Abstract
Studies of human immune diseases are generally limited to the analysis of peripheral blood lymphocytes of heterogenous patient populations. Improved models are needed to allow analysis of fundamental immunologic abnormalities predisposing to disease and in which to assess immunotherapies. Immunodeficient mice receiving human fetal thymus grafts and fetal CD34+ cells i.v. produce robust human immune systems, allowing analysis of human T cell development and function. However, to use humanized mice to study human immune-mediated disorders, immune sytsems must be generated from adult hematopoietic cells. Here, we demonstrated robust immune reconstitution in mice with hematopoietic stem cells (HSCs) aspirated from bone marrow of adults with Type 1 diabetes (T1D) and healthy control volunteers. In these humanized mice, cryopreservation of HLA allele-matched fetal thymic tissue prevented allogeneic adult HSC rejection. Newly generated T cells, which included regulatory T cells, were functional, self-tolerant, and had a diverse repertoire. The immune recognition of these mice mimicked that of the adult CD34+ cell donor, but the T cell phenotypes were more predominantly “naïve” than those of the adult donors. HSCs from T1D and control donors generated similar numbers of natural Tregs intrathymically; however, peripheral T cells from T1D subjects showed increased proportions of activated or memory cells compared to controls, suggesting possible HSC-intrinsic differences in T cell homeostasis that might underly immune pathology in T1D. This “Personalized Immune” (PI) mouse provides a new model for individualized analysis of human immune responses that may provide new insights into not only T1D, but other forms of immune function and dysfunction as well.
Introduction
While large-scale studies of human populations have provided important clues to the genetic basis of immune diseases and responses, little is known about the mechanisms by which these genes exert their effects. The ability to dissect these mechanisms in patient populations is currently limited largely to the analysis of peripheral blood lymphocytes from individuals with diverse disease characteristcs, duration, treatments and environments, and in whom immunological causes and effects of inflammatory cascades cannot be readily distinguished. Thus, there is a need for models that eliminate all of these inter-individual variables while allowing analysis of individuals with demonstrated disease. While human peripheral blood mononuclear cells (PBMC) can populate immunodeficient mice (1), the function of T cells is limited in this setting and complicated by xenogeneic graft-vs-host reactivity (2). Human T cells develop in human fetal thymus (THY) grafts implanted with fetal liver under the kidney capsule (3). The combination of intravenous human hematopoietic stem cell (HSC) infusion with human fetal thymus and liver (THY/LIV) grafts under the kidney capsule allows human immune reconstitution with high levels of peripheral human T cells, B cells, and both myeloid and plasmacytoid dendritic cells (4), with antigen-specific immune responses in vivo(4-6). Normal thymic development of regulatory T cells (Tregs) with suppressive function (7) and homeostatic peripheral expansion of human T cells occurs (8). However, this model is limited to the analysis of fetal HSC-derived immune systems.
Humanized mice provide the opportunity to analyze the effects of autoimmunity-associated genetic polymorphisms on immune regulation. Recently-defined non-HLA-linked genes collectively confer substantial autoimmune disease risk (9-12). In humans with autoimmune diseases, however, underlying immunoregulatory defects arising from non-HLA-associated genes are largely undefined. Given that many of these loci contain immunoregulatory genes, such as cytokines, costimulatory and inhibitory molecules (9-12), intrinsic abnormalities in the cells of the immune system, which originate from hematopoietic stem cells (HSCs), likely contribute to the development of autoimmunity. Consistently, diabetes disease susceptibility is transferred via hematopoietic cells in NOD mice (13) and possibly in humans (14). However, studies of patients with disease cannot distinguish underlying causes from effects of disease evolution, disease treatment or precipitating environmental factors.
Fulfillment of the above basic research potentialities of humanized mice would require achievement of human immune reconstitution and function with adult HSCs obtained from patients. However, these cells are not available in large quantities from study volunteers, and adult HSCs engraft less efficiently than fetal CD34+ cells in immunodeficient mice (15). Furthermore, even if obtained in large quantities for therapeutic applications, adult HSCs may be rejected by allogeneic thymocytes pre-existing in fetal thymus grafts. Here, we report the development of a new humanized mouse model that supports robust peripheral reconstitution of T cells and APCs from small numbers of adult, allogeneic bone marrow CD34+ cells. This “Personalized Immune” (PI) mouse model could potentially be used to identify HSC-intrinsic immune abnormalities predisposing to autoimmunity as well as model individual human immune responses in both health and disease.
Results
Overcoming the immune barrier imposed by mature T cells in fetal thymus grafts
To assess human immune reconstitution from adult HSCs in immunodeficient mice, CD34+ cells were isolated from discarded human bone marrow infusion filters and given i.v. to sublethally irradiated nonobese diabetic-severe combined immunodeficient (NOD/SCID) mice receiving fetal THY transplantation. Recipients of untreated fetal human THY grafts showed low peripheral T cell reconstitution during the first weeks after transplantation, which declined markedly over time, suggesting that these cells emigrated from the graft (average CD3+ cell reconstitution 7.0% ± 8.22% of PBMC at 6 weeks, n=5 vs 2.34% ± 2.26% at 16 weeks post-transplantation). Non-T cells did not reconstitute from injected allogeneic CD34+ cells, suggesting that these were rejected. Moreover, some animals reconstituted with fetal human THY grafts and CD34+ cells have developed a late-onset graft-versus-host disease (GVHD)-like wasting syndrome that resulted in mortality (Fig. S1). We hypothesized that thymocytes pre-existing in the THY grafts might reject allogeneic CD34+ cells and expand to attack recipient tissues, preventing immune reconstitution and causing xenogeneic GVHD, respectively. We therefore tested methods for depleting graft thymocytes in an effort to prevent these phenomena.
Fetal thymus organ culture (FTOC) with 2’-deoxyguanosine (dGuo) depletes thymocytes while preserving stromal elements (16) that can support thymopoiesis (17). NOD/SCID mice received allogeneic adult CD34+ cells plus fetal THY tissue cultured for 7 or 21 days in the presence of dGuo. Control animals received fetal liver CD34+ cells from the thymic tissue donor. Mice that received 5×105 adult CD34+ cells without a THY graft reconstituted an average of 20% human PBMCs by Week 10 (Figure 1A), with robust B cell reconstitution (Figure 1B), but CD3+ cells were undetectable (Figure 1C). In mice that received 7 day dGuo-cultured THY tissue plus allogeneic CD34+ cells, CD3+ levels averaging ~7% of PBMC were detectable by 6 weeks and subsequently declined (Figure 1C), but CD19+ cells did not appear (Figure 1B). These data suggest that mature T cells escaping dGuo depletion may have rejected the infused allogeneic CD34+ cells. Cells within the THY grafts did not achieve human non-T cell reconstitution or a high level of T cell reconstitution (Figure 1C).
Figure 1. Peripheral human cell reconstitution in NOD/SCID mice following transplantation of dGuo-treated thymic tissue.
