
Values are means (SE). N = 80 at 5 years and 63 at 11 years for delay; N = 32 for trace. Data for one child from the 5-year cohort who only completed session 1 were omitted due to listwise deletion.
Processing of the tone conditioned stimulus was assessed by measuring alpha or startle responses (SR; Kadlac and Grant, 1977), which were defined as blinks that peaked within the 150 ms after tone onset. A sessions × diagnostic group × age repeated measures ANOVA of the SRs to the tone indicated no main effect for session, F (1,137) = 1.10, p = .297, or interaction effects involving session, all ps > 20. The data were, therefore, again pooled across sessions. The absence of a session effect in the SR measure is consistent with the historical eyeblink conditioning literature (Spence and Ross, 1959), our previous 5-year report (Jacobson et al., 2008), and the animal literature on ethanol exposure (Green, 2004; Goodlett, Stanton, & Steinmetz, 2000). There were no differences among diagnostic groups, F (2,137) = 0.99, p =.376), and no main effect for age, (F (1,137) = 1.55, p =.215) or interaction for age × diagnostic group, (F (2,137) = 1.58, p =.209). The absence of differences across groups in both the UR and alpha measures suggests that prenatal alcohol exposure did not alter sensory processing of the tone or air puff or the ability to perform the eyeblink response. These findings are also consistent with the animal literature (e.g., Stanton & Goodlett, 1998).
Trace conditioning
As with the delay procedure, there were no main, F (1,29) = 0.42, p = .521, or interaction effects, F (2,29) = 0.53, p = .597, for session for URs, and the data for the two sessions were, therefore, pooled. Children in the three diagnostic groups again all showed a similar high rate of URs to the air puff during the trace condition for the two sessions, F (2,29) = 0.23, p = .797 (Table 2). There was a decrease in SRs to the tone from an average of 9.5 (SE = 1.4) in Session 1 to 6.1 (SE = 1.5) in Session 2, which fell short of statistical significance, F (1,29) = 3.90, p = .058, but there was no session × diagnosis interaction, F (2,29) = 0.25, p = .779. There was a main effect for diagnostic group, F (2,29) = 7.11, p = .003, with the HE group exhibiting more SRs than either the FAS, p < .004, or control groups to the tone, p = .009, which were not different from each other, p = .270. The implications of this effect are unclear, particularly since it was not also seen in the delay EBC condition (see above).
Delay and trace conditioning effects
Delay conditioning
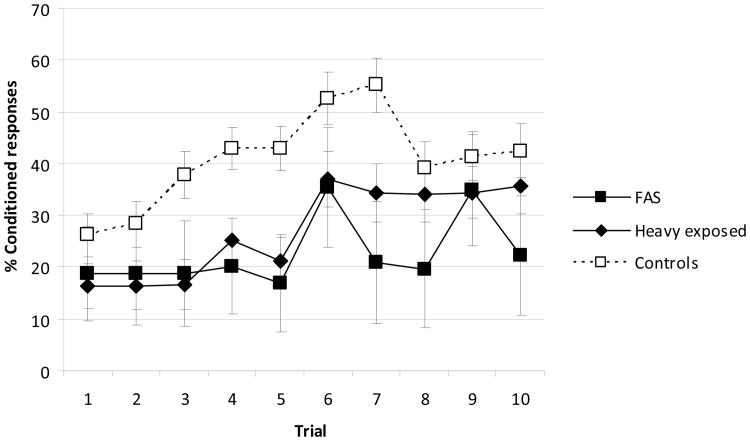
Acquisition of delay CRs was impaired in the alcohol-exposed children at 11 years of age. Prenatally exposed children were significantly less likely to meet criterion for delay conditioning than the controls within the first two sessions (Table 3). Only 2 of 6 children with FAS met criterion, as contrasted with 79.3% of the controls; performance in the HE group was also poor, with only 42.9% of the children reaching criterion. Odds ratio data from a logistic regression analysis show that the children with FAS were 7.7 times more likely to fail to meet criterion on the delay task compared with controls and that the HE group was 5.1 times more likely to fail to meet criterion. The effects of alcohol exposure on delay EBC remained significant after controlling for each of the potential confounders, education and smoking (ps = .015 and .032, respectively).1 When percent CRs was examined as a continuous measure, all groups improved between Sessions 1 and 2, F (1,60) = 12.55, p < .001. Figure 2 shows the acquisition curves for percentage CRs for each of the groups in 10-trial blocks for the first two 50-trial sessions of delay conditioning.
Table 3. Number (%) of children meeting criterion for eyeblink conditioning at school age by diagnosis.
| FAS | Other heavily exposed | Controls | χ2 | |
|---|---|---|---|---|
| Delay | 2 | 12 | 23 | 9.96** |
| (33.3%) | (42.9%) | (79.3%) | ||
| N= 6 | N = 28 | N = 29 | ||
| Trace | 1 | 3 | 8 | 7.11* |
| (16.7%) | (21.4%) | (66.7%) | ||
| N= 6 | N = 14 | N = 12 |
p = .029
p = .007
Figure. 2.
Percent conditioned responses in each block of delay conditioning by fetal alcohol diagnostic group. Error bars are standard error of the mean. Blocks 1 to 5 = Session 1; Blocks 6 to 10 = Session 2 (N = 63).
A repeated measures ANOVA was used to compare effects of diagnosis on percent CRs examined as a continuous measure at the two ages (5 and 11 years). All groups improved between Sessions 1 and 2, F (1,137) = 32.74, p < .001, but the controls performed better than the two alcohol-exposed groups, both ps < .001, which did not differ from each other, p = .137 (Table 4). There was a main effect for diagnosis, F (2,137) = 11.79, p < .001, and age with increased percent CRs at the older age, F (1,137) = 8.23, p = .005. There were no significant interaction effects except for a session × age × diagnosis interaction that fell short of significance, F (2,137) = 2.90, p = .058. The 11-year-old control children performed better than their 5-year-old counterparts in session 1 but not 2. By contrast, the alcohol exposed children at 11 years performed better than their 5-year counterparts in both sessions.
Table 4. Percent CRs for delay and trace conditioning.
| FAS | Heavy exposed | Controls | Mean | |
|---|---|---|---|---|
| Delay | ||||
| 5 years | 12.5 (5.5) | 20.5 (2.5) | 34.4 (3.6) | 22.4 (2.3) |
| 11 years | 25.0 (7.1) | 30.9 (3.3) | 43.0 (3.2) | 33.0 (2.8) |
| Mean | 18.7 (4.5) | 25.7 (2.1) | 38.7 (2.4) | 27.7 (1.8) |
| Trace | 20.1 (6.3) | 25.6 (4.1) | 32.6 (4.4) | 26.0 (2.9) |
Values are means (SE). N = 80 at 5 years and 63 at 11 years for delay; N = 32 for trace.
