Abstract
Puberty is a critical risk period for binge eating and eating disorders characterized by binge eating. Previous research focused almost entirely on psychosocial risk factors during puberty to the relative exclusion of biological influences. The current study addressed this gap by examining the emergence of binge eating during puberty in a rat model. We predicted that there would be minimal differences in binge eating proneness during pre-early puberty, but significant differences would emerge during puberty. Two independent samples of female Sprague-Dawley rats (n = 30 and n = 36) were followed longitudinally across pre-early puberty, mid-late puberty, and adulthood. Binge eating proneness was defined using the binge eating resistant (BER)/binge eating prone (BEP) model of binge eating that identifies BER and BEP rats in adulthood. Across two samples of rats, binge eating proneness emerged during puberty. Mixed linear models showed little difference in palatable food intake between BER and BEP rats during pre-early puberty, but significant group differences emerged during mid-late puberty and adulthood. Group differences could not be accounted for by changes in non-palatable food intake or body weight. Similar to patterns in humans, individual differences in binge eating emerge during puberty in female rats. Findings provide strong confirming evidence for the importance of biological risk factors in developmental trajectories of binge eating risk across adolescence.
Keywords: binge eating, puberty, animal models, bulimia nervosa, eating disorders
Studies of girls provide evidence that puberty is a critical risk period for the eventual development of eating disorders characterized by binge eating (e.g., bulimia nervosa (BN)) (American Psychiatric Association, 2000; Bulik, 2002). Bulimic syndromes rarely occur in prepubertal girls, and puberty marks an increase in risk for bulimic symptoms (American Psychiatric Association, 2000; Bulik, 2002). Rates of bulimic symptoms increase significantly with advancing pubertal development (Bulik, 2002; Garber, Brooks-Gunn, Paikoff, & Warne, 1994; Killen, Hayward, Hammer, Wilson, Miner, Taylor et al., 1992) and predict the development of BN later in adolescence (Bulik, 2002; Striegel-Moore, Silberstein, & Rodin, 1986). Studies further suggest that risk for bulimia nervosa (BN) is associated with the timing of pubertal development. Early maturing girls are at increased risk for BN and binge eating both during (Attie & Brooks-Gunn, 1989; Kaltiala-Heino, Marttunen, Rantanen, & Rimpela, 2003; Kaltiala-Heino, Rimpela, Rissanen, & Rantanen, 2001) and after puberty (Bulik, 2002; Fairburn, Welch, Doll, Davies, & al., 1997; Zehr, Culbert, Sisk, & Klump, 2007). Thus, although the onset of full bulimic syndromes is typically in late adolescence (American Psychiatric Association, 2000), symptoms contributing to their development appear to increase during puberty.
Theories accounting for pubertal risk focus almost exclusively on the psychosocial effects of the physical changes of puberty (e.g., increased body dissatisfaction) (Bulik, 2002; Garber, Brooks-Gunn, Paikoff, & Warrne, 1994). Although these psychological processes play a role, puberty is associated with numerous biological changes (Wilson, Foster, Kronenberg, & Larsen, 1998) that are very likely to contribute as well. Animal models represent powerful tools for confirming a role for biological influences on behavioral phenotypes without the confounds of psychosocial factors. The presence of increased pubertal risk for binge eating in animals would provide strong confirming evidence of biological influences, as animals do not experience key psychological risk factors (e.g., increased body dissatisfaction) during puberty.
Fortunately, rat models for binge eating in adulthood are well-established (Corwin & Buda-Levin, 2004) and several exhibit face validity for binge eating in humans. The Boggiano rat model of binge eating (Boggiano et al., 2007) is promising in this regard. This model identifies binge eating resistant (BER) and binge eating prone (BEP) female rats based on the consumption of intermittently presented, highly palatable food (PF; foods that are high in fat and sugar with little nutritional value, e.g., vanilla frosting) in adulthood. BER rats are those that consistently consume small amounts of intermittently presented PF across testing days, while BEP rats are those that consistently consume high amounts of PF across testing days. Importantly, BER/BEP differences in PF intake are present upon the very first feeding test (Boggiano et al., 2007); thus, group differences are not simply learned preferences resulting from repeated exposure to PF, but are instead pre-existing differences that are immediately manifested upon first experience with PF.
The BER/BEP model shows good face validity for the binge eating that occurs in BN syndromes. BEP rats binge eat on highly PF, but do not binge eat on standard rat chow (a less PF) (Boggiano et al., 2007; Oswald, Murdaugh, King, & Boggiano, in press). The consumption of chow is equal in BER and BEP rats, suggesting that while both BER and BEP rats prefer PF over chow (as do humans), only BEP rats do not limit their caloric intake in the presence of PF (Boggiano et al., 2007; Oswald et al., in press). BEP rats also may experience a lack of control over their binge episodes, as they endure increasingly high levels of pain (via foot shock) in order to consume PF (Oswald et al., in press). In contrast, BER rats do not endure incremental foot shock in order to consume PF (Oswald et al., in press). In women, binge episodes are intermittent and discrete (i.e., lasting only a few hours) (Boggiano et al., 2007). The BER/BEP framework models this time course by alternating feeding test days with chow only days (making binge eating intermittent) and focusing on the first 4 hours of PF access (making binge eating discrete) (Boggiano et al., 2007).
The BEP rats do not differ significantly from BER rats in body weight (Boggiano et al., 2007). A similar proportion (i.e., 50% each) of BER and BEP rats are prone to diet-induced obesity (Boggiano et al., 2007), suggesting that the BEP phenotype is not simply an obesity prone group of rats. The distribution of binge eating in the BER/BEP model also resembles that observed in women. While binge eating exists on a continuum (from low to high), some women are binge prone while other women are binge resistant. The BER/BEP model includes a continuum of binge eating (from low to high), but also identifies rats who are resistant to binge eating, and rats who are prone to binge eating (Boggiano et al., 2007). Finally, BEP rats are more likely to binge eat in the presence of risk factors, such as stress (Boggiano et al., 2007; Smyth et al., 2007). Indeed, the effects of stress in BER/BEP rats resemble those in women for both the BER group (i.e., they are unlikely to binge eat, even in the presence of stress) and the BEP group (e.g., they are more likely to binge eat and increase binge eating in the presence of stress).
