Abstract
Among wide spectrum of biomolecules induced by various stress factors low molecular mass protein called metallothionein (MT) is suitable for assessment of the heavy metal environmental pollution. The aim of this work was to determine the metallothionein and total thiols content in larvae of freshwater midges (Chironomus riparius) sampled from laboratory exposure to cadmium(II) ions and from field studies using differential pulse voltammetry Brdicka reaction. Unique electrochemical instrument, stationary electrochemical analyser Autolab coupled with autosampler, was utilized for the analysis of the samples. The detection limit for MT was evaluated as 5 nM. The larvae exposed to two doses (50 ng/g or 50 μg/g) of cadmium(II) ions for fifteen days under laboratory controlled conditions were at the end of the exposure killed, homogenized and analysed. MT content in control samples was 1.2 μM, in larvae exposed to 50 ng Cd/g it was 2.0 μM and in larvae exposed to 50 μg Cd/g 2.9 μM. Moreover at field study chironomid larvae as well as sediment samples have been collected from eight field sites with different levels of pollution by heavy. The metals content (chromium, nickel, copper, zinc, arsenic, molybdenum, cadmium, tin and lead) in the sediment and or MT content in the chironomid larvae were determined by inductively coupled plasma mass spectrometry or Brdicka reaction, respectively.
Keywords: Catalytic Hydrogen Evolution, Brdicka Reaction, Differential Pulse Voltammetry, Environmental Marker, Thiols, Metallothionein, Heavy Metal Contamination
1. Introduction
The on-line monitoring of a specific pollutant can be performed for only a short time interval, thus the alternative methods for long-term monitoring of pollution are developing. Various species of plants and animals, which are sensitive to higher levels of pollutants or which can synthesize easily detectable biomolecules as a response to environmental pollution, have been used to assess the effects of long-term environmental stress [1-8]. Among wide spectrum of biomolecules induced by various stress factors low molecular mass thiols are suitable for assessment of the environmental pollution because of their main physiological functions in scavenging of reactive oxygen species and detoxification of toxic organic and inorganic molecules via binding with free –SH groups [9]. Metallothioneins (MT) as a group of intracellular, low molecular mass, free of aromatic amino acids and rich in cysteine proteins with molecular weight from 6 to 10 kDa can be considered members of forementioned thiols biomarkers [10-14]. These proteins are abundant through whole animal kingdom, and they were also found in higher plants, eukaryotic microorganisms and some prokaryotes. MT can be found mostly in liver, kidney, pancreas and intestines at animal species. Moreover, MT is accumulated in lysosomes and was found also in nuclei [15].
It has been shown that the MT level increases, when an organism is affected by heavy metals ions. This event can be used for monitoring of environmental contamination by heavy metals [16-18]. Besides stress factors MT level strongly depends on animal specie, analysed tissue, age of an animal, eating habits and likely on others, not yet fully understood and identified factors.
Various analytical techniques and methods can be used to determine MT [11,16,18-41]. One of the most sensitive techniques called Brdicka reaction belongs to wide group of electrochemical methods and measures the hydrogen evolution catalyzed by a protein containing free –SH groups in the presence of Co(III) complex. This method was discovered by Brdicka in 1933 [42-44]. Since the discovery Brdicka reaction has been utilized for determination of MT levels in various animal species (fish, mussels, gastropods) [45-50]. A modification of this method to improve its sensitivity and selectivity has been recently proposed [10,51].
The aim of this work was to determine the metallothionein and total thiols content in larvae of freshwater midges (Chironomus riparius) using Brdicka reaction.
2. Material and Methods
2.1. Chemicals and instruments
Rabbit liver MT (MW 7143), containing 5.9 % Cd and 0.5 % Zn, was purchased from Sigma Aldrich (St. Louis, USA). Tris(2-carboxyethyl)phosphine (TCEP) is produced by Molecular Probes (Evgen, Oregon, USA). Co(NH3)6Cl3 and other used chemicals were purchased from Sigma Aldrich in ACS purity unless noted otherwise. The stock standard solutions of MT at 10 μg/ml was prepared with ACS water (Sigma-Aldrich, USA), reduced by adding of 1 mM TCEP [52] and stored in the dark at − 20 °C. Working standard solutions were prepared daily by dilution of the stock solutions. Deionised water underwent demineralization by reverse osmosis using the instruments Aqua Osmotic 02 (Aqua Osmotic, Tisnov, Czech Republic) and then it was subsequently purified using Millipore RG (Millipore Corp., USA, 18 M′Ω) – MiliQ water. The pH value was measured using WTW inoLab Level 3 with terminal Level 3 (Weilheim, Germany), controlled by personal computer program (MultiLab Pilot; Weilheim). The pH-electrode (SenTix-H) was calibrated by set of WTW buffers (Weilheim).
2.2. Larvae of freshwater midges
The larvae of freshwater midges Chironomus riparius were used in this study. The laboratory populations have been cultured according to standard procedures in siliceous sand overlaid by defined media at 20+-2°C, with constant humidity and 16 h light: 8 h dark photoperiod (US EPA, 2000). The samples for analysis of thiols were collected after fifteen days of exposure of chironomid larvae to sediment artificially contaminated by cadmium (Cd2+ ) at concentrations of 50 ng/g or 50 μg/g. The control samples were reared under same conditions without exposure to heavy metal. In the second part of the study, environmentally exposed chironomid larvae (3rd instar) have been collected from eight field sites with various level of pollution by heavy metals. All the sampling sites were located at smaller streams with abundant chironomid populations. Several sites were in a close proximity of large industrial works, which are the potential sources of pollution including heavy metals (Fig. 1). The control samples were the same as in the first part of the study.