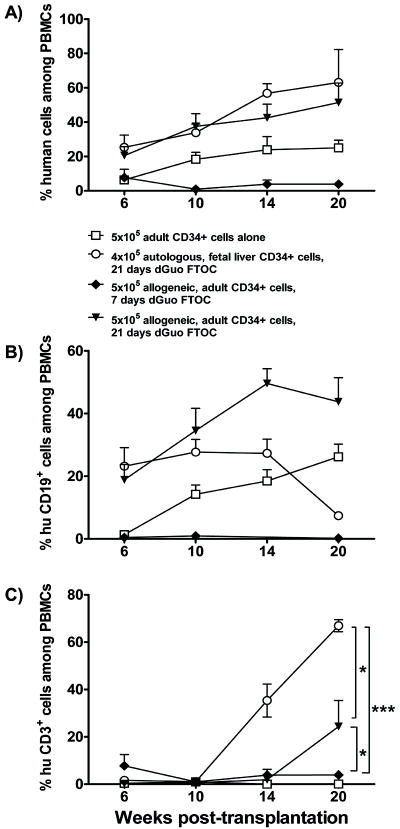
Fetal thymic tissue was treated with dGuo for 7 (black diamonds, n=4) or 21 days (black triangles, n=5) before transplantation into sublethally irradiated NOD/SCID that received 5×105 allogeneic, adult CD34+ cells or 4×105 autologous fetal liver CD34+ cells (open circles, n=5) intravenously. Age-matched control animals received 5×105 adult CD34+ cells alone (open squares, n=5). The mice were bled to measure human (hu) cell reconstitution in (total mouse plus human) peripheral blood mononuclear cells (PBMCs) at the indicated time points. A: total human chimerism; B: percentage of human B cells; C: percentage of human T cells among PBMCs at indicated time points.
In contrast, successful thymic engraftment with human thymopoiesis as well as peripheral CD19+ cell reconstitution occurred after intravenous infusion of 5×105 allogeneic adult CD34+ cells with a 21-day dGuo-cultured THY graft, (average ~25% human CD3+ cells among PBMC at 20 weeks, Figure 1B,C). Since recipients of CD34+ cells without a THY graft did not reconstitute T cells and THY grafts treated with dGuo for even 7 days did not contain enough progenitors to permit long-term (>14 weeks) T cell reconstitution (Figure 1C), we conclude that progenitors derived from peripherally-infused allogeneic adult CD34+ cells populated 21-day dGuo-treated thymi, underwent thymopoiesis, and emigrated to the periphery. Control recipients of dGuo-treated fetal thymus tissue with 4×105 autologous fetal liver CD34+ cells instead of allogeneic adult marrow CD34+ cells showed more rapid T cell reconstitution (Figure 1C) than was achieved with allogeneic adult CD34+ cells.
While the above recipients of 21-day FTOC grafts plus 5×106 adult CD34+ cells exhibited high levels of long-term (>20 weeks) human chimerism (Figure 1), a lower dose of adult CD34+ cells did not achieve robust immune reconstitution (average 3.89% ± 9.89% human cells in PBMC at 20 weeks in recipients of 2×105 CD34+ cells, n=9). Only limited HSCs are available through volunteer bone marrow aspiration. Therefore, we used NOD/SCID/IL2 receptor γ chainnull (NSG) mice, which lack NK cells and are more permissive for engraftment of human HSCs (18), for the ensuing experiments.
We evaluated irradiation of THY grafts to deplete pre-existing thymocytes. NSG mice receiving 7 Gy irradiated THY plus 3×105 adult CD34+ cells showed excellent B cell and monocyte reconstitution, but low numbers of peripheral T cells by 20 weeks (Fig. S2). THY grafts were so small as to be barely visible under the kidney capsule upon laparotomy (Fig S3A). T cells eventually reconstituted the periphery by 34 weeks post implantation (Fig. S2).
Cryopreservation and thawing of fetal THY grafts to allow peripheral reconstitution of T cells and multiple hematopoietic lineages from allogeneic, adult human hematopoietic stem cells
Transplantation of cryopreserved and thawed mouse thymus tissue can restore immune function (19;20). Since the studies above suggested that human fetal THY tissue contains viable, alloreactive and xenoreactive thymocytes, we evaluated the ability of cryopreservation of intact fetal thymic tissue fragments to deplete these thymocytes. As shown in Supplemental Figure 4, cryopreservation of thymic tissue indeed led to marked depletion (1567 fold decrease in total cellularity) of all thymocyte subsets from fetal thymic tissue.
To test the utility of cryopreserved fetal thymic tissue, sublethally irradiated NSG mice received cryopreserved and thawed fetal human THY grafts plus 3-5×105 allogeneic, adult CD34+ cells i.v. To further assure depletion of T cells derived from pre-existing graft thymocytes, the mice received a depleting anti-human CD2 mAb. As shown in Figure 2, all mice achieved human B cell and monocyte chimerism by 6 weeks. Unlike recipients of CD34+ cells alone, which showed minimal T cell reconstitution, recipients of cryopreserved and thawed THY grafts generated peripheral T cells, which appeared by 6 weeks and peaked at ~10% and ~30% of PBMC at 16 weeks following infusion with 3×105 or 5×105 CD34+ cells, respectively (Figure 2A, lower right panel). While it took 20 weeks to achieve T cell reconstitution with 21-day dGuo-treated grafts, similar T cell levels were reconstituted by 10 weeks with cryopreserved and thawed THY grafts. At the time of animal sacrifice, these cryopreserved grafts were markedly enlarged, showing evidence of robust human thymopoiesis with predominant CD4/CD8 double positive thymocytes (Figure 2B, C).
Figure 2. Multilineage human cell reconstitution in NSG mice receiving cryopreserved and thawed thymic grafts and allogeneic, adult CD34+ cells.
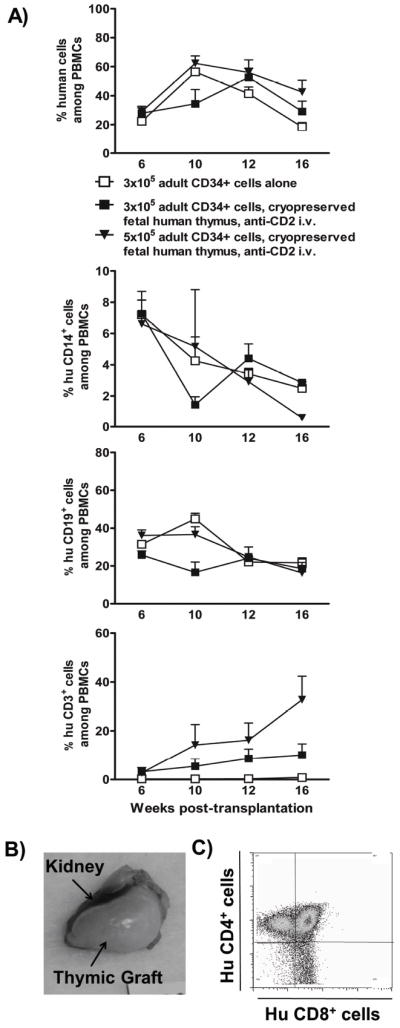
A) Sublethally irradiated NSG mice that received a cryopreserved and thawed fetal thymus graft in combination with 3×105 (black squares, n= 6) or 5×105 (black triangles, n=6) adult CD34+ cells and two doses of anti-CD2 mAb i.v. were bled to measure human cell reconstitution in (total mouse plus human) PBMCs at the indicated time points. Age-matched control animals received 3×105 adult HSCs alone (white squares, n=6). Single cell suspensions of PBMCs were stained for markers of human hematopoietic cells (CD45), T cells (CD3), B cells (CD19) and monocytes (CD14). Dead cells and mouse red blood cells were excluded from the analysis. B) Representative THY graft appearance 20 weeks post-transplantation. NSG mice that were transplanted with cryopreserved and thawed fetal thymus tissue had abundant, viable thymic tissue underneath the kidney capsule. C) FCM analysis of thymocytes (representative of 12 grafts in a single experiment).