In summary, the BER/BEP model represents many of the phenomenological and distributional qualities of binge eating in women. Like other animal models of binge eating (Corwin & Buda-Levin, 2004), binge eating in BEP rats cannot map entirely onto the binge eating observed in humans, as it is difficult to design an experiment to test all of the cognitive and behavioral aspects (e.g., loss of control) of binge eating in animals. Nonetheless, the BER/BEP model represents several key features of binge eating in women. The model is also highly amenable to a developmental design, as it is relatively easy to implement and does not require extensive or lengthy pre-testing manipulations (e.g., repeated cycles of food restriction) (Corwin & Buda-Levin, 2004) that would be difficult to perform across the brief pre-pubertal and pubertal periods in rats.
In the current study, we used the BER/BEP model to conduct a longitudinal investigation of binge eating risk across pre-puberty, puberty, and adulthood in female rats. We predicted that binge eating phenotypes would emerge during puberty, such that the two phenotypes would be indistinguishable during pre-puberty but would emerge during puberty and persist into adulthood. In order to confirm that observed effects were robust, we examined this hypothesis in two independent samples of rats followed longitudinally across pubertal development.
Methods
Animals
Sixty six (n = 30 for Experiment1, n = 36 for Experiment 2) weanling female Sprague-Dawley rats were obtained from Harlan (Madison, Wisconsin) at postnatal day 19 (i.e., P19). Animals were singly housed in clear Plexiglas cages (45 × 23 × 21 cm) and given ad lib access to water and chow (Rodent diet 8640; Harlan Teklad Global Diets, Madison, WI). Animals were maintained on a 12/12hr light-dark cycle (lights on at 2400h; off at 1200h) and the temperature was maintained at 21 ± 2°C. All animals were treated in accordance with the NIH Guide for the Care and use of Laboratory Animals, and all protocols were approved by the Michigan State Institutional Animal Care and Use Committee.
Experimental Design
Experiment 1
Feeding tests followed standard BER/BEP protocols (Boggiano et al., 2007; Oswald et al., in press) and began on postnatal day 23 (P23). At the beginning of each test, approximately 15–25 grams (with increasing amounts with age) of Betty Crocker Vanilla Frosting (General Mills Inc., Minneapolis, MN) was placed in a small petri dish in the subject’s home cage along with premeasured chow (~300–400 grams) placed into a well on the wire cage top. The petri dish was secured to a wire hook that was hung over the cage wall, and the dish could not easily fall into the bedding on the cage floor. These foods were given just prior to lights out, and the petri dish was weighed before it was given to the rat, and again 1, 2, 4, and 24 hr later. Rats were given open access to both the frosting and the chow during the 24-hour feeding test period. The chow and petri dishes with any remaining frosting were then removed at the 24 hr time point. Body weight was also assessed every day prior to lights out. Feeding tests were given 3x/week (Monday, Wednesday, Friday) from P23 through P69, and chow was provided ad lib between feeding test days. However, during puberty (P39 and P58; see definition below), the 3x/wk feeding tests were decreased to a single maintenance feeding test that was given 1 day/week only. The frequency of feeding tests was decreased during the pubertal period so that preferences for PF could continue to be tracked during this time while minimizing access to high-fat food during this period of rapid body weight gain and hormonal changes associated with reproductive maturation.
Rats were examined daily upon arrival in the laboratory and the age at which vaginal opening occurred was used as the indicator of the onset of puberty. All rats experienced the onset of puberty between feeding test 6 (P34) and 7 (P39), and so feeding tests 1–6 (P23-P34) fell in the pre-early puberty classification, and feeding tests 7–10 (P39-P58) were classified as mid-late puberty. Following standard classifications of adulthood in female rats (Spear, 2000), adulthood was defined as starting at postnatal day 60 and included feeding tests 11–15 (P60-69). Thus, rats received 6 feeding tests in pre-early puberty, 4 feeding tests in mid-late puberty, and 5 feeding tests in adulthood.
Experiment 2
Experiment 2 was identical to Experiment 1 with two exceptions. First, we continued feeding tests 3x/week during puberty rather than conducting 1x/week maintenance tests. The purpose of this modification was to ensure that effects observed in Experiment 1 were not due to differences in the spacing of the feeding tests during mid-late puberty (i.e., 1x/week) versus pre-early puberty and adulthood (i.e., 3x/week).
Second, in Experiment 2, pubertal onset (day of vaginal opening) was more variable, as 11 rats experienced the onset of puberty between feeding tests 5 (P32) and 6 (P34), 21 rats showed puberty onset between feeding tests 6 and 7 (P37), and 4 rats fell between feeding tests 7 and 8 (P39). We therefore classified which feeding tests occurred in the pre-early puberty and mid-late puberty stages for each rat individually (e.g., for the 11 early onset rats, pre-early puberty was feeding tests 1–5, and mid-late puberty was classified as feeding tests 6–16). Adulthood was again defined at postnatal day 60 in all rats and included feeding tests 17–21 (P60-P69). Thus, rats received 5–7 feeding tests during pre-early puberty, 9–11 feeding tests during mid-late puberty, and 5 feeding tests in adulthood.
Statistical Analyses
Statistical analyses were identical in Experiments 1 and 2. We followed the Boggiano et al. method (Boggiano et al., 2007; Oswald et al., in press) for identifying BER and BEP rats by examining tertiles of 4-hour PF intake across the five feeding tests that took place in adulthood. Our focus on the 4-hour intakes comes from previous research with the BER/BEP model (Boggiano et al., 2007; Oswald et al., in press) and other rat models of binge eating (Boggiano et al., 2005; Hagan, Chandler, Wauford, Rybak, & Oswald, 2003; Hagan et al., 2002) confirming that measurable binge eating can be consistently observed and measured during this time interval. After establishing tertiles for PF intake on each individual feeding test day, we identified BER rats as those that ate in the lowest tertile of PF intake on at least three out of the five feeding test days (i.e., 60% of the feeding tests), and who never ate in the highest tertile for any feeding test. By contrast, BEP rats were those that ate in the highest tertile of PF intake on at least 3 out of 5 testing days, and never ate in the lowest tertile during any feeding test. Notably, the proportion of BER or BEP rats in a population could range from 0–100% (i.e., there is no constraint on the number of BER/BEP rats identified) since the BER/BEP definition is based on the frequency and consistency of PF intake across testing days rather than PF tertiles for any given day. This focus on binge eating frequency and consistency closely follows methods for defining binge eating status in eating disorders (e.g., binge eating disorder) where women who binge eat at least 2x/week for three consecutive months (American Psychiatric Association, 2000) are considered to be binge eaters or “binge prone” while those who rarely binge eat are considered non-binge eaters or “binge resistant”. Much like the BER/BEP model, the cut-offs for determining these binge eating groups were based on statistical comparisons of women at the high versus low end of the binge eating distribution (American Psychiatric Association, 1997).
