Abstract
AIM:
Alzheimer's disease (AD) is characterized by large deposits of amyloid β (Aβ) peptide. Aβ is known to increase reactive oxygen species (ROS) production in neurons, leading to cell death. In this study, we screened 15 plant seeds’ aqueous extracts (PSAE) for inhibitory effects on Aβ (25-35)-induced cell death using hippocampus neurons (HIPN).
MATERIALS AND METHODS:
Fifteen chosen plants were nine medical herbs (Japanese honeywort, luffa, rapeseed, Chinese colza, potherb mustard, Japanese radish, bitter melon, red shiso, corn, and kaiware radish) and six general commercial plants (common bean, komatsuna, Qing geng cai, bell pepper, kale, and lettuce). PSAE were measured for total phenolic content (TPC) with the Folin–Ciocalteu method, and the 2-diphenyl-1-picryl-hydrazyl (DPPH) radical scavenging effect of each seed extract was measured. To find a protectant against Aβ-induced oxidative stress, we screened 15 PSAE using a 2’, 7’-dichlorofluorescein diacetate assay. To further unravel the anti-inflammatory effects of PSAE on Aβ-induced inflammation, PSAE were added to HIPN. The neuroprotective effects of the PSAE were evaluated by Cell Counting Kit-8 assay, measuring the cell viability in Aβ-induced HIPN.
RESULTS:
TPC of 15 PSAE was in the range of 0.024-1.96 mg of chlorogenic acid equivalents/gram. The aqueous extracts showed antioxidant activities. Furthermore, intracellular ROS accumulation resulting from Aβ treatment was reduced when cells were treated with some PSAE. Kale, bitter melon, kaiware radish, red shiso, and corn inhibited tumor necrosis factor-alpha secretion by the Aβ-stimulated neurons and all samples except Japanese honeywort showed enhancement of cell survival.
CONCLUSION:
From these results, we suggest that some plant seed extracts offer protection against Aβ-mediated cell death.
KEY WORDS: 2-diphenyl-1-picryl-hydrazyl, amyloid β, cell viability, hippocampal neurons, phenolic compounds, plant seeds, radical scavenging, tumor necrosi factor-alpha
Alzheimer's disease (AD), the most common neurodegenerative disorder, is characterized by the accumulation of amyloid β (Aβ) peptide and the hyper-phosphorylation of tau protein. Increased levels of Aβ and phosphorylated tau, oxidative stress, inflammation, and cell death contribute to the neurodegenerative features of AD.[1] Moreover, the overproduction of Aβ leads to Aβ-associated reactive oxygen species (ROS) production, inflammation, and cell death. The accumulation of Aβ is suggested to be the trigger of the neurodegenerative processes that lead to the development of AD. In addition, experimental evidence on this accumulation suggests links between deposition of Aβ, oxidative stress, and apoptosis associated with AD.[2,3] Although the mechanisms of neuronal cell loss in AD have not yet been fully revealed, increased oxidative stress[4] and inflammation[5] are considered important initiators/mediators of neuronal damage in AD. Not only does Aβ increase oxidative stress, but its generation is also increased as a result of oxidative stress, which in turn causes more oxidative damage.
The potential beneficial roles of dietary antioxidants have been emphasized in various diseases including AD. Plants and plant-derived products in foods contain antioxidants which are thought to provide health benefits in decreasing the risk of disease. Extensive studies have been focused on the positive role of fruit and vegetable polyphenols as free radical scavengers and in disease prevention.[6,7] Experimental evidence indicates that polyphenols have neuroprotective effects in animals.[8] Plant seeds possess considerably stronger antioxidant activity. For example, Canadian black currant (Ribes nigrum L.) seed is a source of γ-linolenic acid and has a high content of antioxidants such as flavonoids, phenolic acid, p-coumaric acid, and tocopherol.[9] Furthermore, flavonoids and phenolic compounds are now accepted as providing an anti-inflammatory function.[10] For example, amygdalin (d-mandelonitrile-β-gentiobioside) in apricot seeds is one of the cyanogenic glycosides, possibly having antinociceptive and anti-inflammatory effects.[11]
We recently conducted a large-scale screening of ethanol extracts from the dried plant seeds for inhibitory activity on tumor necrosis factor-alpha (TNF–α) in vitro.[12] The study showed that some plant seed extracts effectively decreased TNF–α levels. Furthermore, it examined the effects on 2-diphenyl-1-picryl-hydrazyl (DPPH) radical scavenging activities in the plant seed extracts, which clearly displayed radical scavenging activity. Considering these findings, in the present study, we investigated the effects of plant seed extracts on Aβ-induced neurotoxicity and on the regulation of cell death processing in hippocampus neurons (HIPN).