Control animals receiving cryopreserved THY grafts without i.v. CD34+ cells (with [n=5] or without [n=4] anti-CD2 mAb) did not repopulate significant human T cells or non-T cells in the periphery (0.46% ± 0.34% human cell reconstitution 20 weeks post-transplantation). Thus, the majority of pre-existing graft thymocytes were depleted by cryopreservation and administration of CD34+ cells was necessary for human T cell and non-T cell reconstitution. Additional control NSG animals receiving fresh thymic tissue with allogeneic CD34+ cells showed human T cells (mean 2.4% of PBMC) but no human chimerism in any other lineage by 5 weeks (<0.006%), whereas recipients of autologous CD34+ cells showed significant human T cell (mean 3.4%), B cell (mean 2.1%) and monocyte (mean 0.2%) reconstitution by this time (Fig. S5). These data are consistent with the interpretation that T cells from fresh thymic tissue implanted into NSG mice rejected allogeneic CD34+ cells.
None of the long-term animals (17 of 17 followed for 20 weeks) that received cryopreserved and thawed THY plus anti-CD2 mAb developed wasting syndrome or other evidence of GVHD. Mouse class II+ cells were present in the long-term human THY grafts (Fig S6). Anti-CD2 mAb was not required to prevent rejection of allogeneic donor stem cells, as no difference was seen in the level of human reconstitution by 15 weeks when groups of mice receiving cryopreserved thymic grafts and allogeneic CD34+ cells with or without anti-CD2 mAb treatment were compared (Fig. S7).
Human immune reconstitution from a bedside bone marrow aspirate from control and T1D volunteers
We next evaluated reconstitution capabilities of adult CD34+ cells isolated from bedside bone marrow aspiration. An aspiration of 15ml bone marrow yielded 3.6×105 and 2.7×106 CD34+ cells from an initial healthy control and T1D volunteer subject, respectively. Sublethally irradiated NSG mice received 1.8×105 adult CD34+ cells each plus a cryopreserved and thawed human fetal THY graft and anti-human CD2 mAb. Control irradiated mice received CD34+ cells without THY tissue. Human chimerism was detectable by Week 6 and peaked at ~25%-80%. Recipients of THY grafts plus i.v. CD34+ cells from the control and T1D volunteers developed substantial CD3+ cell levels by 8 weeks, while control mice (no THY graft) had markedly delayed T cell reconstitution (Figure 3). CD19+ cells and CD14+ cells also developed from the HSCs of the T1D and control volunteers. Similar results were obtained in 3 additional experiments, in each of which 4-14 NSG mice were each reconstituted with 2-3×105 CD34+ cells from a single volunteer aspirate. Composite data from these experiments are presented in Figure 3A. Splenic T cell reconstitution was also rapid and robust, with a mean of 2.47×106 (SEM 0.6×106) human CD3 cells per spleen in a group of 3 T1D cell-reconstituted mice and 106 CD3 cells in a healthy control-reconstituted mouse spleen that were sacrificed at 9 weeks. Figure 3B shows the robust thymopoiesis from adult CD34+ cells, including normal proportions of CD4/CD8 double positive and single positive cells and similar proportions of CD45RO+ and CD45RA+ cells among single positive thymocytes as was seen with fetal thymus and fetal CD34+ cells (7). Although thymocyte numbers tended to be lower in T1D compared to healthy control CD34 cell-reconstituted animals, no statistically significant differences were seen between the two groups.
Figure 3. Multilineage human cell reconstitution in NSG mice receiving cryopreserved/thawed thymic grafts and allogeneic, adult CD34+ cells isolated from bedside bone marrow aspirates.

A) Sublethally irradiated NSG mice received cryopreserved/thawed fetal thymus tissue in combination with 1.8-3.0×105 adult CD34+ cells isolated from bone marrow aspirates from healthy volunteers (black squares, n=3 donors, 6 recipients) and T1D subjects (black triangles, n=4 donors, 29 recipients). Mean levels of human cell reconstitution in (total mouse plus human) PBMCs are shown over time. Control animals received adult HSCs alone from the T1D subjects (open circles, n=2 donors, 7 recipients). Thymus grafts and bone marrow donors were HLA-typed for T1D-associated DRB and DQB alleles and HLA A*201 using SNP genotyping assays. The thymic tissue and bone marrow donors shared at least HLA*A201 and DRB*0302 and/or DQB*0301. ANOVA revealed an effect of THY transplant on CD3 reconstitution comparing T1D CD34+ cells alone with T1D CD34+ plus THY transplantation at early (6-14 weeks) timepoints. Individual timepoint comparisions with Mann-Whitney U test revealed significant differences at 8-14 weeks (*p<0.05). B) Graft thymocytes were analyzed 22-25 weeks post-transplantation in T1D (n=5) and control (n=3) HSC-reconstituted animals. Mean + SEM are shown. No significant differences between T1D and control animals were noted.
T cell function and self-tolerance in mice reconstituted with volunteer donor bone marrow CD34+ cells
We assessed T cell function by transplanting allogeneic human and xenogeneic (pig) skin to THY-grafted mice that received adult CD34+ cells. These mice rapidly rejected allogeneic human and xenogeneic pig skin grafts (Figure 4A), while naive, untreated NSG mice accepted allogeneic human and xenogeneic skin grafts for the duration of follow-up (106 and 50 days, respectively) (Figure 4A) with no infiltrates or evidence for rejection on histology (Fig S8).
Figure 4. Functional and self-tolerant immune systems in NSG mice receiving fetal thymus graft and adult CD34+ cells.

A) NSG mice (n=3) that received a 7 Gy irradiated thymic graft plus 3×105 adult CD34+ cells reconstituted peripheral T cells >30 weeks after transplantation. Thirty-nine weeks after transplantation, they were grafted with allogeneic human and xenogeneic pig skin. Survival of pig and human skin grafts (n=3 and 4, respectively) on untreated control NSG mice “naïve NSG” are also shown. B) Human T cells (>90% pure) were enriched from the spleen and peripheral lymph nodes of NSG mice 20 weeks following transplantation of cryopreserved and thawed THY grafts and allogeneic bone marrow CD34+ cells from a healthy control (black bar) or T1D subject (dotted bars). Control T cells were isolated from PBMC of the same healthy control volunteer (open bar). Supplementary Table 1 shows the naïve/memory cell distribution of CD4 cells in the 3 mice reconstituted from T1D CD34+ cells.