Trace
As expected, trace conditioning, was more difficult than delay conditioning for all of the children at this age; fewer children in all groups reached criterion for conditioning (Table 3). As in the delay paradigm, trace conditioning was also highly sensitive to fetal alcohol diagnosis. Only 1 of 6 children with FAS and 3 of 14 of the HE children met criterion for trace conditioning, compared with two-thirds of the nonexposed controls. Although the HE children appeared to be more sensitive to the tone, as indicated by SRs (Table 2), during the trace conditioning trials when compared with the other two groups, their performance on trace conditioning was consistently intermediate, i.e., better than the FAS group and poorer than controls. An odds ratio analysis indicated that children with FAS were 10.0 times more likely to fail to meet criterion on the trace task compared with controls and that the HE children were 7.3 times more likely to fail to meet criterion. The effect of alcohol exposure on trace conditioning remained significant (p = .017), after control for the sole potential confounder, maternal smoking during pregnancy. A repeated measures ANOVA showed that percent CRs for trace conditioning increased across session, F (1,29) = 10.84, p = .003, but the effect of diagnostic group on the continuous CR measure was not significant, F (2,29) = 1.51, p = .237 (Table 4).
A large proportion of the children who failed to meet criterion for delay conditioning (78.6%) subsequently failed to acquire trace conditioning, which is consistent with evidence from the animal literature indicating that the cerebellar-based neural circuitry involved in delay is also critical for successful trace conditioning (see Woodruff-Pak & Disterhoft, 2008). When the data for the 32 children assessed on both delay and trace conditioning were compared, the magnitude of the effect of diagnostic group on trace conditioning (Cramer's V = 0.47) was similar to the magnitude of the effect on delay (Cramer's V = 0.44), suggesting that alcohol exposure impairs performance on both delay and trace conditioning to a similar extent and that the more difficult trace task is not more sensitive to alcohol exposure.
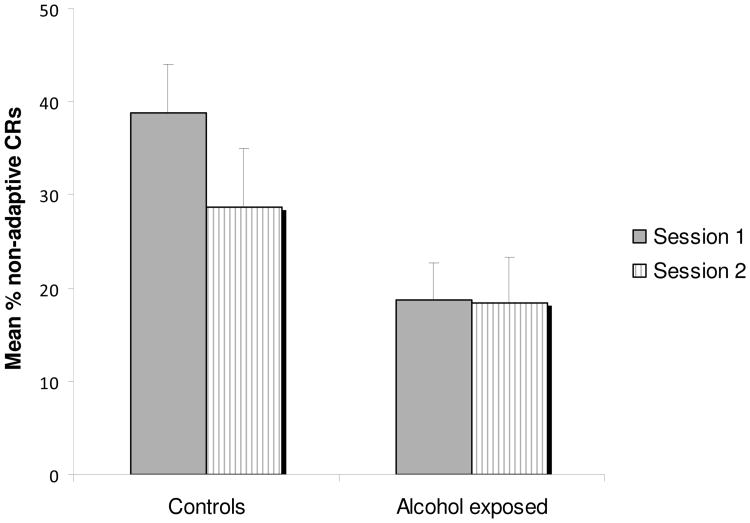
A substantial number of children who met the delay conditioning criterion within two sessions at 11 years showed prematurely-timed (non-adaptive) CRs during session 1 of the trace conditioning trials. These non-adaptive CRs occurred between 300 and 650 ms after the CS, which corresponds to the adaptive CR in short delay conditioning, indicating retention of the short-delay CR over a 1.5-year period. Non-adaptive CRs were examined in a repeated measures ANOVA based on exposure group (exposed, controls) and session (first, second). There was a main effect for session, F (1,30) = 6.53, p = .016, due to extinction of the non-adaptive CRs across the two trace conditioning sessions. There was also a main effect for alcohol exposure group, with controls showing almost twice as many non-adaptive CRs than the exposed group, F (1,30) = 4.62, p = .040, and an interaction effect for session × alcohol group, F (1,30) = 5.80, p = .022. When sessions 1 and 2 were examined separately, the control children showed more non-adaptive CRs than the alcohol exposed children during the first session of trace conditioning, F (1,30) = 9.06, p = .005, presumably because more of them had been successfully conditioned during the delay conditioning procedure, but these differences were no longer significant by the second session, F (1,30) = 1.66, p = .207 (see Fig. 3). There were virtually no non-adaptive CRs among the children who had failed to reach criterion on the delay conditioning task. By contrast, the controls exhibited substantial retention, and exposed children who had met criterion for conditioning showed some evidence of retention as well, even after 1.5 years.
Figure 3.
Mean percent of non-adaptive CRs during trace conditioning by session and exposure group.
Regarding the hypothesis that the relation between alcohol exposure and EBC might be mediated by child IQ, we examined the relation between IQ and EBC outcome. IQ did not differentiate the children who reached criterion on delay and trace EBC from those who failed, t (61) = 1.38, p = .172, and t (30) = 1.18, p = .249, respectively, indicating that it could not be a mediator of the effect of fetal alcohol exposure on performance on either EBC task. These data confirm a similar finding for delay conditioning at 5 years (Jacobson et al., 2008).
Latency to Onset and Peak CR
Precision of timing of eyeblinks in delay conditioning was assessed at 5 and 11 years by examining latency to onset and to peak CR during the CS tone-alone trials. As shown in Figure 1, onset of the air puff in the delay task was 650 ms after the onset of the tone. The main effect for age fell short of significance, F (1,118) = 3.18, p = .077, with average CR onset occurring more than 650 ms post-tone onset (i.e., after when US onset occurred in the paired trials) at 5 years but very close to time for US onset at 11 years. There was a main effect for diagnostic group, F (2,118) = 3.90, p = .023 (Table 5), with the FAS group having significantly later onset than controls or other exposed children, p < .004 and p < .018, respectively. Average CR occurred after US onset for the FAS group at both ages; for the HE group, it occurred after US onset at 5 years and at onset at 11 years. By contrast, onset of the CR for the controls occurred at or in anticipation of the US at both ages. The age × diagnostic group interaction was not significant, F (2,118) = 0.80, p = .450.
Table 5. Latency to onset and peak CR on delay and trace conditioning (ms).
| FAS | Heavy exposed | Controls | Mean | |
|---|---|---|---|---|
| Delay | ||||
| Latency to onset | ||||
| 5 years | 821.4 (53.5) | 668.7 (22.2) | 649.1 (27.9) | 713.0 (21.4) |
| 11 years | 696.2 (58.6) | 651.7 (25.2) | 624.0 (24.3) | 657.3 (22.8) |
| Mean | 758.8 (39.7) | 660.2 (16.8) | 636.6 (18.5) | 685.2 (15.6) |
| Latency to peak | ||||
| 5 years | 899.9 (52.5) | 753.8 (21.7) | 735.0 (27.4) | 786.9 (19.9) |
| 11 years | 788.1 (57.5) | 743.6 (24.8) | 710.0 (23.9) | 747.2 (21.1) |
| Mean | 844.0 (38.9) | 748.7 (16.5) | 722.5 (18.2) | 771.7 (15.3) |
| Trace (11 years) | ||||
| Latency to onset | 1187.8 (41.9) | 1165.1 (29.6) | 1084.1 (29.6) | 1145.7 (19.7) |
| Latency to peak | 1252.8 (41.6) | 1241.9 (29.4) | 1162.6 (29.4) | 1219.1 (19.6) |
Values are means (SE). N = 63 at 5 years and 61 at 11 years for delay; N = 30 for trace.