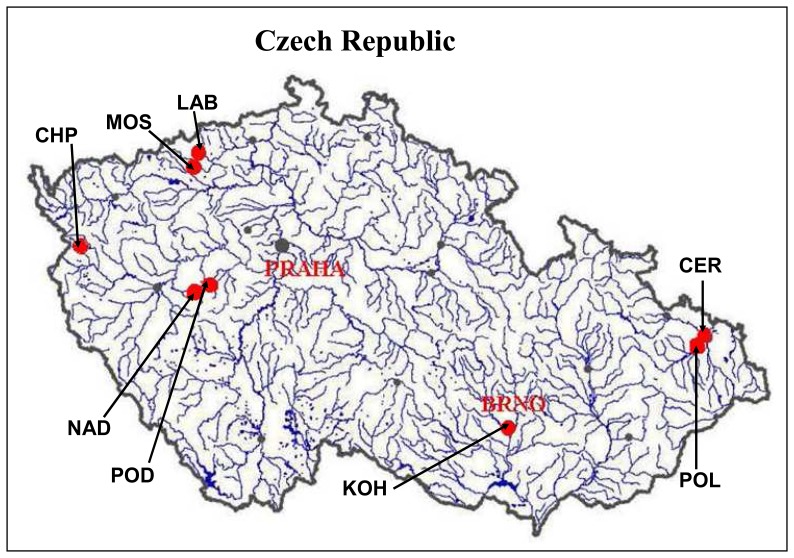
Figure 1.
Map of sampling sites: CHP – Planenský potok (creek), MOS – Bílina: U Mostu, LAB – Bílina: U Labutí, NAD – Červený potok (creek), POD – Červený potok (creek), KOH – Kohoutovický potok (creek), POL – Polančice, CER – Černý potok (creek).
2.3. Heavy metals analysis
Heavy metals content in sediment samples was evaluated based on Aqua Regia leaching process and after total decomposition of silicate matrix. Inductively coupled plasma – mass spectrometry (ICP-MS) (Agilent 7500ce, Agilent Technologies, Japan) was used for determination of heavy metals in Aqua Regia leachate and total decomposed sediment samples. Elements (isotopes) suffering from polyatomic interferences were measured in He collision mode using Octopole Reaction System.
2.4. Preparation of biological samples for electrochemical analysis
Larvae of freshwater midges (app. 0.2 g) were homogenized using liquid nitrogen. The homogenate was quantitatively transferred to test tube and vortexed (Vortex Genie, USA) for 15 min at room temperature. The vortexed sample was prepared by heat treatment. Briefly, the sample was kept at 99 °C in a thermomixer (Eppendorf 5430, USA) for 15 min with occasional stirring, and then cooled to 4°C. The denatured homogenates were centrifuged at 4°C, 15 000 g for 30 min (Eppendorf 5402, USA). Heat treatment effectively denature and remove high molecular weight proteins from samples [10,12,53].
2.5. Stationary electrochemical analyser – Adsorptive transfer stripping differential pulse voltammetry Brdicka reaction – MT content
Electrochemical measurements were performed using an AUTOLAB analyser (EcoChemie, The Netherlands) connected to VA-Stand 663 (Metrohm, Switzerland), using a standard cell with three electrodes. The three-electrode system consisted of hanging mercury drop electrode as working electrode, an Ag/AgCl/3 M KCl reference electrode and a glassy carbon auxiliary electrode. For smoothing and baseline correction the software GPES 4.4 supplied by EcoChemie was employed. The Brdicka supporting electrolyte containing 1 mM Co(NH3)6Cl3 and 1 M ammonia buffer (NH3(aq) + NH4Cl, pH = 9.6) was used and changed after five measurements, surface-active agent was not added. AdTS DPV Brdicka reaction parameters were as follows: initial potential of −0.6 V, end potential −1.6 V, modulation time 0.057 s, time interval 0.2 s, step potential of 1.05 mV, modulation amplitude of 250 mV, Eads = 0 V. Temperature of the supporting electrolyte was 4 °C. For other experimental conditions see in Ref. No. [10].
2.6. Stationary electrochemical analyser coupled with autosampler – Differential pulse voltammetry Brdicka reaction – Total content of thiols
Electrochemical measurements were performed with 747 VA Stand instrument connected to 746 VA Trace Analyzer and 695 Autosampler (Metrohm, Switzerland), using a standard cell with three electrodes and cooled sample holder (4 °C). A hanging mercury drop electrode (HMDE) with a drop area of 0.4 mm2 was the working electrode. An Ag/AgCl/3M KCl electrode was the reference and glassy carbon electrode was auxiliary electrode. The Brdicka supporting electrolyte mentioned in Section 2.5 was used and changed per one analysis. The DPV parameters were as follows: initial potential of −0.7 V, end potential of −1.75 V, modulation time 0.057 s, time interval 0.2 s, step potential 2 mV, modulation amplitude -250 mV, Eads = 0 V. All experiments were carried out at temperature 4 °C (Julabo F12, Germany). For smoothing and baseline correction the software GPES 4.9 supplied by EcoChemie was employed.