To assess self-tolerance of T cells generated from adult CD34+ cells of T1D and healthy volunteers, mixed lymphocyte reactions (MLR) were performed using purified T cells isolated from the spleens and lymph nodes. T cells from mice reconstituted from T1D and control subjects showed self-tolerance along with strong responses to allogeneic human stimulators in MLR (Figure 4B, Table S1). Notably, fresh adult donor T cells and T cells from a mouse reconstituted from the same healthy control bone marrow donor showed similar, robust responses to the allogeneic stimulator and similar self-tolerance. Thus, immune responsiveness and self-tolerance to the adult volunteer were recapitulated in these mice. Because they generate immune function from an individual adult bone marrow donor, we refer to these animals henceforth as “Personalized Immune” (PI) mice.
Similar Treg development from T1D and control bone marrow CD34+ cells
We assessed the presence of Tregs in thymus grafts and the periphery of reconstituted mice. As shown in Figure 5A, CD25highFoxP3+ Tregs were present among CD4+CD8- thymocytes of PI mice. Analysis of CD4+CD8-CD25+CD127lo thymocytes demonstrated that the majority of FoxP3+ cells were also Helios+, indicative of thymically-derived “natural” Tregs. Similar numbers and proportions of Tregs were detected in THY grafts reconstituted from control and T1D volunteers. Furthermore, while some studies have suggested that Treg numbers are reduced in the blood of T1D subjects compared to healthy controls (21), similar proportions of Tregs were detected in the peripheral blood of both groups of reconstituted mice (Figure 5B).
Figure 5. Tregs in thymus grafts and periphery of PI mice.

A) 20-22 weeks after transplantation, single cell suspensions were prepared from thymus grafts of NSG mice that received a cryopreserved and thawed THY graft and allogeneic CD34+ cells from one of two healthy volunteers (circles) or one T1D subject (squares) and analysed for CD4+CD8-CD25+FoxP3+ cells by FCM (top row). As a marker for natural Tregs, Helios expression in CD4+CD8-CD25+CD127loFoxP3+ thymocytes is shown in NSG mice derived from a second human donor pair in the bottom row.
B) Similar proportions of Tregs in PBMCs 20 weeks after transplantation of cryopreserved/thawed fetal thymus grafts with CD34+ cells from one of two healthy controls (black squares) or one T1D subject (black triangles) compared to two healthy humans (black circles). Left plots show CD25 and FoxP3 staining on CD4+ T cells from PI mice generated from control and T1D donors.
Diverse TCR repertoire in single positive (SP) thymocytes derived from adult donor CD34+cells
At 20 weeks post-transplantation, we performed spectratyping analysis on CD4 and CD8 SP thymocytes of mice reconstituted from T1D CD34+ cells and a normal volunteer (Figure 6). These human T cells showed a diverse repertoire, with similar utilization of the BV families and a polyclonal CDR3 length distribution for each BV. The reconstituted repertoires resembled those of the average CD4 T cell repertoires of 12 healthy adults, with average Hamming distances for all analyzed BV families in each sample ranging from 14.2 to 26.2, mean 20.6 (Table S2). This is indicative of typical T cell polyclonality as seen in healthy control peripheral blood lymphocytes (PBL).
Figure 6. Diverse repertoire of T cells in PI mice.

Spectratyping (β-chain CDR3 length distribution) of human CD4 and CD8 SP T cells in THY grafts reconstituted with CD34+ cells from one T1D donor or one of two healthy controls 20 weeks after transplantation. Spectratype from one representative animal (#5700) of six (mice) is shown. The vertical axis is relative fluorescence units (full scale=6,000 units). The horizontal axis is nucleotide size. Reference size markers are low fainter peaks. Representative BV is shown from a total of 12 analyzed per sample. The Hamming distances of all 6 samples, a measure of the relative distances of the observed TCR β-chain length distribution from a reference distribution of healthy adult CD4 T cells, are shown in Supplemental Table 1, and each indicates the reconstitution of a polyclonal repertoire.
Naïve vs memory T cell phenotype in PI mice
The T cells populating the peripheral tissues of mice reconstituted from CD34+ bone marrow cells of healthy control and T1D volunteers included both “naïve”-type CD45RA+CD45RO- and “memory”-type CD45RA-CD45RO+ cells (Figure 7A). Comparison of T cells in the blood of a control CD34+ cell donor revealed a marked increase in the proportion of naïve-type CD45RA+CD45RO- CD4, CD8 and Treg subsets in the PI mouse reconstituted from the same donor (Figure 7A,B). Thus, a rejuvenated version of the adult donor’s immune system is generated in PI mice. As shown in Figure 7C, the human thymus was necessary for this rejuvenation, as the proportion of naïve-type T cells in PBMC of recipients of CD34+ cells alone was markedly lower than that in mice that also received thymus grafts.
Figure 7. Naïve vs memory phenotype of T cells in PI mice.

A) and B) Proportions of CD45RA+ CD4 and CD8 T cells (B) and Tregs (C) in PBMCs of healthy volunteers and of PI mice 20 weeks after THY implantation plus i.v. infusion of CD34+ cells from one T1D subject (black squares) or one of two healthy controls (black triangles), including (open circle) the donor of CD34+ cells for the control mouse indicated with an open triangle (*p<0.05, excluding the outlier in the CD8 population of controls from statistical analysis). C) T cell populations were assayed by FCM in NSG mice injected with CD34+ fetal liver cells with or without allogeneic thymus at 7 weeks post-transplant. Mean ± SEM are shown, n=4 for each group. *p<0.05, **p<0.005.
When we compared the proportions of naïve-type and memory-type CD4 and CD8 T cells in the blood of PI mice generated simultaneously from T1D or healthy control donors, the T cells derived from T1D CD34+ cells showed significantly reduced proportions of naïve-type cells compared to those derived from healthy controls (Figure 7A). Tregs derived from CD34+ cells of T1D donors tended more toward the “memory” phenotype than those from healthy controls, but this trend did not achieve statistical significance (Figure 7B; p=0.07).
Discussion
In this study, we sought to develop a means of generating functional immune systems from HSCs of adult humans in immundeficient mice in order to overcome the limitations of current models for the analysis of human immunoregulation in health and disease. We demonstrate here that adult, bone marrow-derived CD34+ cells can reconstitute NSG mice grafted with cryopreserved and thawed allogeneic thymus tissue, generating multiple hematopoietic lineages, including T cells, B cells and myeloid cells. Cryopreserving and thawing the fetal thymus plus administering anti-CD2 mAb depletes mature T cells from the graft, preventing rejection of allogeneic CD34+ cells and GVHD, while preserving thymic function. Thymopoiesis, growth of the thymus graft and reconstitution of a functional, diverse and rejuvenated immune system is achieved. Self-tolerance of the adult donors is recapitulated. While fetal liver fragments were included in the humanized mouse model upon which our studies are based (4;6), these fragments are not required, as thymocyte progenitors from infused CD34+ cells populated the human thymic grafts in the current study.
While in vivo thymopoiesis and peripheral reconstitution were also achieved from dGuo-treated human thymi, T cell reconstitution from infused adult CD34+ cells was slow when thymi were dGuo-treated sufficiently long (21 days) to prevent rejection of allogeneic CD34+ cells. Slow T cell recovery has also been observed in patients with complete DiGeorge syndrome receiving thymic tissue cultured for several weeks in dGuo (22). Our results suggest that cryopreservation of thymic tissue might support more rapid T cell recovery while preventing GVHD.