For the peak CR latency measure, there was no main effect for age, F (1,118) = 2.56, p = .113. There was a main effect for diagnostic group, F (2,118) = 4.01, p = .021, in which children with FAS showed a later peak CR than the controls and HE children, p < .003 and p = .019, respectively. Again, there was no age × diagnostic group interaction, F (2,118) = 0.72, p = .488.
Onset of the air puff during the paired trials in the trace conditioning task occurred 1250 ms after the onset of the tone including a 500-ms stimulus-free interval between the offset of the tone and onset of the air puff (Fig. 1). As in the delay EBC task, timing of eyeblinks in trace conditioning was less precise in the alcohol-exposed children, as indicated in analyses of latency to onset and to peak CR during CS alone trials (Table 5). The main effect of diagnosis on latency to CR onset fell short of significance, F (2,27) = 2.78, p = .08, with children in the FAS and HE group showing later onset than controls, p = .05 and p = .06, respectively, although these alcohol exposed groups were not different from each other, p = .662. The relation of diagnosis to latency to peak CR was not significant, F (2,27) = 2.41, p = .109; peak CR tended to occur later for children in the FAS and HE groups than in the controls, ps = .088 and .067, respectively, but were not different from each other, p = .833. The statistically weaker effects on timing for trace conditioning compared with delay probably reflect the smaller number of children who were assessed on the trace task.
Extended Conditioning
It is possible that more heavily exposed children might meet criterion for conditioning if they are given more extended training on the task. Those children who did not achieve criterion by the end of the first two sessions of short delay conditioning at 11 years were brought back to the laboratory the following day for a third and fourth session to determine if additional training would enable them to meet the criterion of 40% CRs. Table 6 indicates the session during which each child first met this criterion. A few additional children in the exposed groups met criterion by the third or fourth session, but both groups still performed markedly more poorly than the controls, 89.7% of whom were conditioned by the end of the 3rd session.
Table 6. Number (%) of children meeting criteria for delay and trace conditioning by diagnostic group and session during which child first met criterion.
| Alcohol-exposed | Nonexposed | |||
|---|---|---|---|---|
|
|
|
|||
| FAS | Heavy exposed | Control | Total | |
| Delay conditioning | ||||
| Session 1 | 0 (0.0) | 6 (21.4) | 12 (41.4) | 18 (28.6) |
| Session 2 | 2 (33.3) | 6 (21.4) | 11 (37.9) | 19 (30.2) |
| Session 3 | 1 (16.7) | 4 (14.3) | 3 (10.3) | 8 (12.7) |
| Session 4 | 1 (16.7) | 1 (3.6) | 0 (0.0) | 2 (3.2) |
| Total conditioned | 4 (66.7) | 17 (60.7) | 26 (89.7) | 47 (74.6) |
| Total N | 6 | 28 | 29 | 63 |
| Trace conditioning | ||||
| Session 1 | 1 (16.7) | 2 (14.3) | 3 (25.0) | 6 (18.8) |
| Session 2 | 0 (0.0) | 1 (7.1) | 5 (41.7) | 6 (18.8) |
| Session 3 | 1 (16.7) | 4 (28.6) | 0 (0.0) | 5 (15.6) |
| Session 4 | 0 (0.0) | 1 (7.1) | 0 (0.0) | 1 (3.1) |
| Total conditioned | 2 (33.3) | 8 (57.1) | 8 (66.7) | 18 56.3 |
| Total N | 6 | 14 | 12 | 32 |
Values are number of children (%) who met the criterion of at least 40% conditioned responses (CR) in a session.
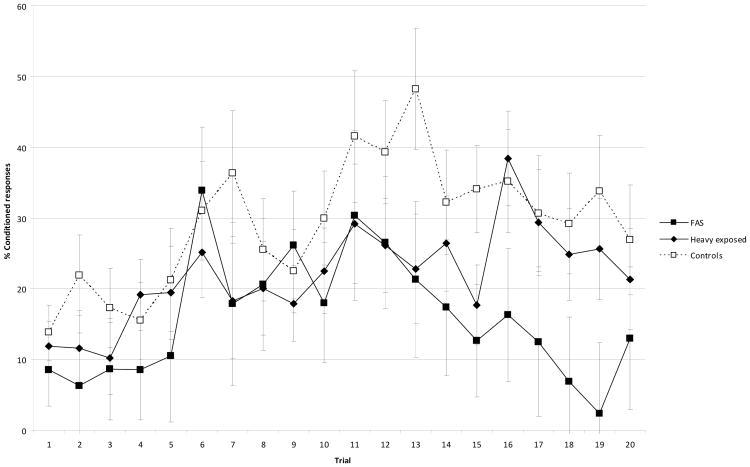
Table 6 also shows the session during which each child first met criterion for trace conditioning. By contrast to the control children, two-thirds of whom met criterion for trace conditioning in two sessions, the majority of the exposed children who met criterion for trace conditioning did not do so until Session 3 or 4. These findings indicate that alcohol-exposed children were slower to acquire trace conditioning. Even with prolonged training, only half as many of the FAS group met criterion for trace conditioning compared to controls. Figure 4 shows the acquisition curves for percentage CRs in 10-trial blocks for the 28 children who completed all four sessions of trace conditioning.
Figure. 4.
Percent conditioned responses in each block of trace conditioning by fetal alcohol diagnostic group. Error bars are standard error of the mean. Blocks 1 to 5 = Session 1; Blocks 6 to 10 = Session 2; Blocks 11 to 15 = Session 3; Blocks 16-20 = Session 4 (N = 28).
Discussion
This study replicates our previous findings showing impaired short delay eyeblink conditioning in heavily alcohol-exposed children at 5 years and extends those findings to a new cohort of school-age children. At 5 years, no children with FAS and only 36.2% of the other heavy exposed children reached criterion for conditioning, compared with 75.0% of the controls; while in the current study of older children, only two of the six children with FAS and 42.9% of the HE children met criterion compared with 79.3% of the controls. The proportion of controls meeting criterion is virtually the same at both ages, while the increase for the children with FAS and HE suggests only limited attenuation of the alcohol effect with age. Moreover, in both our previous study of 5-year-old children and this study of school-age children, there was no relation found between eyeblink conditioning and the child's intellectual ability as measured by IQ, indicating that this form of subcorticallymediated conditioning is not mediated by IQ.
These findings corroborate a report by Coffin et al. (2005), who also found poorer delay eyeblink conditioning—specifically reduced number of CRs and poorer timing—in a small group of school-age, alcohol-exposed children with attention problems and dyslexia, disorders in which the cerebellum is also believed to play an important role (Berquin et al., 1998; Castellanos et al., 2002, Ivry et al., 2001). The contrast between the pattern of EBC deficits in the alcohol exposed children and a comparison group of children with attention deficit hyperactivity disorder (ADHD) in the Coffin et al. study suggests that regionally distinct cerebellar areas are likely affected. Moreover, in contrast to the alcohol-exposed children, children with ADHD produced shorter latency, poorly timed responses; they produced CRs at a rate similar to normal controls but were not able to sustain the learned response. These data suggest that delay EBC may have a high diagnostic sensitivity for discriminating between individuals with different disorders involving cerebellar anomalies who exhibit similar behavioral problems. Together these studies indicate that children with FAS as well as those who were heavily exposed but lack the full syndrome exhibit major deficits in cerebellar function as indicated by their inability to acquire EBC. These findings are consistent with rodent studies, which have shown severe and permanent impairment of the production and timing of responses in eyeblink conditioning in alcohol exposed animals (Brown, Calizo, & Stanton, 2008; Green et al., 2000; Stanton & Goodlett, 1998). These deficits have been linked directly to cerebellar damage and reductions in estimated number of Purkinje cells in the developing rat brain (Goodlett et al., 1990; Thomas et al., 1998; Green et al., 2000; Dunty et al., 2001; Maier & West, 2001).