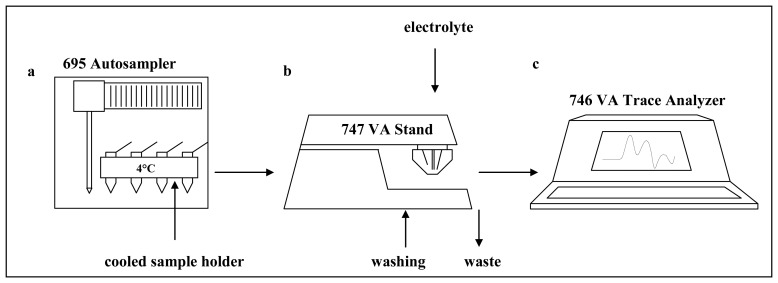
A measurement proceeds as follows: A sample is positioned on the thermostatic sample holder (4 °C). The electrochemical cell is rinsed with distilled water (3 × 25 ml MiliQ water) using three computer controlled pumps. After draining of the water the supporting electrolyte (temperature 4 °C) is pipetted into the washed cell. Further a sample is introduced using the autosampler. The syringe from the autosampler is rinsed and the sample is injected to the cell. The measurement itself consists of a few following processes: At first, the injected sample is accumulated on the surface of hanging mercury drop electrode at open circuit for two minutes. At second, the current responses as function of various potentials are measured. At third, the measured values are processed by 746 VA Trace Analyser and transferred to a personal computer. At fourth, the transferred data is then processed using GPES 4.9 software (Fig. 2).
Figure 2.
Scheme of stationary electrochemical analyser coupled with autosampler: (a) 695 Autosampler with cooled sample holder, (b) 747 VA Stand instrument with potentiostat/galvanostat using a standard cell with three electrodes and (c) 746 VA Trace Analyzer for data processing. Vessels with washing water and the supporting electrolyte are other parts of instrument.
2.7. Statistical analyses
Data were processed using MICROSOFT EXCEL® (USA). Results are expressed as mean ± S.D. unless noted otherwise. Differences with p < 0.05 were considered significant (t-test was applied for means comparison).
3. Results and Discussion
Larvae of freshwater midges (Chironomus riparius) were used for monitoring of pollution of environment by heavy metals already more than 25 years ago [54]. Since then midges and mosquito larvae have been employed for assessment of environmental contamination [55-59]. As mentioned in “Introduction” section, level of MT determined in animal blood and tissues is thought to be a marker of heavy metal stress. Brdicka reaction is very promising analytical method to determine MT due to its very low detection limit [10], however, automated analyser is needed to analyse tens of real samples.
3.1. Stationary electrochemical analyser coupled with autosampler
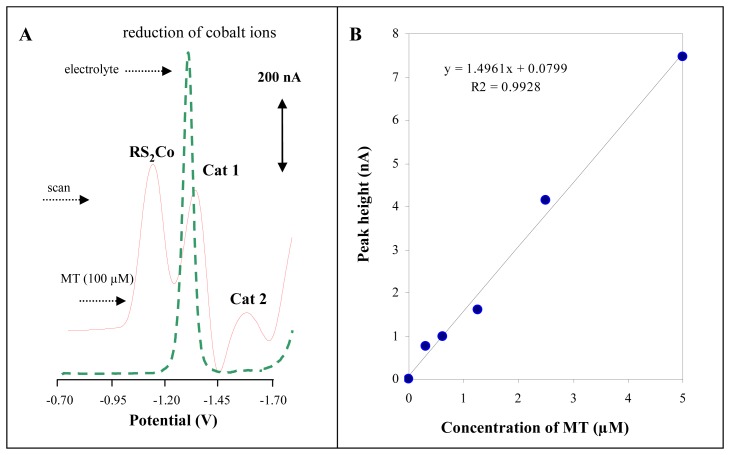
Automatic autosampler injecting low sample volumes (units of μl) coupled with stationary electrochemical analyser was used to overcome a lack of using of Brdicka reaction to analyse tens even hundreds of real samples (Fig. 2). A measurement is carried out automatically under the control of microprocessor within five minutes. The typical voltammograms of the Brdicka supporting electrolyte and MT (100 μM) are shown in Fig. 3A. The calibration curve (dependence of Cat2 peak height on MT concentration, y = 1.4961 + 0.0799, R2 = 0.9928) obtained within the range from 0.25 to 5 μM is shown in Fig. 3B. Relative standard deviation of measurements was 3.6 %. The detection limit for MT estimated as 3 S/N was 5 nM. The detection limits (3 S/N) were calculated according to Long [60], whereas N was expressed as standard deviation of noise determined in the signal domain. The proposed methodology was utilized for determination of MT levels in larvae of freshwater midges exposed to cadmium(II) ions.
Figure 3.
(A) Typical differential pulse voltammograms of the supporting electrolyte (dashed line) and MT (100 μM). (B) Dependence of Cat2 peak height on MT concentration.