Cryopreservation of fetal thymus tissue permits HLA typing of tissue for use with adult CD34+ cells sharing HLA alleles, which is important for optimal immune function. The use of NSG mice allows the engraftment of relatively small numbers of allogeneic adult HSC, allowing reconstitution of multiple mice from a bedside bone marrow aspirate.
The specific tolerance to CD34+ cell donor “self” antigens and the absence of GVHD in our studies most likely reflects intrathymic deletion due to the presence of APCs from the human HSC donor and the murine recipient, respectively, in the human thymus graft, as previously suggested in another thymic xenograft model (23). Although not tested directly, we hypothesize that the inclusion of anti-CD2 mAb was important for the prevention of a wasting syndrome induced by residual xenogeneic GVH-reactive mature T cells emigrating from fetal human thymus grafts. This possibility was suggested by the development of a late-onset (at 22 weeks) GVHD-like syndrome (severe alopecia, skin inflammation, hunched posture and weight loss) in the only mouse that did not receive anti-CD2 mAb within a group of NSG mice receiving cryopreserved THY grafts (plus allogeneic CD34+ cells i.v.).
Immune reconstitution from adult bone marrow CD34+ cells of patients in NSG mice provides an immune system unaltered by disease, allowing comparison of individuals in a controlled and prospective manner. Human immune analyses are typically limited to peripheral blood samples, and underlying immune dysregulation cannot be distinguished from the ensuing cascade of inflammatory events that culminate in disease. Defects in Treg numbers and function have been reported for T1D (21;24-26), systemic lupus erythematosus (27) and rheumatoid arthritis (28), but this has been controversial in T1D (29-31). We observed no gross abnormalities in the T cell populations generated from T1D subjects’ CD34+ cells, which generated Tregs intrathymically in similar proportions as healthy control CD34+ cells. However, we observed significantly reduced proportions of naïve-type T cells in the blood of PI mice generated from T1D compared to healthy control donors, suggesting that abnormalities of T cell homeostasis, as described in NOD mice (32), might be a feature of T1D-derived HSCs. Our model will allow assessment of genetically-programmed, HSC-intrinsic immunoregulatory abnormalities in T1D in relation to predisposing gene alleles.
HLA-transgenic immunocompetent mice have provided insight into the pathogenesis of autoimmune diseases such as rheumatoid arthritis (33), multiple sclerosis (34), celiac disease (35) and T1D (36-39). However, none of these models permit analyses of human HSC-intrinsic, genetically determined immune abnormalities that may contribute to autoimmune pathogenesis. In contrast, the combined administration of i.v. CD34+ cells and fetal THY tissue in immunodeficient mice generated functional human T cells, T-B interactions, class-switched antibody responses, with secondary lymphoid organs containing both plasmacytoid and myeloid dendritic cells (4-6). Since Tregs develop normally (7) and T cell homeostasis can be studied in this model (8), it will allow assessment of HSC-intrinsic immunoregulatory abnormalities associated with autoimmune diseases in HSC donors. The ability to HLA type the thymus before transplantation allows selection for thymi with disease-associated HLA alleles.
Our model has not yet been developed to allow the study of diabetes pathogenesis, as animals reconstituted with HSCs from T1D patients did not develop evidence of insulitis (Figure S9). While we would not expect transplantation of T1D HSCs to cause autoimmune disease in unmodified NSG mice, further development of the model using HLA transgenic NSG mice might permit studies of autoimmune disease pathogenesis.
Our “Personalized Immune” mouse model will also allow the analysis of individual responsiveness of an adult marrow donor to immunotherapeutic agents. In addition, the reconstitution of multiple mice with naïve T cells with a diverse repertoire derived from adult HSCs could potentially provide patients with thymic insufficiency due to immunosuppressants, chemotherapy, irradiation or HIV, with functional, self-tolerant T cells for adoptive transfer. Mice receiving human fetal THY and CD34+ cell grafts generate anti-HIV and other antigen-specific immune responses (5; 40), suggesting the immunotherapeutic potential of this approach. However, robust methods of overcoming the infectious risks of murine pathogens, including endogenous retroviruses (41), would be needed before this approach could be considered.
In summary, we have established a model that permits the development of multilineage peripheral human hematopoietic cells from adult HSCs. The “Personalized Immune” mouse provides an immune system unaltered by disease or its treatment that should allow the analysis of intrinsic defects in immunoregulation associated with autoimmune disorders and of genetically-controlled responses to immunotherapies.
Materials and Methods
Animals and human tissues and cells
Nonobese diabetic-severe combined immunodeficient (NOD/SCID) and NOD/SCID/IL2 receptor γ chain null (NSG) mice were obtained from Jackson Laboratory, and housed in a specific pathogen-free microisolator environment. Human fetal thymus and liver tissues (gestational age 17-20 weeks) were obtained from Advanced Biosciences Resource. Fetal thymus fragments were cryopreserved in 10% DMSO and 90% human AB serum (Atlanta Biologicals), irradiated or cultured, depending on the experimental design. CD34+ cells were isolated from a 15ml bone marrow aspirate, or from discarded human bone marrow filters obtained from the Massachusetts General Hospital (MGH) Bone Marrow Processing Laboratory, or from fetal human liver tissue using a magnetic-activated cell sorter (MACS) separation system with anti-human CD34+ microbeads (Miltenyi Biotec). Human skin was obtained from the National Disease Research Interchange (NDRI) and pig skin kindly provided by Dr. David H. Sachs (MGH). The use of human tissues/cells and animals were approved by the MGH and Columbia University Medical Center (CUMC) Human and Animal research review committees, respectively, and the experiments were performed in accordance with the approved protocols.
Blood samples and bone marrow aspirates from T1D and control volunteers were recruited through the Human Studies Core of the Harvard JDRF Autoimmunity Center from the Joslin Diabetes Institute or from the Naomi Berrie Diabetes Center at Columbia University Medical Center and were collected with written informed consent under protocols approved by the IRBs of the Massachusetts General Hospital and the Columbia University Medical Center, respectively.
Fetal thymus organ culture
Human fetal thymus culture was performed as previously published (16). Briefly, thymus fragments were placed on 0.8μm isopore membrane filters (Millipore) on 1cm2 Gelfoam sponges (Pharmacia & Upjohn Co). To eliminate endogenous thymocytes, organ cultures were grown in the presence of 1.35 mM 2’-deoxyguanosine (Sigma-Aldrich) in Dulbecco’s modified Eagle medium (DMEM, Sigma-Aldrich) at 37°C for 7 or 21 days.
Human tissue transplantation
Mice were conditioned with sublethal (2.5 Gy) total-body irradiation. Human fetal thymus fragments measuring about 1mm3 were implanted underneath the recipient kidney capsule. Within 24 hours, 1-5×105 human CD34+ cells were injected intravenously. Some recipients were treated intravenously with anti-human CD2 mAb (BTI322 (42); 100μg) on Days 0 and 7.
Skin grafting
Split thickness (2.3mm) skin samples from a MHC miniature pig and an allogeneic human donor were grafted on the lateral thoracic wall 39 weeks after human tissue transplantation. Skin grafts were evaluated daily from day 7 onward to 4 weeks and then at least one inspection every third day thereafter. Grafts were defined as rejected when less than 10% of the graft remained viable.