After identifying BER and BEP rats in adulthood, our primary analyses used mixed linear models (MLM) to compare changes in PF intake, chow intake, and body weight across pre-early puberty, mid-late puberty, and adulthood in BER versus BEP rats. We used an autoregressive (lag 1) error structure to model the residual covariance from one feeding trial to the next. In the MLM analysis, the upper-level unit of analysis was the rat (i.e., the level at which observations are independent) and the lower-level unit of analysis was the feeding test (i.e., level at which outcome scores are measured). BER/BEP status was an upper-level predictor (i.e., it varied from rat to rat), and developmental stage was a lower-level predictor (i.e., it varied across feeding tests). When the outcome was PF intake, we expected to find a significant stage by BER/BEP group interaction, where differences in PF intake would be minimal during pre-early puberty and would increase significantly during mid-late puberty and into adulthood. We conducted these same analyses with chow intake and body weight as dependent variables to ensure that observed effects were not due to changes in these other potentially relevant variables.
Similar to previous work (Boggiano et al., 2007; Oswald et al., in press), our primary analyses focused on the 4-hour PF and chow intakes as dependent variables. However, because our pre-pubertal rats were significantly younger than the adult rats examined in previous work (Boggiano et al., 2007), we conducted follow-up analyses using the 24-hour PF intake in the pre-pubertal rats, and the 4-hour intake in pubertal and adult rats. These analyses were used to confirm that biological constraints on the amount of food that could be consumed in a short period of time (i.e., 4 hours) early in development did not unduly influence results. By examining the 24-hour intake in pre-pubertal rats only, we were able to account for pre-pubertal rats’ smaller size and directly test whether effects remain when pre-pubertal rats are given more time to consume PF.1
Although previous work with the BER/BEP model has focused entirely on the categorical BER and BEP groupings, this categorical approach results in a loss of data, as rats that score intermediate to these groups are excluded from analyses. Moreover, methodologists have argued against breaking continuous measures into dichotomies for a number of reasons (see (MacCallum, Zhan, Preacher, & Rucker, 2002). We therefore made use of the continuous “binge proneness” variable that counted the number of times each rat scored in the highest tertile of PF intake during the five adult feeding tests (score range = 0–5). This variable was calculated for all rats in both experiments (n = 30 in Experiment 1, n = 36 in Experiment 2) and then grand mean centered and included as the upper-level predictor in the MLM in place of the BER/BEP categorical variable.
Because of the relatively large number of statistical tests conducted across all analyses, we used a conservative p value of .01.
Results
Experiment 1
We identified 10 BER (10/30; 33%) and 10 BEP (10/30; 33%) rats in adulthood. This proportion is on par with rates observed in previous work (Boggiano et al., 2007; Oswald et al., in press) and suggests that roughly 1/3 of rats consistently consume high levels of PF across multiple testing days (BEP rats), 1/3 consistently consume low levels of PF (BER rats), and the remaining 1/3 were inconsistent in their PF intake and/or ate only moderate amounts of PF.
Table 1 includes means and standard deviations for BER/BEP rats at each developmental stage, and F-tests from the MLM for the main effects of stage, the main effects of BER/BEP status, and the interaction between these two variables. The main effects of developmental stage and BER/BEP group were significant such that 1) all rats ate more PF as they developed, and 2) BEP rats ate more PF than did BER rats. These differences would be expected given the advancing age of the animals and the ways in which the BER/BEP groups were determined. More importantly, there was a significant stage×BER/BEP group interaction, indicating that PF intake differed significantly across stage by BER/BEP group status. As shown in the top panel of Figure 1, in pre-early puberty, BER and BEP rats did not differ in the amount of PF they consumed, but at both mid-late puberty and adulthood, BEP rats ate substantially more PF than did BER rats. Tests of simple main effects confirmed these impressions, as there were no significant differences in PF intake between BER and BEP rats in pre-early puberty, t(31) = 0.08, p = .94, but significant mean differences between groups during mid-late puberty, t(27) = 2.54, p< .01, and adulthood, t(34) = 7.03, p <. 01. These findings remained unchanged when models included 24-hour PF intake in pre-pubertal rats and 4-hour PF intake in pubertal and adult rats. The interaction between BER/BEP status and developmental stage remained statistically significant (F(2,124) = 8.37, p < .01), and mean differences in BER (M = 4.42, SD = 1.12) and BEP (4.78, SD = 1.41) rats’ consumption of PF were non-significant during pre-puberty (F(1,23) = 0.49, p = .52).
Table 1.
Means, Standard Deviations (SD), and F-tests comparing BER Rats to BEP Rats over Three Stages of Development for Experiment 1.
| BER/BEP Group | Pre-Early Puberty |
Mid-Late Puberty |
Adulthood | Stage Main Effect |
BER/BEP Main Effect |
Stage by BER/BEP Interaction |
|
|---|---|---|---|---|---|---|---|
| Palatable Food | F(2,131) = | F(1,89) = | F(2,131) = | ||||
| 217.07** | 41.36** | 17.74** | |||||
| BER | M | 2.20 | 4.07 | 4.97 | |||
| (SD) | (.89) | (1.36) | (1.27) | ||||
| BEP | M | 2.18 | 5.00 | 7.22 | |||
| (SD) | (.93) | (1.72) | (1.69) | ||||
| Chow | F(2,125) = | F(1,63) = | F(2,125) = | ||||
| 33.37** | .70 | .24 | |||||
| BER | M | 2.20 | 4.02 | 4.35 | |||
| (SD) | (1.16) | (1.26) | (1.75) | ||||
| BEP | M | 2.20 | 3.76 | 4.01 | |||
| (SD) | (1.05) | (1.01) | (2.00) | ||||
| Body Weight | F(2,273) = | F(1,15) = | F(2,273) = | ||||
| 46.52** | .001 | .02 | |||||
| BER | M | 67.52 | 150.60 | 193.77 | |||
| (SD) | (19.36) | (23.71) | (10.11) | ||||
| BEP | M | 68.64 | 150.54 | 193.53 | |||
| (SD) | (17.65) | (25.55) | (16.65) | ||||
Note. BER = binge eating resistant; BEP = binge eating prone; Stage = pre-early puberty, mid-late puberty, or adulthood. Pre-Early Puberty included feeding tests 1–6, Mid-Late Puberty included feeding tests 7–10, and Adulthood included feeding tests 11–15.
