Abstract
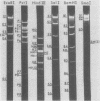
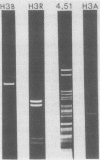
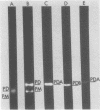
Previously we showed that the mitochondrial deoxyribonucleic acid (DNA) from Paramecium aurelia consists of a linear genome and that replication of this genome is initiated at one terminus and proceeds unidirectionally to the other terminus. Analyses of mitochondria from four closely related species (1, 4, 5, and 7) indicated that the species 1, 5, and 7 DNAs are essentially completely homologous but that the species 4 mitochondrial DNA is only 40 to 50% homologous with that from species 1. The major regions of homology are those containing the genes for ribosomal ribonucleic acid (RNA). To understand the replication and organization of the linear mitochondrial genome better, we compared species 1 (Paramecium primaurelia) and 4 (Paramecium tetraaurelia) DNAs with regard to restriction fragment mapping and homology between initiation regions; we also identified the sites of the genes for ribosomal RNA. In general, the structures of the species 1 and 4 mitochondrial genomes were quite similar. Each ribosomal RNA gene was present in one copy per genome, with the large ribosomal RNA gene located near the terminal region of replication and the small ribosomal RNA gene located more centrally. These two genes were separated by about 10 kilobases in the species 1 genome and by about 12 kilobases in the species 4 genome. In contrast to our previous findings, by using nonstringent hybridization conditions we detected homology between the species 1 and 4 DNA fragments containing the initiation regions. We constructed recombinant DNA clones for many fragments, especially those containing the initiation region and the ribosomal RNA genes. We also constructed restriction enzyme maps for six enzymes for both P. primaurelia and P. tetraaurelia.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adoutte A., Knowles J. K., Sainsard-Chanet A. Absence of detectable mitochondrial recombination in Paramecium. Genetics. 1979 Dec;93(4):797–831. doi: 10.1093/genetics/93.4.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beale G. H., Knowles J. K. Interspecies transfer of mitochondria in Paramecium aurelia. Mol Gen Genet. 1976 Jan 16;143(2):197–201. doi: 10.1007/BF00266922. [DOI] [PubMed] [Google Scholar]
- Bos J. L., Heyting C., Borst P., Arnberg A. C., Van Bruggen E. F. An insert in the single gene for the large ribosomal RNA in yeast mitochondrial DNA. Nature. 1978 Sep 28;275(5678):336–338. doi: 10.1038/275336a0. [DOI] [PubMed] [Google Scholar]
- Cummings D. J. Evolutionary divergence of mitochondrial DNA from Paramecium aurelia. Mol Gen Genet. 1980;180(1):77–84. doi: 10.1007/BF00267354. [DOI] [PubMed] [Google Scholar]
- Cummings D. J., Maki R. A., Conlon P. J., Laping J. Anatomy of mitochondrial DNA from Paramecium aurelia. Mol Gen Genet. 1980;178(3):499–510. doi: 10.1007/BF00337854. [DOI] [PubMed] [Google Scholar]
- Forte M. A., Fangman W. L. Yeast chromosomal DNA molecules have strands which are cross-linked at their termini. Chromosoma. 1979 Apr 30;72(2):131–150. doi: 10.1007/BF00293230. [DOI] [PubMed] [Google Scholar]
- Goddard J. M., Cummings D. J. Mitochondrial DNA replication in Paramecium aurelia. Cross-linking of the initiation end. J Mol Biol. 1977 Jan 15;109(2):327–344. doi: 10.1016/s0022-2836(77)80037-5. [DOI] [PubMed] [Google Scholar]
- Goddard J. M., Cummings D. J. Structure and replication of mitochondrial DNA from Paramecium aurelia. J Mol Biol. 1975 Oct 5;97(4):593–609. doi: 10.1016/s0022-2836(75)80061-1. [DOI] [PubMed] [Google Scholar]
- Goldbach R. W., Bollen-de Boer J. E., van Bruggen E. F., Borst P. Conservation of the sequence and position of the ribosomal RNA genes in Tetrahymena pyriformis mitochondrial DNA. Biochim Biophys Acta. 1978 Nov 21;521(1):187–197. doi: 10.1016/0005-2787(78)90261-7. [DOI] [PubMed] [Google Scholar]
- Goldbach R. W., Borst P., Bollen-de Boer J. E., van Bruggen E. F. The organization of ribosomal RNA genes in the mitochondrial DNA of Tetrahymena pyriformis strain ST. Biochim Biophys Acta. 1978 Nov 21;521(1):169–186. doi: 10.1016/0005-2787(78)90260-5. [DOI] [PubMed] [Google Scholar]
- Howley P. M., Israel M. A., Law M. F., Martin M. A. A rapid method for detecting and mapping homology between heterologous DNAs. Evaluation of polyomavirus genomes. J Biol Chem. 1979 Jun 10;254(11):4876–4883. [PubMed] [Google Scholar]
- Maki R. A., Cummings D. J. Characterization of mitochondrial DNA from Paramecium aurelia with EcoRI and Hae II restriction endonucleases. Plasmid. 1977 Nov;1(1):106–114. doi: 10.1016/0147-619x(77)90011-7. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Pritchard A. E., Herron L. M., Cummings D. J. Cloning and characterization of Paramecium mitochondrial DNA replication initiation regions. Gene. 1980 Oct;11(1-2):43–52. doi: 10.1016/0378-1119(80)90085-2. [DOI] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Sanders J. P., Heyting C., Borst P. The organization of genes in yeast mitochondrial DNA. I. The genes for large and small ribosomal RNA are far apart. Biochem Biophys Res Commun. 1975 Jul 22;65(2):699–707. doi: 10.1016/s0006-291x(75)80202-6. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Suyama Y., Miura K. Size and structural variations of mitochondrial DNA. Proc Natl Acad Sci U S A. 1968 May;60(1):235–242. doi: 10.1073/pnas.60.1.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Etten R. A., Walberg M. W., Clayton D. A. Precise localization and nucleotide sequence of the two mouse mitochondrial rRNA genes and three immediately adjacent novel tRNA genes. Cell. 1980 Nov;22(1 Pt 1):157–170. doi: 10.1016/0092-8674(80)90164-6. [DOI] [PubMed] [Google Scholar]
- Wahl G. M., Stern M., Stark G. R. Efficient transfer of large DNA fragments from agarose gels to diazobenzyloxymethyl-paper and rapid hybridization by using dextran sulfate. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3683–3687. doi: 10.1073/pnas.76.8.3683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittek R., Moss B. Tandem repeats within the inverted terminal repetition of vaccinia virus DNA. Cell. 1980 Aug;21(1):277–284. doi: 10.1016/0092-8674(80)90135-x. [DOI] [PubMed] [Google Scholar]
- Yang R. C., Wu R. BK virus DNA sequence: extent of homology with simian virus 40 DNA. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1179–1183. doi: 10.1073/pnas.76.3.1179. [DOI] [PMC free article] [PubMed] [Google Scholar]