3.2. Determination of MT content in larvae of freshwater midges exposed to cadmium(II) ions
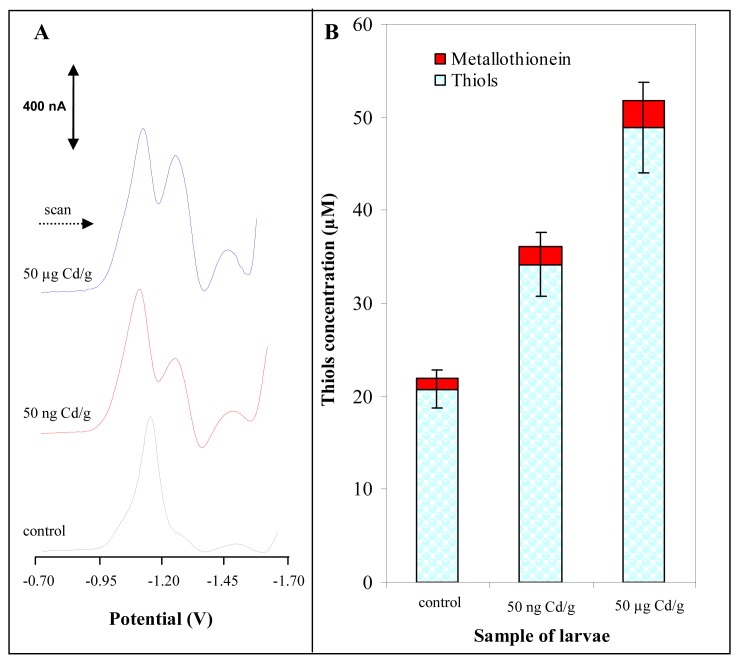
Martinez et al. published a paper on study of morphological deformities in larvae of insect from chironomidae family collected in heavy metal contaminated areas [61]. The authors also determined content of various heavy metals as As, Cd, Ni, Pb, Zn and Ni. They found a significant correlation between metal concentrations and deformity rates for all metals except Ni. To our knowledge a correlation between MT in Chironomids and heavy metals exposure has not been studied so far. In our first laboratory experiments, chironomid larvae have been exposed to sediment contaminated by cadmium(II) ions at concentrations of 50 ng/g or 50 μg/g for fifteen days. One may expected that correlation between MT content and concentration of the heavy metal could be assessed. The larvae exposed to cadmium doses showed no visible marks of heavy metal intoxication because they developed and behaved same as control group. At the very end of the exposure the larvae were killed, washed in distilled water and frozen (-20°C) prior to analysis. The samples of larvae were prepared according to procedure mentioned in “Material and Methods” section. The typical DP voltammograms of real samples are shown in Fig. 4A. The characteristic peaks obtained Cat1 and Cat2 were very well separated and developed. To quantify MT in the samples of interest the Cat2 signal was used. However, we have found previously that if we analysed real samples by the automatic electrochemical analyser (Fig. 2), we quantified not only MT content but also content of all heat stable low molecular mass thiols such as glutathione and others [62,63]. The content of the heat stable thiols in the treated larvae is shown in Fig. 4B. The total content of thiols was enhanced with cadmium(II) dose compared to control samples. Moreover, we attempted to determine MT itself by adsorptive transfer stripping differential pulse voltammetry Brdicka reaction (AdTS DPV Brdicka reaction) [10]. We found that the content of MT also increased with increasing dose of the heavy metal ions but more substantially. MT content in control samples was 1.2 μM, in larvae exposed to 50 ng Cd/g it was 2.0 μM and in larvae exposed to 50 μg Cd/g 2.9 μM. Based on the results obtained it follows that the automated analyser is suitable for routine determination of thiols content in larvae exposed to heavy metals and, thus, to assess heavy metal pollution of environment.
Figure 4.
(A) Typical differential pulse voltammograms of samples obtained from larvae of freshwater midges exposed to 0 ng Cd/g, 50 ng Cd/g and 50 μg Cd/g. (B) The content of total thiols (stationary electrochemical analyser coupled with autosampler) and metallothionein (stationary electrochemical analyser) measured in the larvae exposed to cadmium(II) ions under controlled experimental conditions.
3.3. Determination of the content of heavy metals and thiols in chironomid larvae from the field study
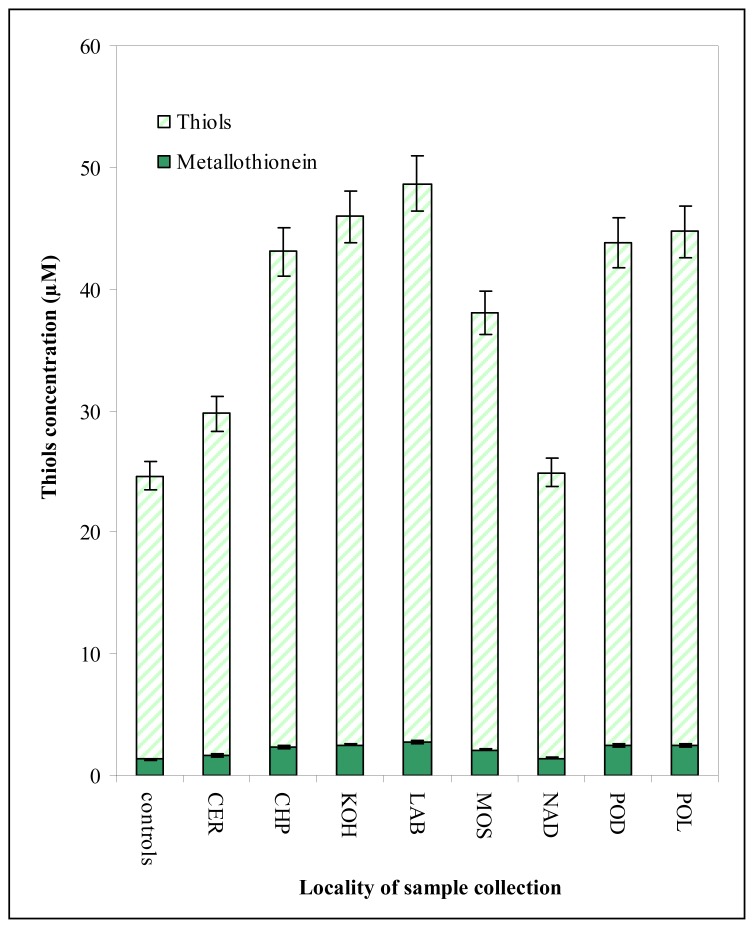
The field study with sampling of chironomids populations from the environment has been conducted to examine the levels of studied thiols and MT in the wild populations. Chironomid larvae as well as sediment samples have been collected from eight field sites with different levels of pollution by heavy metals called LAB, KOH, CER, CHP, NAD, POD, MOS and POL. Most of these sites have been impacted by undergoing or former industrial activities, thus increased concentration of pollutants including heavy metals can be expected. The metals content in sediment extracts were determined by ICP-MS. We determined content of chromium, nickel, copper, zinc, arsenic, molybdenum, cadmium, tin and lead (Tab. 1). Using the simplified criterion for assessment of environmental contamination (sum of concentration of all heavy metals determined in the locality) we evaluated the localities with highest and lowest contamination. The most polluted locality was LAB followed by KOH, CER, CHP, NAD, POD, MOS and POL. Besides the sum of the heavy metal concentrations sediments samples from the LAB locality contained the highest concentrations of seven out of the ten analyzed metals, particularly cobalt, nickel, copper, zinc, arsenic, cadmium and tin.