To our knowledge, this is the first study to examine the impact of prenatal alcohol exposure on trace conditioning in humans, although this type of learning has been shown to be affected by alcohol exposure in several animal studies (Brown et al., 2005; Tran & Goodlett, 2004; Tran & Thomas, 2007). As in the delay paradigm, trace conditioning was also highly sensitive to fetal alcohol exposure. This more difficult form of conditioning was, as expected, more challenging than delay conditioning for all of the children, but especially so for the children with FASD. Only one child with FAS (16.7%) and three heavily exposed children (21.4%) met criterion for trace conditioning at 12.5 years, compared with two-thirds of the controls. These findings are consistent with previous studies documenting the role of development in trace conditioning performance. Whereas college age students show no trace-delay conditioning differences across a wide range of inter-stimulus intervals (Ross & Ross, 1971), trace conditioning is more difficult for normal, middle class children (Werden & Ross, 1972), and infants (Herbert et al., 2003) than in adults.
Of particular interest was the finding that, when the initial session of trace conditioning was administered, non-adaptive CRs (corresponding to the period between 300 and 650 ms post-CS, i.e., the adaptive CR timing period for delay conditioning) persisted in the control children who had been administered delay conditioning 1.5 years earlier. After exposure to the longer interval between the tone and air puff during session 1 in the trace conditioning paradigm, this early CR extinguished in the controls by session 2 and was no longer significant. The lack of non-adaptive CRs in the alcohol-exposed children at 12.5 years confirmed our findings that alcohol exposure had interfered with their acquisition of the short delay CR 1.5 years earlier.
It is of interest that the magnitude of the effect of diagnostic group on the more difficult trace conditioning paradigm (as measured by Cramer's V) was not greater than the effect on delay, suggesting that the impact of fetal alcohol on trace is no greater than on delay. Virtually all of the children who failed delay also failed trace conditioning. These findings are consistent with studies of rats showing that delay and trace conditioning are equally sensitive to developmental alcohol exposure (Brown et al., 2005; Calizo et al., 2006). These rodent studies employed conditioning parameters that are known to make the delay task sensitive to cerebellar injury and the trace task sensitive to hippocampal and cerebellar injury (Stanton et al., 2010). In addition, the task comparison was made across groups, rather than successively in the same subjects as in the present study. The conditioning parameters in the present study were based on previous studies involving human infants and adults (Stanton et al., 2010). Although our delay conditioning task depends on the cerebellum, developmental studies have not yet been conducted with children to identify the trace interval at which the hippocampus becomes essential for trace conditioning. As noted previously, there was evidence for medial temporal lobe involvement in (adult) conditioning at a 500-ms trace interval is mixed (Cheng et al., 2008; McGlinchey-Berroth et al., 1997; Woodruff-Pak, 1993) and future studies involving longer trace intervals (e.g., 1000 ms; Clark & Squire, 1998) might show that trace conditioning is more impaired than delay conditioning in alcohol exposed children. Nevertheless, the most parsimonious account of the rodent and human findings to date is that impairment in the cerebellar circuitry required for both delay and trace conditioning is sufficient to account for impaired performance on both tasks following developmental exposure to alcohol. However, less parsimonious explanations involving targeting of other brain structures by alcohol cannot be entirely ruled out at this point.
When precision of timing of the eyeblink CRs was examined in the tone only trials in the delay task, latency to onset and peak tended to be longer at 5 than at 11 years for all of the children. For the alcohol-exposed children at 5 years, CR onset actually occurred after the time when the air puff was expected (based on the timing in the paired trials), indicating less precise timing and a failure to produce the blink in anticipation of the air puff, by contrast to the controls whose mean CRs occurred in anticipation to the US at both ages. At 11 years the CR in the delay task continued to occur after the time of US onset for the FAS group. In the trace task, which was only administered at 11 years, CR onset was also significantly later in the alcohol-exposed children, although none of the group means occurred after US onset. Developmental research on eyeblink CR timing in children (Coffin et al., 2005) and rodents has also found that neonatal alcohol exposure causes CR latencies to be longer than in controls (Green et al., 2000; Brown et al., 2008). By contrast to the delayed onset in the fetal alcohol-exposed child and animal research, empirical findings and computational models attribute CR timing to interactions between cerebellar cortex and the deep nuclei (Ohyama et al., 2003), with cerebellar cortical injury leading to prematurely timed CRs. That fetal alcohol exposure causes CR-timing deficits characterized by CR latencies that occur after rather than before the time of US onset suggests that developmental exposure to alcohol impairs cerebellar function via a mechanism that differs fundamentally from what is seen after adult injuries to cerebellar cortex or “disconnection” of cerebellar cortex and deep nuclei. Alternatively, the patient known as H.M., the most thoroughly studied subject with bilateral lesions of the hippocampus, was also characteristically slow to respond with no eyeblinks occurring within the paired CS-US trials (Woodruff-Pak, 1993). Like the alcohol-exposed children in our study, H.M.'s mean response occurred after US onset and, therefore, constituted a UR. Only after extensive training on Sessions 12 and 13 did H.M.'s average response attain the latency of a CR. However, H.M. was not considered a “pure” case with bilateral hippocampal removal since he also had atrophy of the cerebellar vermis and hemispheres. A second subject, who had contracted herpes simplex encephalitis and had bilateral hippocampal lesions without cerebellar damage, conditioned more rapidly than H.M. in both the delay and trace paradigms, suggesting that H.M.'s slower performance may have resulted from the cerebellar cortical rather than the hippocampal lesions (Woodruff-Pak, 1993). As suggested by Keele and Ivry (1990) and Woodruff-Pak (1993), the cerebellar cortex may provide the critical temporal computation necessary for tasks, such as EBC and finger tapping, and damage to this area may result in the slower responses seen in the children with FASD. Further work is required to elucidate the underlying mechanisms of CR timing effects in FASD. The timing deficits reported here are consistent with previous reports of slower reaction time and information processing in alcohol-exposed infants (Jacobson et al., 1993, 1994; Kable & Coles, 2004) and children (Streissguth et al., 1984 Streissguth et al., 1986 Streissguth et al., 1994; Burden et al., 2005; Ma et al., 2005).