p < .01
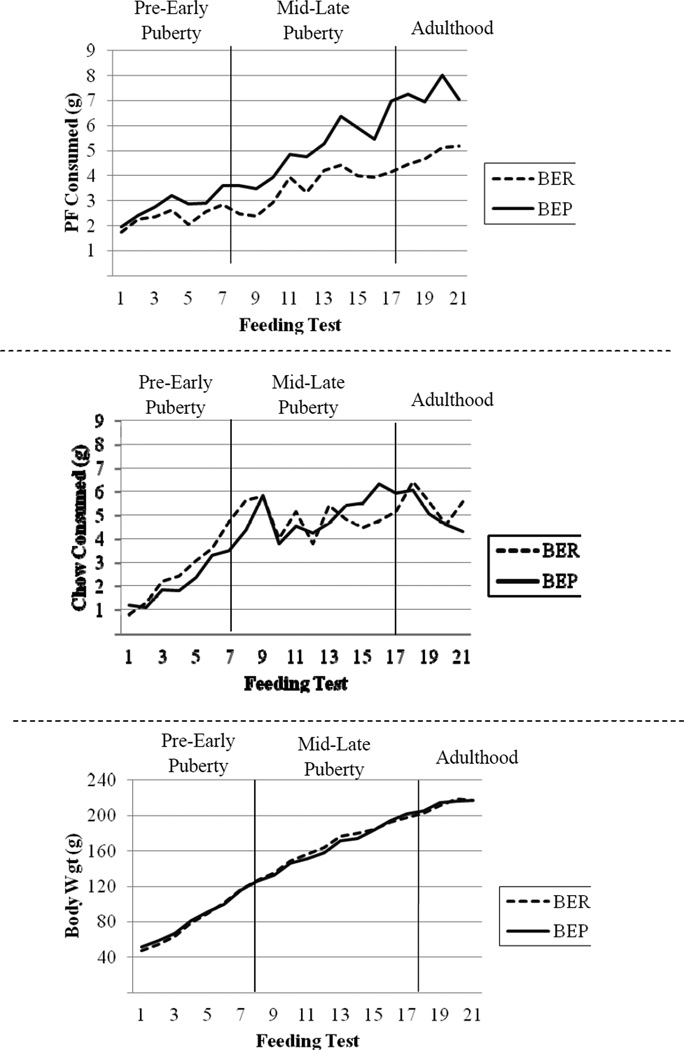
Figure 1.
Patterns of Mean PF Intake (top panel), Chow Intake (middle panel) and Body Weight (bottom panel) as a function of Binge Eating Resistant (BER) and Binge Eating Prone (BEP) Status across Development for Experiment 1. Due to a scale malfunction, chow intake is missing for feeding test 1 in all rats.
Importantly, BER/BEP group differences also could not be accounted for by differences in chow intake or body weight during any stage. Although there were significant main effects of stage, there were no main effects of BER/BEP group status, and the stage×BER/BEP group interaction was non-significant for both variables (see Table 1, and middle and bottom panels of Figure 1). The lack of significant difference in body weight is similar to what has been observed previously (Boggiano et al., 2007) and is likely due to the fact that: 1) access to PF is only provided intermittently every 3 days; and 2) BEP rats did not consume larger amounts of chow on non-feeding test days, F(1,205) = 0.81, p = .37. Overall, these findings suggest that differences in PF intake by stage and BER/BEP status are unlikely to be due to differences in chow intake or body weight.
Identical results were obtained using the continuous binge proneness variable in the full sample of rats (n = 30). As with the categorical BER/BEP groupings, the main effects of stage, F(2,199) = 338.37, p < .01, and binge proneness, F(1,131) = 41.14, p < .01, and the stage×binge proneness interaction, F(2,199) = 22.21, p < .01, were significant for PF intake. By contrast, for chow intake and body weight, there were no significant main effects of binge proneness [Chow: F(1,97) = .02, p = .90; Body weight: F(1, 24) = .00, p = .95] and no significant stage×binge proneness interactions [Chow: F(2,197) = .55, p = .58; Body weight: F(2,412) = .04, p = .96]. These findings suggest that our results are not due to the dichotomization of BER and BEP rats, but instead reflect the full distribution of binge proneness.
Experiment 2
In Experiment 2, we again identified a sizable number of BER (7/36; 19%) and BEP (11/36; 30%) rats in adulthood. The proportion of BER rats was somewhat smaller in Experiment 2 (19%) than Experiment 1 (30%); this highlights the fact that, similar to what is observed in women, the proportion of BER and BEP rats can vary by population despite general trends toward particular prevalence rates (e.g., ~20–30%) across samples (Boggiano et al., 2007; Oswald et al., in press).
In the MLM, there were significant main effects of stage and BER/BEP group status, and the stage×BER/BEP group interaction was again statistically significant (see Table 2). Figure 2 and tests of simple effects confirmed that BEP/BER differences in PF intake increased across development, as group differences were not statistically significant during pre-early puberty, t(17) = 2.04, p = .06, but were statistically significant during both mid-late puberty, t(23) = 3.26, p < .01, and adulthood, t(25) = 6.94, p < .01. Consistent with Experiment 1, these findings did not appear to be unduly influenced by our use of 4- versus 24-hour intakes of PF during prepuberty. When 24-hour PF intakes during pre-puberty only were included in the models, the stage by BER/BEP group interaction continued to be significant (F(2,166) = 4.56, p = .01). Although group differences in pre-puberty, 24-hour PF intakes between BER (M = 4.69, SD = 0.80) and BEP (M = 5.77, SD = 1.26) rats were larger than those observed in Experiment 1 (t(20) = 2.86, p = .01), they were smaller than the group differences observed during mid-late puberty and adulthood (see Tables 1 and 2).