Table 1.
Content of heavy metals in the sediments from the studied field sites.
| Locality | Heavy metals (mg/kg)* | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Cr | Co | Ni | Cu | Zn | As | Mo | Cd | Sn | Pb | Sum | |
| MOS | 23.7 | 28.9 | 44.0 | 32.0 | 153 | 33.8 | 1.5 | 0.7 | 0.8 | 23.9 | 342.3 |
| LAB | 74.4 | 170* | 249* | 346* | 715* | 81.7* | 17.9 | 2.8* | 5.8* | 74.0 | 1736.6 |
| CER | 243* | 21.0 | 38.7 | 114 | 417 | 9.6 | 8.1 | 0.9 | 1.5 | 152 | 1005.8 |
| POL | 17.3 | 4.9 | 14.8 | 23.9 | 132 | 3.9 | 0.6 | 0.3 | 0.5 | 15.4 | 213.6 |
| NAD | 57.0 | 24.3 | 33.6 | 38.3 | 336 | 10.2 | 118 | 1.3 | 3.8 | 39.2 | 661.7 |
| POD | 51.1 | 20.3 | 41.6 | 46.9 | 264 | 19.2 | 133* | 2.5 | 3.4 | 41.6 | 623.6 |
| CHP | 66.6 | 10.0 | 39.9 | 43.1 | 567 | 7.6 | 3.3 | 0.6 | 1.0 | 22.7 | 761.8 |
| KOH | 121 | 23.3 | 41.7 | 93.1 | 667 | 5.9 | 4.0 | 0.6 | 2.2 | 86.9* | 1045.7 |
… The highest concentration of the metal.
Moreover total content of thiols and MT in the chironomid larvae (3rd instar) sampled at the same sites as sediments was determined electrochemically (Fig. 5). The thiols and MT content determined in the larvae sampled was higher in comparison to control ones (from 102 to 198 % of content in control group). However, the content of the target molecules in the larvae samples at CER and NAD localities was lower about 30-40 % compared to other field localities. This difference could be associated with many factors hardly identified due to natural origin of the samples. After exclusion of results obtained from these two localities (CER and NAD) due to the lowest levels of MT, the good correlation (y(sum of content of heavy metals as mg/kg) = 0.0004×(content of MT, μM) + 2.0917; R2 = 0.8294) between metallothionein content and observed contamination expressed as total heavy metal concentration was obtained.
Figure 5.
The content of total thiols and metallothionein measured in the larvae sampled from eight field sites with various level of pollution by heavy metals.
4. Conclusion
We have shown that the level of thiols in larvae of freshwater midges can be determined by using stationary electrochemical analyser coupled with autosampler. Moreover, we have compared the total thiols content and MT level determined by AdTS DPV Brdicka reaction and found that these values well correlated.
Acknowledgments
This experimental work was supported by grant MSMT INCHEMBIOL 0021622412.
References
- 1.Houtman C.J., Booij P., van der Valk K.M., van Bodegom P.M., van den Ende F., Gerritsen A.A.M., Lamoree M.H., Legler J., Brouwer A. Biomonitoring of estrogenic exposure and identification of responsible compounds in bream from Dutch surface waters. Environ. Toxicol. Chem. 2007;26:898–907. doi: 10.1897/06-326r.1. [DOI] [PubMed] [Google Scholar]
- 2.Dardenne F., Smolders R., De Coen W., Blust R. Prokaryotic gene profiling assays to detect sediment toxicity: Evaluating the ecotoxicological relevance of a cell-based assay. Environ. Sci. Technol. 2007;41:1790–1796. doi: 10.1021/es062162m. [DOI] [PubMed] [Google Scholar]
- 3.Tripathi A.K., Gautam M. Biochemical parameters of plants as indicators of air pollution. J.Environ.Biol. 2007;28:127–132. [PubMed] [Google Scholar]
- 4.Ervin G.N., Herman B.D., Bried J.T., Holly D.C. Evaluating non-native species and wetland indicator status as components of wetlands floristic assessment. Wetlands. 2006;26:1114–1129. [Google Scholar]
- 5.Misik M., Micieta K., Solenska M., Misikova K., Pisarcikova H., Knasmuller S. In situ biomonitoring of the genotoxic effects of mixed industrial emissions using the Tradescantia micronucleus and pollen abortion tests with wild life plants: Demonstration of the efficacy of emission controls in an eastern European city. Environ. Pollut. 2007;145:459–466. doi: 10.1016/j.envpol.2006.04.026. [DOI] [PubMed] [Google Scholar]
- 6.Tomasevic M., Rajsic S., Dordevic D., Tasic M., Krstic J., Novakovic V. Heavy metals accumulation in tree leaves from urban areas. Environ. Chem. Lett. 2004;2:151–154. [Google Scholar]
- 7.Sasmaz A., Yaman M. Distribution of chromium, nickel, and cobalt in different parts of plant species and soil in mining area of Keban, Turkey. Commun. Soil Sci. Plant Anal. 2006;37:1845–1857. [Google Scholar]
- 8.Rusu A.M., Jones G.C., Chimonides P.D.J., Purvis O.W. Biomonitoring using the lichen Hypogymnia physodes and bark samples near Zlatna, Romania immediately following closure of a copper ore-processing plant. Environ. Pollut. 2006;143:81–88. doi: 10.1016/j.envpol.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 9.Huska D., Zitka O., Adam V., Beklova M., Krizkova S., Zeman L., Horna A., Havel L., Zehnalek J., Kizek R. A sensor for investigating the interaction between biologically important heavy metals and glutathione. Czech J. Anim. Sci. 2007;52:37–43. [Google Scholar]