Flow Cytometry (FCM)
Levels of human hematopoietic cells in transplanted mice were assessed by multicolor flow cytometry. Mice were tail bled at regular intervals after transplantation to obtain peripheral blood mononuclear cells (PBMC), which were prepared with Histopaque-1077 (Sigma-Aldrich). Flourochrome-labelled mAbs, purchased from BD Pharmingen, were used in different combinations: anti-mouse CD45, anti-mouse Ter119, anti-human CD4, anti-human CD8, anti-human CD14, anti-human CD19, anti-human CD45, anti-human CD3, anti-human CD45RA, anti-human CD45RO, anti-human CD127, anti-human FoxP3, anti-human CD25 and isotype control mAbs. FCM analysis was performed using a FACSCalibur, FACSCanto or LSRII (BD), and analysis was carried out by FlowJo software (TreeStar). Dead cells were excluded from the analysis by gating out low forward scatter and high propidium iodide (PI) – retaining cells. Murine erythroid cells were excluded by gating out mouse Ter119+ cells.
Mixed lymphocyte reactions
Splenocytes and lymph nodes were harvested from humanized mice and mononuclear cell suspensions were isolated by Ficoll separation. Human T cells were enriched by depletion of mouse cells using anti-mouse CD45 and anti-Ter-119 microbeads (Miltenyi Biotec) followed by T cell purification using the Pan T cell isolation kit II (Miltenyi Biotec) according to the manufacturer’s instructions. Purity was >90%. Responder T cells (105 per well) were cultured with irradiated human allogeneic PBMCs (3000rad, 105 cells per well) as stimulators for 5 days and proliferation was measured via [3H] thymidine incorporation as we have described (43). In self-stimulated control cultures, responder cells were incubated with autologous PBMCs from the same humanized mouse, depleted of mouse CD45+ and Ter119+ cells. Data are shown as mean [3H] thymidine incorporation in triplicate cultures.
Spectratyping
Total RNA was extracted directly from 1 to 2×104 CD4 or CD8 single positive thymocytes (purity >80%), reverse transcribed and single-strand complementary cDNA synthesis was performed as described (44). Amplification reactions were performed using a TCR β-chain constant region primer and individual variable region primers as described (44). Products were then used in run-off reactions with a Cβ-specific FAM-labeled primer (Integrated DNA Technologies) as described (44). The labelled products were then used to determine the length distribution of the TCR β-chain length. The size and area of the peaks corresponding to the DNA products were determined using an ABI 3100 Genetic Analyzer (Applied Biosystems) and analyzed using Applied Biosystems Genotyper 3.7 NT. Hamming distances to assess the quantitative difference between the experimental and reference β-chain length distributions of peripheral blood CD4 T cells in normal humans were calculated as described (44).
Statistical analysis
Statistical analysis and comparisons were performed with GraphPad Prism version 4.0 (GraphPad Software). Data in bar graphs are expressed as mean ± SEM. Student’s t-test for parametric data sets, or Mann-Whitney test for nonparametric data sets were used to compare groups. ANOVA was used to resolve overall effects between transplant groups over time, and Mann-Whitney test used for individual time point comparisons. A p value less than 0.05 was considered to be statistically significant.
Supplementary Material
Methods (Immunohistochemistry)
Fig. S1 Death due to late onset graft-versus-host disease (GVHD)-like wasting syndrome in humanized mice.
Fig. S2 Late T cell reconstitution in NSG mice receiving irradiated THY transplants and adult CD34+ cells.
Fig. S3 Irradiation of THY graft inhibits growth.
Fig. S4 Cryopreservation depletes thymocytes in human fetal graft.
Fig. S5 Human cell reconstitution with autologous versus allogeneic CD34+ fetal liver cells and tranplantation of fresh fetal thymus.
Fig. S6 Antigen-presenting cells from the recipient mouse in human thymic graft of PI mouse.
Fig. S7 Effect of anti-CD2 mAB BTI322 on chimerism in humanized mice.
Fig. S8 Human allografts are accepted by unmanipulated NSG mice.
Fig. S9 Normal islet histology in PI mice reconstituted with T1DM CD34+ cells.
Table S1 T cell percentages in PI mice reconstituted with T1D marrow used for MLR in Fig 4b.
Table S2 TCR Vbeta distribution and Hamming distances for Figure 6.
Acknowledgments
We thank Drs. Jessica Sachs and Hui Wang for critical review of this manuscript, Mr. Orlando Moreno for outstanding animal husbandry, Ms. Shavree Washington for expert assistance with the manuscript, and Dr. George Eisenbarth and Taylor Armstrong at the Barbara Davis Center for Childhood Diabetes for HLA typing.
Funding: Supported by the JDRF Autoimmunity Center at Harvard University and by NIH grant #RO1 AI084903. H.K. was supported by the Deutsche Forschungsgemeinschaft (German Research Foundation) and ND was supported by the American Diabetes Association.
Footnotes
Author contributions: HK designed and performed experiments, analysed results and wrote the paper; ND designed and performed experiments, analysed results and wrote the paper; TO assisted with the design and performance of experiments; TF participated in the performance of experiments; RW designed, conducted and analysed spectratyping experiments; RG assisted with regulatory approval, volunteer selection criteria and recruitment; EG assisted with and coordinated volunteer recruitment and procurement of tissue samples; TRS assisted with regulatory approval and procured human bone marrow samples; DS procured human bone marrow samples; HT designed, performed and analysed skin grafting experiments; Y-G Yang provided significant intellectual input; MS provided funding, oversaw the design, conduct and interpretation of all experiments and, with HK and ND, wrote the paper.
The authors declare that they have no competing interests.