Table 2.
Means, Standard Deviations, and F-tests comparing BER Rats to BEP Rats over Three Stages of Development for Experiment 2.
| BER/BEP Group | Pre-Early Puberty |
Mid-Late Puberty |
Adulthood | Stage Main Effect |
BER/BEP Main Effect |
Stage by BER/BEP Interaction |
|
|---|---|---|---|---|---|---|---|
| Palatable Food | F(2,159) = | F(1,47) = | F(2,159) = | ||||
| 69.69** | 39.20** | 7.86** | |||||
| BER | M | 2.25 | 3.41 | 4.72 | |||
| (SD) | (0.47) | (1.18) | (1.26) | ||||
| BEP | M | 2.72 | 4.69 | 7.25 | |||
| (SD) | (0.76) | (1.38) | (1.26) | ||||
| Chow | F(2,138) = | F(1,83) = | F(2,138) = | ||||
| 93.5** | .70 | .34 | |||||
| BER | M | 2.04 | 4.83 | 5.47 | |||
| (SD) | (1.13) | (1.30) | (1.66) | ||||
| BEP | M | 1.88 | 4.80 | 5.19 | |||
| (SD) | (0.91) | (1.47) | (1.81) | ||||
| Body Weight | F(2,354) = | F(1,14) = | F(2,354) = | ||||
| 15.27** | .000 | .37 | |||||
| BER | M | 81.09 | 164.59 | 214.15 | |||
| (SD) | (19.97) | (25.90) | (15.65) | ||||
| BEP | M | 85.14 | 163.42 | 214.39 | |||
| (SD) | (20.02) | (25.90) | (13.32) | ||||
Note. BER = binge eating resistant; BEP = binge eating prone; Stage = pre-early puberty, mid-late puberty, or adulthood. Pre-early puberty and mid-late puberty varied by rat, but occurred between feeding tests 1–7 and 6–16, respectively, for the full sample. Adulthood occurred between feeding tests 17 and 21 for all rats.
p < .01
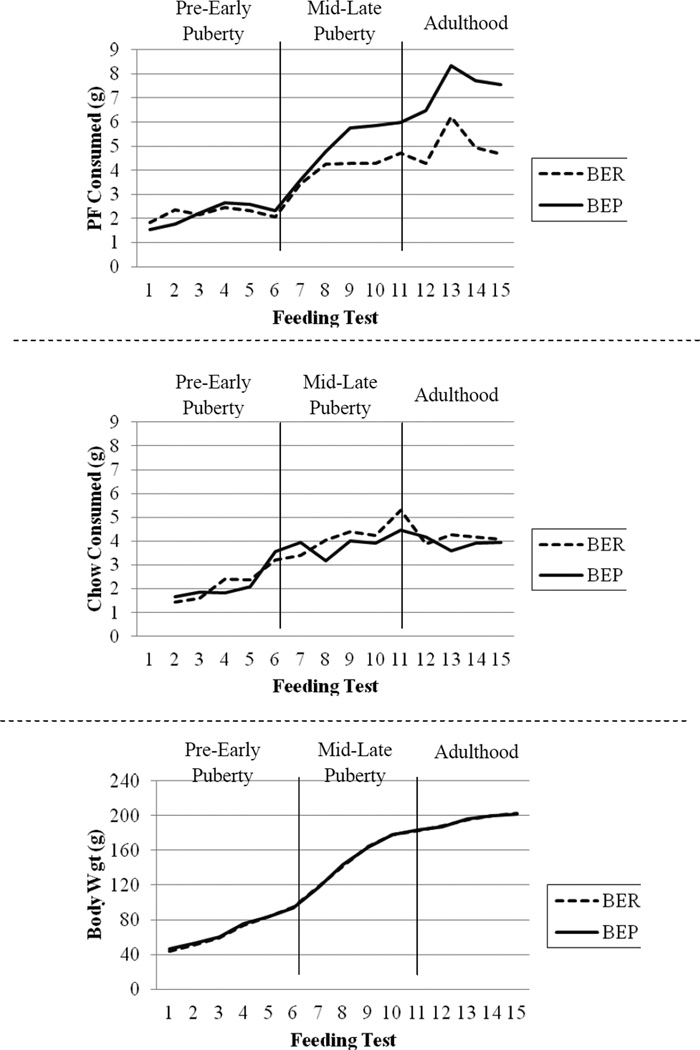
Figure 2.
Patterns of Mean PF Intake (top panel), Chow Intake (middle panel) and Body Weight (bottom panel) as a Function of Binge Eating Resistant (BER) and Binge Eating Prone (BEP) Status across Development for Experiment 2. Because of the large number of feeding tests in Experiment 2 (i.e., 21 tests), every other feeding test (rather than every feeding test) is pictured in the graph.
Also similar to findings for Experiment 1, the main effects of BER/BEP group and the stage by BER/BEP group interactions were non-significant for both chow intake and body weight (see Table 2 and Figure 2). The lack of significant BER/BEP group differences in body weight again appears to be due to the fact that that BEP rats did not consume significantly larger amounts of chow than BER on non-feeding test days, F(1,129) = 4.04, p = .05). Indeed, the small group differences that were observed were due to slightly larger amounts of 24-hour chow intakes in the BER (M = 13.45, SD = 0.21) relative to the BEP rats (M = 12.93, SD = 0.16) on non-feeding test days.
Finally, analyses of the continuous binge proneness variable in the full sample of rats (N = 36) yielded very similar findings. As with the categorical BER/BEP groupings, the main effects of stage [F(2,300) = 193.38, p < .01] and binge proneness [F(1,103) = 77.06, p < .01], and the stage×binge proneness interaction [F(2,299) = 11.02, p < .01] were significant for PF intake. Chow consumption and body weight showed no significant main effects of binge proneness [Chow: F(1,181) = .13, p = .72; Body weight: F(1,30) = .01, p = .91] and no significant stage by binge proneness interactions [Chow: F(2,287) = .34, p = .72; Body weight: F(2,712) = .29, p = .75].