- 10.Petrlova J., Potesil D., Mikelova R., Blastik O., Adam V., Trnkova L., Jelen F., Prusa R., Kukacka J., Kizek R. Attomole voltammetric determination of metallothionein. Electrochim. Acta. 2006;51:5112–5119. [Google Scholar]
- 11.Kukacka J., Vajtr D., Huska D., Prusa R., Houstava L., Samal F., Diopan V., Kotaska K., Kizek R. Blood metallothionein, neuron specific enolase, and protein S100B in patients with traumatic brain injury. Neuroendocrinol. Lett. 2006;27:116–120. [PubMed] [Google Scholar]
- 12.Kizek R., Trnkova L., Palecek E. Determination of metallothionein at the femtomole level by constant current stripping chronopotentiometry. Anal. Chem. 2001;73:4801–4807. doi: 10.1021/ac010126u. [DOI] [PubMed] [Google Scholar]
- 13.Adam V., Krizkova S., Zitka O., Trnkova L., Petrlova J., Beklova M., Kizek R. Determination of apo-metallothionein using adsorptive transfer stripping technique in connection with differential pulse voltammetry. Electroanalysis. 2007;19:339–347. [Google Scholar]
- 14.Studnickova M., Turanek J., Zabrsova H., Krejci M., Kysel M. Rat liver metallothioneins are metal dithiolene clusters. J. Electroanal. Chem. 1997;421:25–32. [Google Scholar]
- 15.Tsujikawa K., Imai T., Kakutani M., Kayamori Y., Mimura T., Otaki N., Kimura M., Fukuyama R., Shimizu N. Localization of Metallothionein in Nuclei of Growing Primary Cultured Adult-Rat Hepatocytes. FEBS Lett. 1991;283:239–242. doi: 10.1016/0014-5793(91)80597-v. [DOI] [PubMed] [Google Scholar]
- 16.Prusa R., Svoboda M., Blastik O., Adam V., Zitka O., Beklova M., Eckschlager T., Kizek R. Increase in content of metallothionein as marker of resistence to cisplatin treatment. Clin. Chem. 2006;52:A174–A175. [Google Scholar]
- 17.Petrlova J., Potesil D., Zehnalek J., Sures B., Adam V., Trnkova L., Kizek R. Cisplatin electrochemical biosensor. Electrochim. Acta. 2006;51:5169–5173. [Google Scholar]
- 18.Strouhal M., Kizek R., Vecek J., Trnkova L., Nemec M. Electrochemical study of heavy metals and metallothionein in yeast Yarrowia lipolytica. Bioelectrochemistry. 2003;60:29–36. doi: 10.1016/s1567-5394(03)00043-4. [DOI] [PubMed] [Google Scholar]
- 19.Adam V., Baloun J., Fabrik I., Trnkova L., Kizek R. An electrochemical detection of metallothioneins in nanolitres at zeptomole level. Sensors. 2008;8:2293–2305. doi: 10.3390/s8042293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Adam V., Beklova M., Pikula J., Hubalek J., Trnkova L., Kizek R. Shapes of differential pulse voltammograms and level of metallothionein at different animal species. Sensors. 2007;7:2419–2429. doi: 10.3390/s7102419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Adam V., Blastik O., Krizkova S., Lubal P., Kukacka J., Prusa R., Kizek R. Application of the Brdicka reaction in determination of metallothionein in patients with tumours. Chem. Listy. 2008;102:51–58. [Google Scholar]
- 22.Adam V., Fabrik I., Nakielna J., Hrdinova V., Blahova P., Krizkova S., Kukacka J., Prusa R., Kizek R. New perspectives in electrochemical determination of metallothioneins. Tumor Biol. 2007;28:79–79. [Google Scholar]
- 23.Adam V., Krizkova S., Fabrik I., Zitka O., Horakova Z., Binkova H., Hrabeta J., Eckschlager T., Kukacka J., Prusa R., Sykorova E., Kizek R. Metallothioneins as a new potential tumour marker. Tumor Biol. 2007;28:43–43. [Google Scholar]
- 24.Adam V., Petrlova J., Potesil D., Zehnalek J., Sures B., Trnkova L., Jelen F., Kizek R. Study of metallothionein modified electrode surface behavior in the presence of heavy metal ions-biosensor. Electroanalysis. 2005;17:1649–1657. [Google Scholar]
- 25.Diopan V., Baloun J., Adam V., Macek T., Havel L., Kizek R. Determination of expression of metallothionein at transgenic tobacco plants. Listy Cukrov. Reparske. 2007;122:325–327. [Google Scholar]
- 26.Huska D., Krizkova S., Beklova M., Havel L., Zehnalek J., Diopan V., Adam V., Zeman L., Babula P., Kizek R. Influence of cadmium(II) ions and brewery sludge on metallothionein level in earthworms (Eisenia fetida) – Bio-transforming of toxic wastes. Sensors. 2008;8:1039–1047. doi: 10.3390/s8021039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kizek R., Vacek J., Trnkova L., Klejdus B., Havel L. Application of catalytic reactions on a mercury electrode for electrochemical detection of metallothioneins. Chem. Listy. 2004;98:166–173. [Google Scholar]