Reference List and Notes
- 1.Mosier DE, Gulizia RJ, Baird SM, Wilson DB. Transfer of a functional human immune system to mice with severe combined immunodeficiency. Nature. 1988;335:256–259. doi: 10.1038/335256a0. [DOI] [PubMed] [Google Scholar]
- 2.Tary-Lehmann M, Lehmann P, Schols D, Roncarolo MG, Saxon A. Anti-SCID mouse reactivity shapes the human CD4+ T cell repertoire in hu-PBL-SCID chimeras. J Exp Med. 1994;180:1817–1827. doi: 10.1084/jem.180.5.1817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McCune JM, Namikawa R, Kaneshima H, Shultz LD, Lieberman M, Weissman IL. The SCID-hu mouse: Murine model for the analysis of human hematolymphoid differentiation and function. Science. 1988;241:1632–1639. doi: 10.1126/science.241.4873.1632. [DOI] [PubMed] [Google Scholar]
- 4.Lan P, Tonomura N, Shimizu A, Wang S, Yang YG. Reconstitution of a functional human immune system in immunodeficient mice through combined human fetal thymus/liver and CD34+ cell transplantation. Blood. 2006;108:487–492. doi: 10.1182/blood-2005-11-4388. [DOI] [PubMed] [Google Scholar]
- 5.Tonomura N, Habiro K, Shimizu A, Sykes M, Yang YG. Antigen-specific human T-cell responses and T cell-dependent production of human antibodies in a humanized mouse model. Blood. 2008;111:4293–4296. doi: 10.1182/blood-2007-11-121319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lan P, Wang L, Diouf B, Eguchi H, Su H, Bronson R, Sachs DH, Sykes M, Yang YG. Induction of human T cell tolerance to porcine xenoantigens through mixed hematopoietic chimerism. Blood. 2004;103:3964–3969. doi: 10.1182/blood-2003-10-3697. [DOI] [PubMed] [Google Scholar]
- 7.Onoe T, Kalscheuer H, Danzl N, Chittenden M, Zhao G, Yang YG, Sykes M. Human natural regulatory T cell development, suppressive function, and postthymic maturation in a humanized mouse model. J Immunol. 2011;187:3895–3903. doi: 10.4049/jimmunol.1100394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Onoe T, Kalscheuer H, Chittenden M, Zhao G, Yang Y-G, Sykes M. Homeostatic expansion and phenotypic conversion of human T cells depend on peripheral interactions with APC. J Immunol. 2010;184:6756–6765. doi: 10.4049/jimmunol.0901711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ueda H, Howson JM, Esposito L, Heward J, Snook H, Chamberlain G, Rainbow DB, Hunter KM, Smith AN, DiGenova G, Herr MH, Dahlman I, Payne F, Smyth D, Lowe C, Twells RC, Howlett S, Healy B, Nutland S, Rance HE, Everett V, Smink LJ, Lam AC, Cordell HJ, Walker NM, Bordin C, Hulme J, Motzo C, Cucca F, Hess JF, Metzker ML, Rogers J, Gregory S, Allahabadia A, Nithiyananthan R, Tuomilehto-Wolf E, Tuomilehto J, Bingley P, Gillespie KM, Undlien DE, Ronningen KS, Guja C, Ionescu-Tirgoviste C, Savage DA, Maxwell AP, Carson DJ, Patterson CC, Franklyn JA, Clayton DG, Peterson LB, Wicker LS, Todd JA, Gough SC. Association of the T-cell regulatory gene CTLA4 with susceptibility to autoimmune disease. Nature. 2003;423:506–511. doi: 10.1038/nature01621. [DOI] [PubMed] [Google Scholar]
- 10.Steck AK, Bugawan TL, Valdes AM, Emery LM, Blair A, Norris JM, Redondo MJ, Babu SR, Erlich HA, Eisenbarth GS, Rewers MJ. Association of non-HLA genes with type 1 diabetes autoimmunity. Diabetes. 2005;54:2482–2486. doi: 10.2337/diabetes.54.8.2482. [DOI] [PubMed] [Google Scholar]
- 11.Svejgaard A. The immunogenetics of multiple sclerosis. Immunogenetics. 2008;60:275–286. doi: 10.1007/s00251-008-0295-1. [DOI] [PubMed] [Google Scholar]
- 12.Danska JS, Poussier P. After the GWAS rush: nuggets of insight into the pathogenesis of autoimmune disease. Semin Immunol. 2009;21:313–317. doi: 10.1016/j.smim.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 13.Serreze DV, Leiter EH, Worthen SM, Shultz LD. NOD marrow stem cells adoptively transfer diabetes to resistant (NOD × NON)F1 mice. Diabetes. 1988;37:252–255. doi: 10.2337/diab.37.2.252. [DOI] [PubMed] [Google Scholar]
- 14.Lampeter EF, McCann SR, Kolb H. Transfer of diabetes type 1 by bone-marrow transplantation. Lancet. 1998;351:568–569. doi: 10.1016/S0140-6736(05)78555-X. [DOI] [PubMed] [Google Scholar]
- 15.Lepus CM, Gibson TF, Gerber SA, Kawikova I, Szczepanik M, Hossain J, Ablamunits V, Kirkiles-Smith N, Herold KC, Donis RO, Bothwell AL, Pober JS, Harding MJ. Comparison of human fetal liver, umbilical cord blood, and adult blood hematopoietic stem cell engraftment in NOD-scid/gammac-/-, Balb/c-Rag1-/-gammac-/-, and C.B-17-scid/bg immunodeficient mice. Hum Immunol. 2009;70:790–802. doi: 10.1016/j.humimm.2009.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jenkinson EJ, Anderson G. Fetal thymic organ cultures. Curr Opin Immunol. 1994;6:293–297. doi: 10.1016/0952-7915(94)90104-x. [DOI] [PubMed] [Google Scholar]
- 17.Jenkinson EJ, Franchi LL, Kingston R, Owen JJ. Effect of deoxyguanosine on lymphopoiesis in the developing thymus rudiment in vitro: application in the production of chimeric thymus rudiments. Eur J Immunol. 1982;12:583–587. doi: 10.1002/eji.1830120710. [DOI] [PubMed] [Google Scholar]
- 18.Shultz LD, Ishikawa F, Greiner DL. Humanized mice in translational biomedical research. Nat Rev Immunol. 2007;7:118–130. doi: 10.1038/nri2017. [DOI] [PubMed] [Google Scholar]
- 19.Cacheiro LH, Glover PL, Perkins EH. Restoration of immune competence with cryopreserved thymus. Transplantation. 1985;40:110–112. [PubMed] [Google Scholar]
- 20.Cheers C, Leuchars E, Davies AJ, Wallis V. Restoration of thymectomized irradiated mice by frozen and stored thymus grafts. Transplantation. 1970;10:505–511. doi: 10.1097/00007890-197012000-00007. [DOI] [PubMed] [Google Scholar]
- 21.Kukreja A, Cost G, Marker J, Zhang C, Sun Z, Lin-Su K, Ten S, Sanz M, Exley M, Wilson B, Porcelli S, Maclaren N. Multiple immuno-regulatory defects in type-1 diabetes. J Clin Invest. 2002;109:131–140. doi: 10.1172/JCI13605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Davis CM, McLaughlin TM, Watson TJ, Buckley RH, Schiff SE, Hale LP, Haynes BF, Markert ML. Normalization of the peripheral blood T cell receptor V beta repertoire after cultured postnatal human thymic transplantation in DiGeorge syndrome. J Clin Immunol. 1997;17:167–175. doi: 10.1023/a:1027382600143. [DOI] [PubMed] [Google Scholar]
- 23.Nikolic B, Gardner JP, Scadden DT, Arn JS, Sachs DH, Sykes M. Normal development in porcine thymus grafts and specific tolerance of human T cells to porcine donor MHC. J Immunol. 1999;162:3402–3407. [PubMed] [Google Scholar]