Conclusions
This is the first study to examine the emergence of binge eating proneness during puberty in animals. Results revealed dramatic increases in the binge prone phenotype across puberty, such that there was little evidence of individual differences in binge proneness during pre-early puberty, but significant differences during mid-late puberty and adulthood. Developmental effects were robust across two independent samples and categorical as well as continuous definitions of binge prone status. These findings are significant in suggesting that increases in binge eating and eating disorders characterized by binge eating during and after puberty may be at least partially due to biological factors. Indeed, the presence of these phenotypic effects in animals strongly suggests that factors other than psychological influences (e.g., increased body dissatisfaction) contribute to individual differences in binge eating risk in females during puberty.
The BER/BEP rats we identified in adulthood closely resembled those identified previously in terms of their patterns of PF intake, chow intake, and body weight. The BEP phenotype also resembles several aspects of binge eating observed in humans, including a preferential increases in PF (but not chow) consumption, particularly in response to stress (Boggiano et al., 2007; Oswald et al., in press). Nonetheless, the percentage (~30%) of adult rats identified as binge prone in our and previous research (Boggiano et al., 2007; Oswald et al., in press) is higher than estimates of binge eating in older adolescent and young adult women (~10–19%) (Gauvin, Steiger, & Brodeur, 2009; Haines, Neumark-Sztainer, Eisenberg, & Hannan, 2006; Hay, Mond, Buttner, & Darby; Jones, Bennett, Olmsted, Lawson, & Rodin, 2001). In addition, the BEP rats do not experience the weight losses and gains commonly observed in women with binge eating. These differences highlight the continuing gaps in our knowledge regarding the face validity of the BER/BEP and other animal models of binge eating.
However, an alternative interpretation is that these differences may not be surprising when one considers that BEP rats do not experience the negative environmental (e.g., social disapproval) and psychological (e.g., guilt, self-blame, fears of weight gain) consequences (Fairburn, Marcus, & Wilson, 1993) of binge eating that are common in women who binge eat. These negative consequences likely act as social and physical constraints on binge eating in women, such that a smaller proportion of women develop binge eating, and they engage in compensatory behaviors (e.g., dieting) that cause physical (i.e., weight fluctuations) and physical/psychological (e.g., weight suppression; Butryn, Lowe, Safer, & Agras, 2006; Lowe, Thomas, Safer, & Butryn, 2007) features that we do not observe in BEP rats. In essence, the BEP rats may represent a model of “pure” binge eating proneness as it exists in the population before moderation by environmental/psychological factors that decrease the likelihood of binge eating in all women. Clearly, this hypothesis requires empirical testing, but is an intriguing possibility to consider in future research. Indeed, although no animal model is an exact replica of all binge eating characteristics in humans, the BER/BEP model appears promising for understanding the biological basis of several aspects of human binge eating.
The emergence of the binge prone phenotype during puberty further contributes to the face validity of the model. All rats prefer PF, regardless of developmental stage; this is similar to what we see in humans where a preference for PF (e.g., candy) is present in most girls across development. Importantly, however, we begin to see a divergence in the preference for PF intake during puberty, with BEP rats consuming much more PF than BER rats. This increase in individual differences is similar to what is observed in humans, where binge eating symptoms increase during puberty in some, but not all, girls (Corcos et al., 2000; Killen, Hayward, Hammer, Wilson, Miner, C.B. et al., 1992). This emergence previously has been attributed to psychological (e.g., increased negative affect, body dissatisfaction), physiological (e.g., caloric deprivation from increases in dieting), and psychosocial (e.g., increased pressures for thinness) factors (Bulik, 2002; Garber, Brooks-Gunn, Paikoff, & Warne, 1994). The emergence of individual differences in binge eating proneness in female rats suggests that biological factors related to puberty are critical for this developmental pattern as well.
The biological factors contributing to puberty’s effects on individual differences in binge proneness are not yet known. Hormone-independent processes cannot be ruled out, but given the myriad hormonal changes associated with puberty in combination with robust hormonal influences on food intake (Asarian & Geary, 2006), hormones are likely candidates. For example, ovarian hormones and leptin both become elevated during puberty and are linked to homeostatic regulation of food intake (Wilson et al., 1998). Of these two classes of hormones, it seems more likely that ovarian hormones play a role in the pubertal manifestation of binge eating phenotypes. Ovarian hormones drive pubertal development in females in both rats and humans (Wilson et al., 1998) and show phenotypic (Edler, Lipson, & Keel, 2007; Klump, Culbert, Edler, & Keel, 2008) as well as genetic associations (Klump, Keel, Sisk, & Burt, 2010) with binge eating. For example, binge eating is negatively associated with estradiol levels, and positively associated with progesterone levels, in clinical (i.e., BN women) (Edler et al., 2007) and non-clinical (Klump et al., 2008) samples of women. Associations have been observed across the menstrual cycle where it can be confirmed that changes in ovarian hormones drive changes in binge eating rather than the reverse. These apparent causal relationships are not surprising given extant animal data showing that experimental manipulations of both hormones (via ovariectomy, hormone administration) cause predictable changes in food intake in a variety of species (Asarian & Geary, 2006). Indeed, a recent study confirmed that estradiol reduces fat intake under binge-like conditions in ovariectomized, adult female rats (Yu, Geary, & Corwin, 2008).
Ovarian hormones also exhibit genetic associations with binge eating. Previous research suggests that the heritability of disordered eating is activated at puberty, such that genes account for 0% of the heritability during pre-puberty and ~50% during and after puberty (Culbert, Burt, McGue, Iacono, & Klump, 2009; Klump, McGue, & Iacono, 2003; Klump, Perkins, Burt, McGue, & Iacono, 2007). Follow-up research has confirmed a role for estradiol in these effects. After dividing twins by high versus low estradiol levels during puberty,Klump et al. (2010) found no evidence for genetic effects on binge eating and disordered eating symptoms in twins with low estradiol levels, but significant genetic effects in twins with high estradiol levels. Findings remained unchanged when controlling for age, body mass index, and the physical changes of puberty (e.g., breast development), suggesting direct effects of estradiol on genetic risk for binge eating and disordered eating.