Coffin et al. (2005) noted that one limitation of their study was the relative brevity of their conditioning protocol, which consisted of a single session, and raised the question of whether alcohol-exposed children would acquire the conditioned response if conditioning were extended to several daily sessions. In our previous 5-year study we showed that no children with FAS met criterion after three sessions of 50 trials each. By contrast, at the older age of assessment in the present study, extended training did yield beneficial effects for some of the alcohol-exposed children. Two additional children with FAS and 17.9% additional from the HE group reached criterion for conditioning by session 4, yet 30-40% of the exposed children failed to improve even after four sessions. By contrast, a large majority of the control children (79.3%) met criterion by the second session and nearly all (89.7%) did so by the end of the third. In trace conditioning, all of the control children who reached criterion did so by the end of the second session. By contrast, a majority of the relatively few alcohol-exposed children to reach criterion did not do so until the third or fourth session. These findings suggest that some alcohol exposed children can benefit from more extensive training, although the data on precision of timing (latency to onset and peak CRs) suggest that the quality of the CRs may still be impaired. Future studies will need to determine whether conditioning resulting from extended training in alcohol-exposed children is retained over a 1.5 year period, as it was in controls.
In our previous report on an EBC deficit in alcohol-exposed children, we found a dose dependent relation between fetal alcohol exposure and severity of diagnosis. Mothers of children with FAS drank more frequently and more drinks per occasion at time of conception and during pregnancy compared with mothers of children with partial FAS, who in turn drank more than mothers of heavily exposed nondysmorphic children, who drank more than controls (Jacobson et al., 2008). In that study maternal alcohol exposure data were collected prospectively during pregnancy, an approach which we have previously found to be more valid than retrospective maternal report (Jacobson et al., 2002). In the present study we collected information on maternal drinking during pregnancy retrospectively. Although we found significant differences between drinkers and controls, there was no difference between mothers of children with FAS and the other heavy drinking mothers of nonsyndromal children in terms of drinks per occasion or frequency of drinking. It is most likely that this lack of difference is attributable to the diminished accuracy of retrospective alcohol ascertainment. Despite the lack of an alcohol dose response relation at school age, the EBC performance of heavily exposed nonsyndromal children at both 5 years and now again at 11 and 12.5 years was consistently intermediate between that of children with the full FAS syndrome and normal controls.
In both the previous study of 5-year-olds and now in this study of older children, no relation was found between eyeblink conditioning and the child's intellectual ability as measured by IQ, which ranged from lowest among children with FAS, intermediate for heavily exposed children, and best for normal controls. Given the low IQs seen in both the alcohol-exposed children and the controls, the validity of the IQ assessment and the role of sociodemographic or nutritional factors were considered. The validity of the IQ assessment was supported by the strong relation (r = .77) reported above between WISC IQ scores obtained at 9 years and those based on the 5-year JSAIS, which is normed for South African children. Although the IQ scores are low and the educational opportunities limited, the mean for the control group is comparable to IQ scores reported for U.S. inner-city children, whose scores are consistently lower than suburban, nonminority children (e.g., Burden et al., 2010). Nor were the lower IQ scores attributable to malnutrition. As expected, the children with heavy prenatal alcohol exposure were smaller in weight and height by about 0.4 standard deviations than controls (Carter et al., in press-b). However, in both the alcohol-exposed children and controls, moderate and severe thinness, an indicator of food insecurity, was rare. Only one child, a control, met WHO criteria for thinness (body mass index z-score < -2) at 9 years. Thus, moderate-to-severe malnutrition is unlikely to be responsible for the alcohol-related EBC deficits reported here. In addition, no differences were seen in percent body fat at 9 years (Carter et al., in press-b). Despite the relatively low IQ scores in this cohort, there is a dose-dependent relation between severity of FASD diagnosis and performance on both EBC tasks, as well as between degree of alcohol exposure and EBC performance. Moreover, as indicated above, in their study of U.S. school-age children, Coffin et al. (2005) found similar impaired delay conditioning in alcohol-exposed children compared to controls, indicating that the alcohol-related deficits reported here are not seen only in disadvantaged samples or attributable to poorer socioeconomic conditions or malnutrition.
The diagnosis of FASD in childhood is difficult because information regarding prenatal exposure is often lacking, many affected children do not exhibit facial anomalies, and no distinctive behavioral phenotype has been identified. Research on FASD treatment has been hampered by the lack of specificity in behavioral diagnostic criteria and limited understanding of the pathophysiology of the disorder. Given the impairment in conditioning and the particular damage to the cerebellum caused by prenatal alcohol exposure, eyeblink conditioning appears to provide a well-characterized model system for assessment of the degree of cerebellar-related learning and memory dysfunction in fetal alcohol exposed children.
Acknowledgments
Supported by grants from the NIH Fogarty International Center (R03 TW007030); National Research Foundation of South Africa (FA2005040800024); South African Research Chairs Initiative of the Department of Science and Technology and National Research Foundation of South Africa; and seed money grants from the University of Cape Town, Wayne State University, and the Joseph Young, Sr., Fund, State of Michigan. The dysmorphology assessments were conducted by H.E. Hoyme, L.K. Robinson, and N. Khaole under grants from the National Institute on Alcohol Abuse and Alcoholism Collaborative Initiative on Fetal Alcohol Spectrum Disorder (U01-AA014790 and U24AA014815). We acknowledge the major contribution of the late Andrea Hay, who helped set up and conducted the initial eyeblink conditioning assessments in Cape Town. We thank members of our University of Cape Town staff, Mariska Pienaar, Maggie September, Mandy Cronje, Jan Chamberlain, Lisa Aitken, and John Minnies for their help in collecting the data; and our Wayne State University staff, Renee Sun; and R. Colin Carter, Children's Hospital Boston/Harvard Medical School, for his many contributions to the Cape Town study. We also thank the mothers and children in the cohort for their contribution to the study. The FAS clinical diagnostic assessments were supported by grants awarded in conjunction with the National Institute on Alcohol Abuse and Alcoholism Collaborative Initiative on Fetal Alcohol Spectrum Disorder (U01-AA014790 to S.W. Jacobson and U24AA014815 to K.L. Jones).