- 28.Krizkova S., Zitka O., Adam V., Beklova M., Horna A., Svobodova Z., Sures B., Trnkova L., Zeman L., Kizek R. Possibilities of electrochemical techniques in metallothionein and lead detection in fish tissues. Czech J. Anim. Sci. 2007;52:143–148. [Google Scholar]
- 29.Kukacka J., Petrlova J., Prusa R., Adam V., Sures B., Beklova M., Havel L., Kizek R. Changes of content of glutathione and metallothionein at plant cells and invertebrate treated by platinum group metals. Faseb J. 2006;20:A75–A75. [Google Scholar]
- 30.Petrlova J., Krizkova S., Zitka O., Hubalek J., Prusa R., Adam V., Wang J., Beklova M., Sures B., Kizek R. Utilizing a chronopotentiometric sensor technique for metallothionein determination in fish tissues and their host parasites. Sens. Actuator B-Chem. 2007;127:112–119. [Google Scholar]
- 31.Prusa R., Blastik O., Potesil D., Trnkova L., Zehnalek J., Adam V., Petrlova J., Jelen F., Kizek R. Analytic method for determination of metallothioneins as tumor markers. Clin. Chem. 2005;51:A56–A56. [Google Scholar]
- 32.Prusa R., Kizek R., Vacek J., Trnkova L., Zehnalek J. Study of relationship between metallothionein and heavy metals by CPSA method. Clin. Chem. 2004;50:A28–A29. [Google Scholar]
- 33.Trnkova L., Kizek R., Vacek J. Catalytic signal of rabbit liver metallothionein on a mercury electrode: a combination of derivative chronopotentiometry with adsorptive transfer stripping. Bioelectrochemistry. 2002;56:57–61. doi: 10.1016/s1567-5394(02)00048-8. [DOI] [PubMed] [Google Scholar]
- 34.Vajtr D., Kukacka J., Adam V., Kotaska K., Houstava L., Toupalik P., Kizek R., Prusa R. Serum levels of metallothionein in expansive contusions, interleukine-6 and TNF-α during reparative phase of the blood brain barrier damage. Tumor Biol. 2007;28:44–44. [Google Scholar]
- 35.Szpunar J. Bio-inorganic speciation analysis by hyphenated techniques. Analyst. 2000;125:963–988. doi: 10.1039/a909137h. [DOI] [PubMed] [Google Scholar]
- 36.Lobinski R., Chassaigne H., Szpunar J. Analysis for metallothioneins using coupled techniques. Talanta. 1998;46:271–289. doi: 10.1016/s0039-9140(97)00343-3. [DOI] [PubMed] [Google Scholar]
- 37.Dabrio M., Rodriguez A.R., Bordin G., Bebianno M.J., De Ley M., Sestakova I., Vasak M., Nordberg M. Recent developments in quantification methods for metallothionein. J. Inorg. Biochem. 2002;88:123–134. doi: 10.1016/s0162-0134(01)00374-9. [DOI] [PubMed] [Google Scholar]
- 38.Vodickova H., Pacakova V., Sestakova I., Mader P. Analytical methods for determination of metallothioneins. Chem. Listy. 2001;95:477–483. [Google Scholar]
- 39.Sestakova I., Vodickova H., Mader P. Voltammetric methods for speciation of plant metallothioneins. Electroanalysis. 1998;10:764–770. [Google Scholar]
- 40.Werner J., Palace V., Baron C., Shiu R., Yarmill A. A real-time PCR method for the quantification of the two isoforms of metallothionein in lake trout (Salvelinus namaycush) Arch. Environ. Contam. Toxicol. 2008;54:84–91. doi: 10.1007/s00244-007-9000-x. [DOI] [PubMed] [Google Scholar]
- 41.Ndayibagira A., Sunahara G.I., Robidoux P.Y. Rapid isocratic HPLC quantification of metallothionein-like proteins as biomarkers for cadmium exposure in the earthworm Eisenia andrei. Soil Biol. Biochem. 2007;39:194–201. [Google Scholar]
- 42.Brdicka R. Polarographic Studies with the Dropping Mercury Kathode. -Part XXXII. - Activation of Hydrogen in Sulphydryl Group of Some Thio-Acids in Cobalt Salts Solutions. Coll. Czech. Chem. Commun. 1933;5:148–164. [Google Scholar]
- 43.Brdicka R. Polarographic Studies with the Dropping Mercury Kathode. -Part XXXI. - A New Test for Proteins in The presence of Cobalt Salts in Ammoniacal Solutions of Ammonium Chloride. Coll. Czech. Chem. Commun. 1933;5:112–128. [Google Scholar]
- 44.Brdicka R. Polarographic studies with the dropping mercury kathode.- Part XXXIII.- The microdetermination of cysteine and cystine in the hydrolysates of proteins, and the course of the protein decomposition. Coll. Czech. Chem. Commun. 1933;5:238–252. [Google Scholar]
- 45.Dragun Z., Erk M., Raspor B., Ivankovic D., Pavicic J. Metal and metallothionein level in the heat-treated cytosol of gills of transplanted mussels Mytilus galloprovincialis Lmk. Environ. Int. 2004;30:1019–1025. doi: 10.1016/j.envint.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 46.Raspor B., Dragun Z., Erk M., Ivankovic D., Pavicic J. Is the digestive gland of Mytilus galloprovincialis a tissue of choice for estimating cadmium exposure by means of metallothioneins? Sci. Total Environ. 2004;333:99–108. doi: 10.1016/j.scitotenv.2004.05.008. [DOI] [PubMed] [Google Scholar]