Taken together, previous data suggest that the emergence of individual differences in binge eating during puberty may be due to increases in ovarian hormones in females during this important developmental stage. These increases may “activate” genetic risk in vulnerable individuals and lead to increased expression of, and individual differences in, binge eating in both rats and humans. Unfortunately, our data are unable to directly examine this hypothesis, as we did not directly manipulate ovarian hormone exposure or examine gene expression. Future animal research should directly examine these possibilities by experimentally manipulating ovarian hormones (e.g., via ovariectomy) before, during, and after puberty to determine whether the emergence of individual differences in binge proneness is dependent upon the presence of these hormones. Ideally, these investigations would also investigate gene expression patterns within the central nervous system (CNS) in order to identify neural systems that contribute to individual differences in binge proneness.
Despite the strengths of our study (e.g., longitudinal data, replication across two samples), there are limitations that must be noted. First, although the BER/BEP model appears promising for understanding biological influences on binge eating, it cannot be determined for certain that the phenotype is the same as that observed in humans. Additional work examining the validity of the model is needed, including continued efforts to model cognitive (e.g., loss of control) and behavioral (e.g., weight suppression; Butryn et al., 2006; Lowe et al., 2007) symptoms of eating disorders in BEP versus BER rats.
Second, sample sizes in our BER/BEP groups were small in both experiments. Although our use of categorical and continuous measures of binge proneness partially addressed this concern, future research should examine larger samples of rats to replicate our results. Finally, because of limited resources for this internally funded project, we were unable to identify causal mechanisms underlying puberty’s effects. Additional research should investigate the role of ovarian hormones and other biological factors to understand the mechanisms underlying developmental changes in binge eating across puberty.
Acknowledgements
The research was supported in part by a National Institute of Mental Health Grant (084470) awarded to Kristen Culbert. All authors had full access to the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. The authors would like to thank Rayson Figueira for his invaluable help in collecting study data.
Footnotes
We also conducted these analyses using 24-hour PF intake across all three developmental states (i.e., pre-puberty, puberty, and adulthood). Results remained largely unchanged from those reported herein (data not shown), albeit with somewhat decreased BER/BEP group differences in PF intake during puberty in Study 2 only (p = .03 versus p< .01).
None of the authors have financial conflicts of interest.
References
- American Psychiatric Association. DSM-IV Sourcebook, Volume 3. Washington, DC: American Psychiatric Association; 1997. [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition - Text Revision (DSM-IV-TR) Washington, DC: American Psychiatric Association; 2000. [Google Scholar]
- Asarian L, Geary N. Modulation of appetite by gonadal steroid hormones. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences. 2006;361(1471):1251–1263. doi: 10.1098/rstb.2006.1860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attie I, Brooks-Gunn J. Development of eating problems in adolescent girls: A longitudinal study. Developmental Psychology. 1989;25:70–79. [Google Scholar]
- Boggiano MM, Artiga AI, Pritchett CE, Chandler-Laney PC, Smith ML, Eldridge AJ. High intake of palatable food predicts binge-eating independent of susceptibility to obesity: an animal model of lean vs. obese binge-eating and obesity with and without binge-eating. International Journal of Obesity (London) 2007;31(9):1357–1367. doi: 10.1038/sj.ijo.0803614. [DOI] [PubMed] [Google Scholar]
- Boggiano MM, Chandler PC, Viana JB, Oswald KD, Maldonado CR, Wauford PK. Combined dieting and stress evoke exaggerated startle responsons to opioids in binge-eating rats. Behavioral Neuroscience. 2005;119:1207–1214. doi: 10.1037/0735-7044.119.5.1207. [DOI] [PubMed] [Google Scholar]
- Bulik CM. Eating disorders in adolescents and young adults. Child and Adolescent Psychiatric Clinics. 2002;11:201–218. doi: 10.1016/s1056-4993(01)00004-9. [DOI] [PubMed] [Google Scholar]
- Butryn ML, Lowe MR, Safer DL, Agras WS. Weight suppression is a robust predictor of outcome in the cognitive-behavioral treatment of bulimia nervosa. Journal of Abnormal Psychology. 2006;115:62–67. doi: 10.1037/0021-843X.115.1.62. [DOI] [PubMed] [Google Scholar]
- Corcos M, Flament MF, Giraud MJ, Paterniti S, Ledoux S, Atger F, et al. Early psychopathological signs in bulimia nervosa. A retrospective comparison of the period of puberty in bulimic and control girls. European Child and Adolescent Psychiatry. 2000;9:115–121. doi: 10.1007/s007870050006. [DOI] [PubMed] [Google Scholar]
- Corwin RL, Buda-Levin A. Behavioral models of binge-type eating. Physiol Behav. 2004;82(1):123–130. doi: 10.1016/j.physbeh.2004.04.036. [DOI] [PubMed] [Google Scholar]
- Culbert KM, Burt SA, McGue M, Iacono WG, Klump KL. Puberty and the genetic diathesis of disordered eating. Journal of Abnormal Psychology. 2009;118:201–218. doi: 10.1037/a0017207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edler C, Lipson SF, Keel PK. Ovarian hormones and binge eating in bulimia nervosa. Psychological Medicine. 2007;37(1):131–141. doi: 10.1017/S0033291706008956. [DOI] [PubMed] [Google Scholar]
- Fairburn CG, Marcus MD, Wilson GT, editors. Cognitive-behavioral therapy for binge eating and bulimia nervosa: A comprehensive treatment manual. New York: The Guilford Press; 1993. [Google Scholar]
- Fairburn CG, Welch SL, Doll HA, Davies BA, et al. Risk factors for bulimia nervosa: A community-based case-control study. Archives of General Psychiatry. 1997;54:509–517. doi: 10.1001/archpsyc.1997.01830180015003. [DOI] [PubMed] [Google Scholar]