Footnotes
Studies have shown that iron deficient children are at risk for poor cognitive and emotional development (e.g., Lozoff et al., 2008; Carter et al., in press-a). In a recent study, McEchron et al. (2008) found that perinatal iron deficiency in rats was related to hippocampus-dependent impairment in trace EBC but only minor impairment in delay conditioning. At the 5-year assessment of the Cape Town longitudinal cohort (Jacobson et al., 2008), we collected blood samples from 166 children and tested them for iron deficiency. Of the 166 children, only 2 (1.2%) met criteria for iron deficiency anemia (IDA) at this age, suggesting that these preschool children were not characterized by iron deficiency. Iron deficiency was more prevalent during infancy, particularly for the alcohol exposed infants (Carter et al., 2007). Iron deficiency data were collected for 37 infants at 13 months who were later tested on the delay task at 5 years. There was no relation between infant iron deficiency and EBC performance at 5 years, χ2 = 0.32, p = .575
Conflict of interest: None of the authors have any conflicts of interest related to this study
References
- Archibald SL, Fennema-Notestine C, Gamst A, Riley EP, Mattson SN, Jernigan TL. Brain dysmorphology in individuals with severe prenatal alcohol exposure. Dev Med Child Neurol. 2001;43:148–154. [PubMed] [Google Scholar]
- Astley SJ, Clarren SK. Measuring the facial phenotype of individuals with prenatal alcohol exposure: correlations with brain dysfunction. Alcohol. 2001;36:147–159. doi: 10.1093/alcalc/36.2.147. [DOI] [PubMed] [Google Scholar]
- Berquin PC, Giedd JN, Jacobsen JK, Hamburger SD, Krain AL, Rapoport JL, Castellanos JX. Cerebellum in attention-deficit hyperactivity disorder: A morphometric MRI study. Neurol. 1998;50:1087–1093. doi: 10.1212/wnl.50.4.1087. [DOI] [PubMed] [Google Scholar]
- Brown KL, Calizo LH, Stanton ME. Differential effects of neonatal alcohol exposure on eyeblink conditioning and spatial delayed alternation. Dev Psychobiol. 2005;47:420. [Google Scholar]
- Brown KL, Pagani JH, Stanton ME. The ontogeny of interstimulus interval (ISI) discrimination of the conditioned eyeblink response in rats. Behav Neurosci. 2006;120:1057–1070. doi: 10.1037/0735-7044.120.5.1057. [DOI] [PubMed] [Google Scholar]
- Brown KL, Calizo LH, Stanton ME. Dose-dependent deficits in dual interstimulus interval classical eyeblink conditioning tasks following neonatal binge alcohol exposure in rats. Alcohol Clin Exp Res. 2008;32:277–293. doi: 10.1111/j.1530-0277.2007.00579.x. [DOI] [PubMed] [Google Scholar]
- Brown SM, Kieffaber PD, Carroll CA, Vohs JL, Tracy JA, Shekhar A, O'Donnell BF, Steinmetz JE, Hetrick WP. Eyeblink conditioning deficits indicate timing and cerebellar abnormalities in schizophrenia. Brain Cog. 2005;58:94–108. doi: 10.1016/j.bandc.2004.09.011. [DOI] [PubMed] [Google Scholar]
- Burden MJ, Jacobson JL, Westerlund AJ, Lundahl LH, Klorman R, Nelson CA, Avison MJ, Jacobson SW. An event-related potential study of response inhibition in ADHD with and without prenatal alcohol exposure. Alcohol Clin Exp Res. 2010;34:617–627. doi: 10.1111/j.1530-0277.2009.01130.x. [DOI] [PubMed] [Google Scholar]
- Calizo LH, Brown KL, Stanton ME. Differential effects of US intensity on impairment of delay and trace eyeblink conditioning in juvenile rats by neonatal binge alcohol exposure. Alcohol Clin Exp Res. 2006;30:32A. doi: 10.1111/j.1530-0277.2007.00579.x. [DOI] [PubMed] [Google Scholar]
- Carter RC, Duggan CP, Molteno CD, September M, Pienaar M, Hongyu J, Jacobson JL, Jacobson SW. The relation of fetal alcohol exposure to growth and body composition in Cape Town, South African children. Alcohol Clin Exp Res. 34 in press. [Google Scholar]
- Carter RC, Jacobson SW, Molteno CD, Chiodo LM, Viljoen D, Jacobson JL. Effects of prenatal alcohol exposure on infant visual acuity. J Pediatr. 2005;147:473–479. doi: 10.1016/j.jpeds.2005.04.063. [DOI] [PubMed] [Google Scholar]
- Carter RC, Jacobson SW, Molteno CD, Jacobson JL. Fetal alcohol exposure, iron deficiency anemia, and infant growth. Pediatrics. 2007;120:559–567. doi: 10.1542/peds.2007-0151. [DOI] [PubMed] [Google Scholar]
- Castellanos FX, Lee PP, Sharp W, Jeffrier NO, Greenstein DK, Clasen LS, Blumenthal JD, James RS, Ebens CL, Walter JM, Zijdenbos A, Evans AC, Giedd JN, Rapoport JL. Developmental trajectories of brain volume abnormalities in children and adolescents with attention deficit/hyperactivity disorder. JAMA. 2002;288:1740–1748. doi: 10.1001/jama.288.14.1740. [DOI] [PubMed] [Google Scholar]
- Center for Disease Control and Prevention. Alcohol use among women of childbearing age—United States 1991-1999. MMWR. 2002;51:273–276. [PubMed] [Google Scholar]
- Cheng DT, Disterhoft JF, Power JM, Ellis DA, Desmond JE. Neural substrates underlying human delay and trace eyeblink conditioning. Proc Natl Acad Sci USA. 2008;105:8108–8113. doi: 10.1073/pnas.0800374105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christian KM, Thompson RF. Neural substrates of eyeblink conditioning: Acquisition and retention. Learn Mem. 2003;11:427–455. doi: 10.1101/lm.59603. [DOI] [PubMed] [Google Scholar]
- Claflin D, Stanton ME, Herbert J, Greer J, Eckerman CO. Effect of delay-interval on classical eyeblink conditioning in 5-month-old human infants. Dev Psychobiol. 2002;41:329–340. doi: 10.1002/dev.10050. [DOI] [PubMed] [Google Scholar]
- Clark RE, Squire LR. Classical conditioning and brain systems: The role of awareness. Science. 1998;280:77–81. doi: 10.1126/science.280.5360.77. [DOI] [PubMed] [Google Scholar]
- Clarren SK. Central nervous system malformations in two offspring of alcoholic women. Birth Defects Original Article Series. 1977;13:151–153. [PubMed] [Google Scholar]
- Clarren SK, Smith DW. The fetal alcohol syndrome. N Eng J Med. 1978;298:1063–1067. doi: 10.1056/NEJM197805112981906. [DOI] [PubMed] [Google Scholar]
- Coffin JM, Baroody S, Schneider K, O'Neill J. Impaired cerebellar learning in children with prenatal alcohol exposure: A comparative study of eyeblink conditioning in children with ADHD and dyslexia. Cortex. 2005;41:389–398. doi: 10.1016/s0010-9452(08)70275-2. [DOI] [PubMed] [Google Scholar]
- Croxford J, Viljoen D. Alcohol consumption by pregnant women in the Western Cape. South African Med J. 1999;89:962–965. [PubMed] [Google Scholar]
- Dikranian K, Qin YQ, Labruyere J, Nemmers B, Olney JW. Ethanol-induced neuroapoptosis in the developing rodent cerebellum and related brain structures. Dev Brain Res. 2005;155:1–13. doi: 10.1016/j.devbrainres.2004.11.005. [DOI] [PubMed] [Google Scholar]