- 24.Arif S, Tree TI, Astill TP, Tremble JM, Bishop AJ, Dayan CM, Roep BO, Peakman M. Autoreactive T cell responses show proinflammatory polarization in diabetes but a regulatory phenotype in health. J Clin Invest. 2004;113:451–463. doi: 10.1172/JCI19585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lindley S, Dayan CM, Bishop A, Roep BO, Peakman M, Tree TI. Defective Suppressor Function in CD4+CD25+ T-Cells From Patients With Type 1 Diabetes. Diabetes. 2005;54:92–99. doi: 10.2337/diabetes.54.1.92. [DOI] [PubMed] [Google Scholar]
- 26.Brusko TM, Wasserfall CH, Clare-Salzler MJ, Schatz DA, Atkinson MA. Functional defects and the influence of age on the frequency of CD4+ CD25+ T-cells in type 1 diabetes. Diabetes. 2005;54:1407–1414. doi: 10.2337/diabetes.54.5.1407. [DOI] [PubMed] [Google Scholar]
- 27.Bonelli M, Savitskaya A, Steiner CW, Rath E, Smolen JS, Scheinecker C. Phenotypic and functional analysis of CD4+CD25-FoxP3+ T cells in patients with systemic lupus erythematosis. J Immunol. 2009;182:1689–1695. doi: 10.4049/jimmunol.182.3.1689. [DOI] [PubMed] [Google Scholar]
- 28.Flores-Borja F, Jury EC, Mauri C, Ehrenstein MR. Defects in CTLA-4 are associated with abnormal regulatory T cell function in rheumatoid arthritis. Proc Natl Acad Sci U S A. 2008;105:19396–19401. doi: 10.1073/pnas.0806855105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Berzins SP, Venanzi ES, Benoist C, Mathis D. T-cell compartments of prediabetic NOD mice. Diabetes. 2003;52:327–334. doi: 10.2337/diabetes.52.2.327. [DOI] [PubMed] [Google Scholar]
- 30.Tang Q, Bluestone JA. Regulatory T-cell physiology and application to treat autoimmunity. Immunol Rev. 2006;212:217–237. doi: 10.1111/j.0105-2896.2006.00421.x. [DOI] [PubMed] [Google Scholar]
- 31.Brusko T, Wasserfall C, McGrail K, Schatz R, Viener HL, Schatz D, Haller M, Rockell J, Gottlieb P, Clare-Salzler M, Akinson M. No Alterations in the Frequency of FOXP3+ Regulatory T-Cells in Type 1 Diabetes. Diabetes. 2007;56:604–612. doi: 10.2337/db06-1248. [DOI] [PubMed] [Google Scholar]
- 32.King C, Ilic A, Koelsch K, Sarvetnick N. Homeostatic expansion of T cells during immune insufficiency generates autoimmunity. Cell. 2004;117:265–277. doi: 10.1016/s0092-8674(04)00335-6. [DOI] [PubMed] [Google Scholar]
- 33.Taneja V, David CS. Role of HLA class II genes in susceptibility/resistance to inflammatory arthritis: studies with humanized mice. Immunol Rev. 2010;233:62–78. doi: 10.1111/j.0105-2896.2009.00858.x. [DOI] [PubMed] [Google Scholar]
- 34.Lang HL, Jacobsen H, Ikemizu S, Andersson C, Harlos K, Madsen L, Hjorth P, Sondergaard L, Svejgaard A, Wucherpfennig K, Stuart DI, Bell JI, Jones EY, Fugger L. A functional and structural basis for TCR cross-reactivity in multiple sclerosis. Nat Immunol. 2002;3:940–943. doi: 10.1038/ni835. [DOI] [PubMed] [Google Scholar]
- 35.Black KE, Murray JA, David CS. HLA-DQ determines the response to exogenous wheat proteins: a model of gluten sensitivity in transgenic knockout mice. J Immunol. 2002;169:5595–5600. doi: 10.4049/jimmunol.169.10.5595. [DOI] [PubMed] [Google Scholar]
- 36.Serreze DV, Niens M, Kulik J, DiLorenzo TP. Bridging mice to men: using HLA transgenic mice to enhance the future prediction and prevention of autoimmune type 1 diabetes in humans. Methods Mol Biol. 2010;602:119–134. doi: 10.1007/978-1-60761-058-8_8. [DOI] [PubMed] [Google Scholar]
- 37.King M, Pearson T, Rossini AA, Shultz LD, Greiner DL. Humanized mice for the study of type 1 diabetes and beta cell function. Ann N Y Acad Sci. 2008;1150:46–53. doi: 10.1196/annals.1447.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gregersen JW, Holmes S, Fugger L. Humanized animal models for autoimmune diseases. Tissue Antigens. 2004;63:383–394. doi: 10.1111/j.0001-2815.2004.00243.x. [DOI] [PubMed] [Google Scholar]
- 39.Wen L, Chen NY, Tang J, Sherwin R, Wong FS. The regulatory role of DR4 in a spontaneous diabetes DQ8 transgenic model. J Clin Invest. 2001;107:871–880. doi: 10.1172/JCI11708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Brainard DM, Seung E, Frahm N, Cariappa A, Bailey CC, Hart WK, Shin HS, Brooks SF, Knight HL, Eichbaum Q, Yang YG, Sykes M, Walker BD, Freeman GJ, Pillai S, Westmoreland SV, Brander C, Luster AD, Tager AM. Induction of robust cellular and humoral virus-specific adaptive immune responses in human immunodeficiency virus-infected humanized BLT mice. J Virol. 2009;83:7305–7321. doi: 10.1128/JVI.02207-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stoye JP, Coffin JM. The four classes of endogenous murine leukemia virus: structural relationships and potential for recombination. J Virol. 1987;61:2659–2669. doi: 10.1128/jvi.61.9.2659-2669.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nizet Y, Chentoufi AA, De La Parra B, Lewalle P, Rouas R, Cornet A, Besse T, Mourad M, Malaise J, Squifflet J-P, Bazin H, Latinne D. The experimental (in vitro) and clincial (in vivo) immunosuppressive effects of a rat IgG2b anti-human CD2 mAb, LO-CD2a/BTI-322. Transplantation. 2000;69:1420–1428. doi: 10.1097/00007890-200004150-00036. [DOI] [PubMed] [Google Scholar]
- 43.Kraus AB, Shaffer J, Toh HC, Preffer F, Dombkowski D, Saidman S, Colby C, George R, MCafee S, Sackstein R, Dey B, Spitzer TR, Sykes M. Early host CD8 T-cell recovery and sensitized anti-donor IL-2-producing and cytolytic T-cell responses associated with marrow graft rejection following nonmyeloablative bone marrow transplantation. Exp Hematol. 2003;31:609–621. doi: 10.1016/s0301-472x(03)00082-1. [DOI] [PubMed] [Google Scholar]
- 44.Wu HD, Maurer MS, Friedman RA, Marboe CC, Ruiz-Vazquez EM, Ramakrishnan R, Schwartz A, Tilson MD, Stewart AS, Winchester R. The lymphocytic infiltration in calcific aortic stenosis predominantly consists of clonally expanded T cells. J Immunol. 2007;178:5329–5339. doi: 10.4049/jimmunol.178.8.5329. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Methods (Immunohistochemistry)
Fig. S1 Death due to late onset graft-versus-host disease (GVHD)-like wasting syndrome in humanized mice.
Fig. S2 Late T cell reconstitution in NSG mice receiving irradiated THY transplants and adult CD34+ cells.
Fig. S3 Irradiation of THY graft inhibits growth.
Fig. S4 Cryopreservation depletes thymocytes in human fetal graft.
Fig. S5 Human cell reconstitution with autologous versus allogeneic CD34+ fetal liver cells and tranplantation of fresh fetal thymus.
Fig. S6 Antigen-presenting cells from the recipient mouse in human thymic graft of PI mouse.
Fig. S7 Effect of anti-CD2 mAB BTI322 on chimerism in humanized mice.
Fig. S8 Human allografts are accepted by unmanipulated NSG mice.
Fig. S9 Normal islet histology in PI mice reconstituted with T1DM CD34+ cells.
Table S1 T cell percentages in PI mice reconstituted with T1D marrow used for MLR in Fig 4b.
Table S2 TCR Vbeta distribution and Hamming distances for Figure 6.