- Garber J, Brooks-Gunn J, Paikoff R, Warne M. Prediction of eating problems: An 8-year study of adolescent girls. Developmental Psychology. 1994;30:823–834. [Google Scholar]
- Garber J, Brooks-Gunn J, Paikoff R, Warrne M. Prediction of eating problems: An 8-year study of adolescent girls. Developmental Psychology. 1994;30:823–834. [Google Scholar]
- Gauvin L, Steiger H, Brodeur JM. Eating-disorder symptoms and syndromes in a sample of urban-dwelling Canadian women: Contributions toward a population health perspective. 2009;42:158–165. doi: 10.1002/eat.20590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagan MM, Chandler PC, Wauford PK, Rybak RJ, Oswald KD. The role of palatable food and hunger as trigger factors in an animal model of stress-induced binge eating. International Journal of Eating Disorders. 2003;34:183–197. doi: 10.1002/eat.10168. [DOI] [PubMed] [Google Scholar]
- Hagan MM, Wauford PK, Chandler PC, Jarrett LA, Rybak RJ, Blackburn K. A new animal model of binge-eating: Key synergistic role of past caloric restriction and stress. Physiology and Behavior. 2002;77:45–54. doi: 10.1016/s0031-9384(02)00809-0. [DOI] [PubMed] [Google Scholar]
- Haines J, Neumark-Sztainer D, Eisenberg ME, Hannan PJ. Weight teasing and disordered eating behaivors in adolescents: Longitudinal findings from Project EAT (Eating Among Teens) Pediatrics. 2006;117:e209–e215. doi: 10.1542/peds.2005-1242. [DOI] [PubMed] [Google Scholar]
- Hay PJ, Mond J, Buttner P, Darby A. Eating disorder behaviors are increasing: Findings from two sequential community surveys in South Australia. PLoS ONE. 3(2):1–5. doi: 10.1371/journal.pone.0001541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones JM, Bennett S, Olmsted MP, Lawson ML, Rodin G. Disordered eating attitudes and behaviours in teenaged girls: A school-based study. Canadian Medical Association Journal. 2001;165(5):547–552. [PMC free article] [PubMed] [Google Scholar]
- Kaltiala-Heino R, Marttunen M, Rantanen P, Rimpela M. Early puberty is associated with mental health problems in middle adolescence. Social Science and Medicine. 2003;57:1055–1064. doi: 10.1016/s0277-9536(02)00480-x. [DOI] [PubMed] [Google Scholar]
- Kaltiala-Heino R, Rimpela M, Rissanen A, Rantanen P. Early puberty and early sexual activity are associated with bulimic-type eating pathology in middle adolescence. Journal of Adolescent Health. 2001;28:346–352. doi: 10.1016/s1054-139x(01)00195-1. [DOI] [PubMed] [Google Scholar]
- Killen J, Hayward C, Hammer L, Wilson D, Miner B, CB T, et al. Is puberty a risk factor for eating disorders? American Journal of Disorders of Childhood. 1992;146:323–325. doi: 10.1001/archpedi.1992.02160150063023. [DOI] [PubMed] [Google Scholar]
- Killen J, Hayward C, Hammer L, Wilson D, Miner B, Taylor C, et al. Is puberty a risk factor for eating disorders? American Journal of Disorders of Childhood. 1992;146:323–325. doi: 10.1001/archpedi.1992.02160150063023. [DOI] [PubMed] [Google Scholar]
- Klump KL, Culbert KM, Edler C, Keel PK. Ovarian hormones and binge eating: Exploring associations in community samples. Psychological Medicine. 2008;38(12):1749–1757. doi: 10.1017/S0033291708002997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klump KL, Keel PK, Sisk CL, Burt SA. Preliminary evidence that estradiol moderates genetic effects on disordered eating attitudes and behaviors during puberty. Psychological Medicine. 2010;40(10):1745–1754. doi: 10.1017/S0033291709992236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klump KL, McGue M, Iacono WG. Differential heritability of eating attitudes and behaviors in prepubertal versus pubertal twins. International Journal of Eating Disorders. 2003;33(3):287–292. doi: 10.1002/eat.10151. [DOI] [PubMed] [Google Scholar]
- Klump KL, Perkins P, Burt SA, McGue M, Iacono WG. Puberty moderates genetic influences on disordered eating. Psychological Medicine. 2007;37:627–634. doi: 10.1017/S0033291707000189. [DOI] [PubMed] [Google Scholar]
- Lowe MR, Thomas JG, Safer DL, Butryn ML. The relationship of weight suppression and dietary restraint to binge eating in bulimia nervosa. International Journal of Eating Disorders. 2007;40(7):640–644. doi: 10.1002/eat.20405. [DOI] [PubMed] [Google Scholar]
- MacCallum RC, Zhan S, Preacher KJ, Rucker DD. On the practice of dichotomization of quantitative variables. Psychological Methods. 2002;7:19–40. doi: 10.1037/1082-989x.7.1.19. [DOI] [PubMed] [Google Scholar]
- Oswald KD, Murdaugh DL, King VL, Boggiano MM. Motivation for palatable food despite consequences in an animal model of binge eating. International Journal of Eating Disorders. doi: 10.1002/eat.20808. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smyth JM, Wonderlich SA, Heron KE, Sliwinski MJ, Crosby RD, Mitchell JE, et al. Daily and momentary mood and stress are associated with binge eating and vomiting in bulimia nervosa patients in the natural environment. Journal of Consulting and Clinical Psychology. 2007;75(4):629–638. doi: 10.1037/0022-006X.75.4.629. [DOI] [PubMed] [Google Scholar]
- Spear LP. The adolescent brain and age-related behavioral manifestations. Neuroscience and Biobehavioral Reviews. 2000;24(4):417–463. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- Striegel-Moore RH, Silberstein LR, Rodin J. Toward an understanding of risk factors for bulimia. American Psychologist. 1986;41:246–263. doi: 10.1037//0003-066x.41.3.246. [DOI] [PubMed] [Google Scholar]
- Wilson JD, Foster DW, Kronenberg HM, Larsen PR. Williams Textbook of Endocrinology. 9th Edition. Philadelphia, PA: W.B. Saunders Company; 1998. [Google Scholar]
- Yu Z, Geary N, Corwin RL. Ovarian hormones inhibit fat intake under binge-type conditions in ovariectomized rats. Physiology & Behavior. 2008;95:501–507. doi: 10.1016/j.physbeh.2008.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zehr JL, Culbert KM, Sisk CL, Klump KL. Long-term effects of early puberty on disordered eating and anxiety in men and women. Hormones and Behavior. 2007;52:427–435. doi: 10.1016/j.yhbeh.2007.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]