- Dodge NC, Jacobson JL, Molteno CD, Meintjes EM, Bangalore S, Diwadkar V, Hoyme EH, Robinson LK, Khaole N, Avison MJ, Jacobson SW. Prenatal alcohol exposure and interhemispheric transfer of tactile information: Detroit and Cape Town findings. Alcohol Clin Exp Res. 2009;33:1628–1637. doi: 10.1111/j.1530-0277.2009.00994.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunty WC, Chen SY, Zucker RM, Dehart DB, Sulik KK. Selective vulnerability of embryonic cell populations to ethanol-induced apoptosis: Implications for alcohol-related birth defects and neurodevelopmental disorder. Alcohol Clin Exp Res. 2001;25:1523–1535. [PubMed] [Google Scholar]
- Freeman JH, Jr, Nicholson DA, Muckler AS, Rabinak CA, DiPietro NT. Ontogeny of eyeblink conditioned response timing in rats. Behav Neurosci. 2003;117:283–291. doi: 10.1037/0735-7044.117.2.283. [DOI] [PubMed] [Google Scholar]
- Goodlett CR, Marcussen BL, West JR. A single day of alcohol exposure during the brain growth spurt induces brain weight restriction and cerebellar Purkinje loss. Alcohol. 1990;7:107–114. doi: 10.1016/0741-8329(90)90070-s. [DOI] [PubMed] [Google Scholar]
- Green JT. The effects of ethanol on the developing cerebellum and eyeblink classical conditioning. Cerebellum. 2004;3:178–187. doi: 10.1080/14734220410017338. [DOI] [PubMed] [Google Scholar]
- Green JT, Johnson TB, Goodlett CR, Steinmetz JE. Eyeblink classical conditioning and interpositus nucleus activity are disrupted in adult rats exposed to ethanol as neonates. Learn Mem. 2002a;9:304–320. doi: 10.1101/lm.47602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green JT, Rogers RF, Goodlett CR, Steinmetz JE. Impairment in eyeblink classical conditioning in adult rats exposed to ethanol as neonates. Alcohol Clin Exp Res. 2000;24:438–447. [PubMed] [Google Scholar]
- Green JT, Tran T, Steinmetz JE, Goodlett CR. Neonatal ethanol produces cerebellar deep nuclear loss and correlated disruption of eyeblink conditioning in adult rats. Brain Res. 2002b;956:302–311. doi: 10.1016/s0006-8993(02)03561-8. [DOI] [PubMed] [Google Scholar]
- Hamre KM, West JR. The effects of the timing of ethanol exposure during the brain growth spurt on the number of cerebellar Purkinje and granule cell nuclear profiles. Alcohol Clin Exp Res. 1993;17:610–622. doi: 10.1111/j.1530-0277.1993.tb00808.x. [DOI] [PubMed] [Google Scholar]
- Herbert JS, Eckerman CO, Stanton ME. The ontogeny of human learning in delay, long-delay, and trace eyeblink conditioning. Behav Neurosci. 2003;117:1196–1210. doi: 10.1037/0735-7044.117.6.1196. [DOI] [PubMed] [Google Scholar]
- Hoyme HE, May PA, Kalberg WO, Kodituwakku P, Gossage JP, Trujillo PM, Buckley DG, Miller JH, Aragon AS, Khaole N, Viljoen DL, Jones KL, Robinson LK. A practical clinical approach to diagnosis of fetal alcohol spectrum disorders: Clarification of the 1996 Institute of Medicine criteria. Pediatr. 2005;115:39–47. doi: 10.1542/peds.2004-0259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivkovich D, Paczkowski CM, Stanton ME. Ontogeny of delay versus trace eyeblink conditioning in the rat. Dev Psychobiol. 2000;36:148–160. doi: 10.1002/(sici)1098-2302(200003)36:2<148::aid-dev6>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- Ivkovich D, Stanton ME. Effects of early hippocampal lesions on trace, delay, and long-delay eyeblink conditioning in developing rats. Neurobiology of Learning and Memory. 2001;76:426–446. doi: 10.1006/nlme.2001.4027. [DOI] [PubMed] [Google Scholar]
- Ivry RB, Justus TC, Middleton C. The cerebellum, timing, and language: Implications for the study of dyslexia. In: Wolf M, editor. Dyslexia, Fluency, and the Brain. York Press; Timonium, MD: 2001. pp. 189–211. [Google Scholar]
- Jacobson JL, Jacobson SW, Molteno CD, Odendaal H. A prospective examination of the incidence of heavy drinking during pregnancy among Cape Coloured South African women. Alcohol Clin Exp Res. 2006;30:233A. [Google Scholar]
- Jacobson SW, Chiodo LM, Jacobson JL, Sokol RJ. Validity of maternal report of alcohol, cocaine, and smoking during pregnancy in relation to infant neurobehavioral outcome. Pediatr. 2002;109:815–825. doi: 10.1542/peds.109.5.815. [DOI] [PubMed] [Google Scholar]
- Jacobson SW, Jacobson JL, Sokol RJ. Effects of fetal alcohol exposure on infant reaction time. Alcohol Clin Exp Res. 1994;18:1125–1132. doi: 10.1111/j.1530-0277.1994.tb00092.x. [DOI] [PubMed] [Google Scholar]
- Jacobson SW, Jacobson JL, Sokol RJ, Martier SS, Ager JW. Prenatal alcohol exposure and infant information processing ability. Child Dev. 1993;64:1706–1721. [PubMed] [Google Scholar]
- Jacobson SW, Stanton ME, Molteno CD, Burden MJ, Fuller DS, Hoyme HE, Robinson LK, Khaole N, Jacobson JL. Impaired eyeblink conditioning in children with fetal alcohol syndrome. Alcohol Clin Exp Res. 2008;32:365–372. doi: 10.1111/j.1530-0277.2007.00585.x. [DOI] [PubMed] [Google Scholar]
- Jones KL, Smith DW. Recognition of the fetal alcohol syndrome in early infancy. Lancet. 1973;2:999–1001. doi: 10.1016/s0140-6736(73)91092-1. [DOI] [PubMed] [Google Scholar]
- Kable JA, Coles CD. The impact of prenatal alcohol exposure on neurophysiological encoding of environmental events at six months. Alcohol Clin Exp Res. 2004;28:489–496. doi: 10.1097/01.alc.0000117837.66107.64. [DOI] [PubMed] [Google Scholar]
- Kadlac JA, Grant DA. Eyelid response topography in differential interstimulus interval conditioning. J Exp Psych Human Learn Mem. 1977;3:345–355. [PubMed] [Google Scholar]
- Keele SW, Ivry R. Does the cerebellum provide a common computation for diverse tasks? A timing hypothesis. In: Diamond A, editor. The development and neural bases of higher cognitive functions. Vol. 608. New York: New York Academy of Sciences; 1990. pp. 179–211. [DOI] [PubMed] [Google Scholar]
- Kim JJ, Thompson RF. Cerebellar circuits and synaptic mechanisms involved in classical eyeblink conditioning. Trends Neurosci. 1997;20:177–181. doi: 10.1016/s0166-2236(96)10081-3. [DOI] [PubMed] [Google Scholar]
- Krupa DJ, Thompson JK, Thompson RF. Localization of a memory trace in the mammalian brain. Science. 1993;260:989–991. doi: 10.1126/science.8493536. [DOI] [PubMed] [Google Scholar]
- Lavond DG, Kim JJ, Thompson RF. Mammalian brain substrates of aversive classical conditioning. Ann Rev Psychol. 1993;44:317–342. doi: 10.1146/annurev.ps.44.020193.001533. [DOI] [PubMed] [Google Scholar]