- 47.Marijic V.F., Raspor B. Metal exposure assessment in native fish, Mullus barbatus L., from the Eastern Adriatic sea. Toxicol. Lett. 2007;168:292–301. doi: 10.1016/j.toxlet.2006.10.026. [DOI] [PubMed] [Google Scholar]
- 48.Marijic V.F., Raspor B. Metal exposure assessment in native fish, Mullus barbatus, from the Eastern Adriatic Sea. Toxicol. Lett. 2006;164:S156–S156. doi: 10.1016/j.toxlet.2006.10.026. [DOI] [PubMed] [Google Scholar]
- 49.Marijic V.F., Raspor B. Age- and tissue-dependent metallothionein and cytosolic metal distribution in a native Mediterranean fish, Mullus barbatus, from the Eastern Adriatic Sea. Comp. Biochem. Physiol. C-Toxicol. Pharmacol. 2006;143:382–387. doi: 10.1016/j.cbpc.2005.05.019. [DOI] [PubMed] [Google Scholar]
- 50.Dragun Z., Raspor B., Erk M., Ivankovic D., Pavicic J. The influence of the biometric parameters on metallothionein and metal level in the heat-treated cytosol of the whole soft tissue of transplanted mussels. Environ. Monit. Assess. 2006;114:49–64. doi: 10.1007/s10661-006-1077-6. [DOI] [PubMed] [Google Scholar]
- 51.Raspor B., Paic M., Erk M. Analysis of metallothioneins by the modified Brdicka procedure. Talanta. 2001;55:109–115. doi: 10.1016/s0039-9140(01)00399-x. [DOI] [PubMed] [Google Scholar]
- 52.Kizek R., Vacek J., Trnkova L., Jelen F. Cyclic voltammetric study of the redox system of glutathione using the disulfide bond reductant tris(2-carboxyethyl)phosphine. Bioelectrochemistry. 2004;63:19–24. doi: 10.1016/j.bioelechem.2003.12.001. [DOI] [PubMed] [Google Scholar]
- 53.Erk M., Ivanković D., Raspor B., Pavičić J. Evaluation of different purification procedures for the electrochemical quantification of mussel metallothioneins. Talanta. 2002;57:1211–1218. doi: 10.1016/s0039-9140(02)00239-4. [DOI] [PubMed] [Google Scholar]
- 54.Lang C., Langdobler B. Melimex, an Experimental Heavy-Metal Pollution Study - Oligochaetes and Chironomid Larvae in Heavy-Metal Loaded and Control Limno-Corrals. Schweizerische Zeitschrift Fur Hydrologie-Swiss Journal of Hydrology. 1979;41:271–276. [Google Scholar]
- 55.Bervoets L., Solis D., Romero A.M., Van Damme P.A., Ollevier F. Trace metal levels in chironomid larvae and sediments from a Bolivian river: Impact of mining activities. Ecotox. Environ. Safe. 1998;41:275–283. doi: 10.1006/eesa.1998.1707. [DOI] [PubMed] [Google Scholar]
- 56.Pourang N. Heavy metal concentrations in surficial sediments and benthic macroinvertebrates from Anzali wetland, Iran. Hydrobiologia. 1996;331:53–61. [Google Scholar]
- 57.Gillis P.L., Reynoldson T.B., Dixon D.G. Metallothionein-like protein and tissue metal concentrations in invertebrates (Oligochaetes and Chironomids) collected from reference and metal contaminated field sediments. J. Gt. Lakes Res. 2006;32:565–577. [Google Scholar]
- 58.Bhattacharyay G., Sadhu A.K., Mazumdar A., Chaudhuri P.K. Antennal deformities of chironomid larvae and their use in biomonitoring of heavy metal pollutants in the River Damodar of West Bengal, India. Environ. Monit. Assess. 2005;108:67–84. doi: 10.1007/s10661-005-3963-8. [DOI] [PubMed] [Google Scholar]
- 59.Mousavi S.K., Primicerio R., Amundsen P.A. Diversity and structure of Chironomidae (Diptera) communities along a gradient of heavy metal contamination in a subarctic watercourse. Sci. Total Environ. 2003;307:93–110. doi: 10.1016/s0048-9697(02)00465-5. [DOI] [PubMed] [Google Scholar]
- 60.Long G.L., Winefordner J.D. Limit of Detection. Anal. Chem. 1983;55:A712–A724. [Google Scholar]
- 61.Martinez E.A., Moore B.C., Schaumloffel J., Dasgupta N. The potential association between mental deformities and trace elements in Chironomidae (diptera) taken from a heavy metal contaminated river. Arch. Environ. Contam. Toxicol. 2002;42:286–291. doi: 10.1007/s00244-001-0190-0. [DOI] [PubMed] [Google Scholar]
- 62.Krizkova S., Fabrik I., Adam V., Kukacka J., Prusa R., Trnkova L., Strnadel J., Horak V., Kizek R. Effects of reduced glutathione, surface active agents and ionic strength on detection of metallothioneinS by using of brdicka reaction. Electroanalysis. 2008 submitted. [Google Scholar]
- 63.Krizkova S., Fabrik I., Adam V., Kukacka J., Prusa R., Chavis G.J., Trnkova L., Strnadel J., Horak V., Kizek R. Utilizing of adsorptive transfer stripping technique Brdicka reaction for determination of metallothioneins level in melanoma cells, blood serum and tissues. Sensors. 2008;8:3106–3122. doi: 10.3390/s8053106. [DOI] [PMC free article] [PubMed] [Google Scholar]