- Lavond DG, Steinmetz JE. Acquisition of classical conditioning without cerebellar cortex. Behav Brain Res. 1989;33:113–164. doi: 10.1016/s0166-4328(89)80047-6. [DOI] [PubMed] [Google Scholar]
- Logan CG, Grafton ST. Functional anatomy of human eyeblink conditioning determined with cerebral glucose metabolism and positron-emission tomography. Proc Natl Acad Sci USA. 1995;92:7500–7504. doi: 10.1073/pnas.92.16.7500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lozoff B, Clark KM, Jing Y, Armony-Sivan R, Angelilli ML, Jacobson SW. Dose-response relations between iron deficiency with or without anemia and infant social-emotional behavior. J Pediatr. 2008;152:696–702. e3. doi: 10.1016/j.jpeds.2007.09.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma X, Coles CD, Lynch ME, LaConte SM, Zurkiya O, Wang D, Hu X. Evaluation of corpus callosum anisotropy in young adults with fetal alcohol syndrome according to diffusion tensor imaging. Alcohol Clin Exp Res. 2005;29:1214–1222. doi: 10.1097/01.alc.0000171934.22755.6d. [DOI] [PubMed] [Google Scholar]
- Madge EM, van den Berg AR, Robinson M, Landman J. Junior South African Individual Scales. Human Sciences Research Council; Pretoria: 1981. [Google Scholar]
- Maier SE, West JR. Regional differences in cell loss associated with binge-like alcohol exposure during the first two trimesters equivalent in the rat. Alcohol. 2001;23:49–57. doi: 10.1016/s0741-8329(00)00133-6. [DOI] [PubMed] [Google Scholar]
- May PA, Brooke L, Gossage JP, Croxford J, Adnams C, Jones KL, Robinson L, Viljoen D. Epidemiology of fetal alcohol syndrome in a South African community in the Western Cape Province. Am J Pub Health. 2000;90:1905–1912. doi: 10.2105/ajph.90.12.1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick DA, Thompson RF. Neuronal responses of the rabbit cerebellum during acquisition and performance of a classically conditioned nictitating membrane-eyelid response. J Neurosci. 1984;3:2811–2822. doi: 10.1523/JNEUROSCI.04-11-02811.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEchron MD, Alexander DN, Gilmartin MR, Paronish MD. Perinatal Nutritional Iron deficiency impairs hippocampus-dependent trace eyeblink conditioning in rats. Dev Neurosci. 2008;30:243–254. doi: 10.1159/000110502. [DOI] [PubMed] [Google Scholar]
- Meintjes EM, Jacobson SW, Molteno CD, Gatenby JC, Warton C, Cannistraci CJ, Gore JC, Jacobson JL. An fMRI study of number processing in children. Magnetic Resonance Imaging. 2010;28:351–362. doi: 10.1016/j.mri.2009.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moyer JR, Deyo RA, Disterhoft JF. Hippocampectomy disrupts trace eye-blink conditioning in rabbits. Behav Neurosci. 1990;104:243–252. doi: 10.1037//0735-7044.104.2.243. [DOI] [PubMed] [Google Scholar]
- Ohyama T, Nores WL, Murphy M, Mauk MD. What the cerebellum computes. Trends Neurosci. 2003;26:222–227. doi: 10.1016/S0166-2236(03)00054-7. [DOI] [PubMed] [Google Scholar]
- Riley EP, Mattson SN, Li TK, Jacobson SW, Coles CD, Kodituwakku PW, Adnams CM, Korkman MI. Neurobehavioral consequences of prenatal alcohol exposure: An international perspective. Alcohol Clin Exp Res. 2003;27:362–373. doi: 10.1097/01.ALC.0000052703.38558.B2. [DOI] [PubMed] [Google Scholar]
- Ross SM, Ross LE. Comparison of trace and delay classical eyelid conditioning as a function of interstimulus interval. J Exp Psychol. 1971;91:165–167. doi: 10.1037/h0031823. [DOI] [PubMed] [Google Scholar]
- Sattler JM. Assessment of Children. 3rd. Jerome M. Sattler, Inc; San Diego: 1992. [Google Scholar]
- Spence KW, Ross LE. A methodological study of the form and latency of eyelid responses in conditioning. J Exp Psych. 1959;58:376–381. doi: 10.1037/h0045837. [DOI] [PubMed] [Google Scholar]
- Stanton ME, Claflin DI, Herbert JS. Ontogeny of multiple memory systems: Eyeblink conditioning in rodents and humans. In: Blumberg MS, Freeman JH Jr, Robinson SR, editors. Oxford Handbook of Developmental Behavioral Neuroscience. Oxford University Press; New York, NY: 2010. pp. 501–526. [Google Scholar]
- Stanton ME, Freeman JH. Developmental studies of eyeblink conditioning in the rat, in Eyeblink Classical Conditioning. In: Woodruff-Pak DS, Steinmetz JH, editors. Animal Models. Vol. 2. Kluwer Academic Publishers; Boston, MA: 2000. pp. 17–49. [Google Scholar]
- Stanton ME, Goodlett CR. Neonatal ethanol exposure impairs eyeblink conditioning in weanling rats. Alcohol Clin Exp Res. 1998;22:270–275. [PubMed] [Google Scholar]
- Steinmetz JE. Brain substrates of classical eyeblink conditioning: A highly localized but also distributed system. Behavioural Brain Res. 2000;110:13–24. doi: 10.1016/s0166-4328(99)00181-3. [DOI] [PubMed] [Google Scholar]
- Thomas JD, Goodlett CR, West JR. Alcohol-induced Purkinje cell loss depends on developmental timing of alcohol exposure and correlates with motor performance. Dev Brain Res. 1998;105:159–166. doi: 10.1016/s0165-3806(97)00164-8. [DOI] [PubMed] [Google Scholar]
- Thompson RF. The neurobiology of learning and memory. Science. 1986;233:941–947. doi: 10.1126/science.3738519. [DOI] [PubMed] [Google Scholar]
- Thompson RF. In search of memory traces. Ann Rev Psychol. 2005;56:1–23. doi: 10.1146/annurev.psych.56.091103.070239. [DOI] [PubMed] [Google Scholar]
- Tran TD, Goodlett CR. Dose-related effects of binge-like ethanol exposure during the early neonatal period on trace eyeblink conditioning in adult rats. Society for Neuroscience; San Diego, CA: 2004. [Google Scholar]
- Tran TD, Thomas JD. Perinatal choline supplementation mitigates trace eyeblink conditioning deficits associated with 3rd trimester alcohol exposure in rodents. Society for Neuroscience Abstracts; 2007. [Google Scholar]
- Werden D, Ross LE. A comparison of the trace and delay classical conditioning performance of normal children. J Exp Child Psychol. 1972;14:126–132. doi: 10.1016/0022-0965(72)90037-9. [DOI] [PubMed] [Google Scholar]
- Woodruff-Pak DS. Eyeblink classical conditioning in H.M.: Delay and trace paradigms. Behav Neurosci. 1993;107:911–925. doi: 10.1037//0735-7044.107.6.911. [DOI] [PubMed] [Google Scholar]
- Woodruff-Pak DS, Disterhoft JF. Where is the trace in trace conditioning? Trends Neurosci. 2008;31:105–112. doi: 10.1016/j.tins.2007.11.006. [DOI] [PubMed] [Google Scholar]
- Woodruff-Pak DS, Steinmetz JE. Eyeblink Classical Conditioning: Volume I—Applications in Humans. Kluwer Academic Publishers; Boston, MA: 2000a. [Google Scholar]
- Woodruff-Pak DS, Steinmetz JE. Eyeblink Classical Conditioning: Volume II—Applications in Animals. Kluwer Academic Publishers; Boston, MA: 2000b. [Google Scholar]