Materials and Methods
Reagents
We purchased reagents from the following sources: HIPN (MB-X0403), nerve cell dispersion medium set, nerve cell culture medium from DS Pharma Biomedical Co., Ltd. (Osaka, Japan); (±)-catechin, 2’,7’-dichlorofluorescein (DCF), 2’,7’-dichlorofluorescein diacetate (DCF-DA), and 2-diphenyl-1-picrylhydrazyl (DPPH) from Sigma-Aldrich (St. Louis, MO, USA); mouse TNF–α immunoassay kit from R and D Systems, Inc. (Minneapolis, MN, USA). We obtained all other analytical grade (or highest grade available) chemicals from Wako Pure Chemical Industries, Ltd. (Osaka, Japan).
Preparation of aqueous extracts from plant seeds (PSAE)
Fifteen chosen plants were nine medical herbs (Japanese honeywort, luffa, rapeseed, Chinese colza, potherb mustard, Japanese radish, bitter melon, red shiso, corn, and kaiware radish) and six general commercial plants (common bean, komatsuna, Qing geng cai, bell pepper, kale, and lettuce). The seeds are sown in Japan from spring to summer, and these are cultivated plants. The dried plant seeds were collected from a local market in Japan. The dried materials were ground into a fine powder with a grinder (Labo Milser LM-PLUS, Osaka Chemical Co., Ltd.). Aqueous extraction was conducted on 10 g of each powder dissolved in 100 mL of distilled water and then boiled for 10 min. After boiling, the mixture was stirred for 60 min. The liquid layer of the mixture obtained was passed through a filter paper and the collected filtrate was stored below −20°C until use. When the filtrate was used in assays, each sample was brought back to room temperature, followed by filtration through a membrane filter (pore size 0.22 μm).
Determination of total phenolics
We measured the total phenolic content (TPC) with a modified version of the Folin–Ciocalteu method[13] using 0-30 mg/L chlorogenic acid as a standard. Briefly, 100 μL of the sample or standard was combined with 100 μL of Folin–Ciocalteu reagent and 100 μL of 2% Na2CO3 solution. We allowed the mixture to stand for 60 min before reading the absorbance at 750 nm using an Ultrospec Visible Plate Reader II 96 (GE Healthcare Ltd., Buckinghamshire, England) and calculating the concentration of PSAE as chlorogenic acid equivalents per gram of dried plant seed.
DPPH radical scavenging activity
Employing the procedure described by Negro,[14] we measured the free radical scavenging activity of PSAE, dissolving the PSAE in water to obtain a series of sample solutions of different concentrations. After mixing a sample solution (100 μL) with 100 μL of 800 μM DPPH–methanol solution and waiting for 30 min, we measured the absorbance of the sample solution at 520 nm against a blank. DPPH radical scavenging activity was determined as follows. The 50% inhibitory concentration (IC50; concentration of sample required to scavenge 50% of DPPH radicals) values were determined by the probit-graphic interpolation method for five concentration levels.
Cell culture
HIPN was cultured for 4 days in a 96-well plate in neuronal cell medium and were maintained at 37°C in a 5% CO2 incubator. On day 4 after the preculture of cells, 4 μL PSAE was added to the culture medium (the concentration usable without inducing cytotoxicity for the purpose of preparing the cells in vitro). Cells were grown for 24 h under 5% CO2 at 37°C. Then, at 24 h after the addition, cells were further cultured for 24 h in a 96-well plate in a medium with or without Aβ (25-35).
Measurement of oxidative stress
The levels of cellular oxidative stress were measured using the DCF-DA assay. HIPN were pretreated for 24 h with various samples, and then exposed to 10 μM Aβ (25-35) for 24 h. At the end of the treatment, the cells were incubated with 50 μM DCF-DA for 50 min and DCF was quantified with a fluorometer (Infinite F200, Tecan, Mδnnedorf, Switzerland) using 485 nm excitation and 535 nm emission filters. The results are given as percent relative to the oxidative stress of the control cells set to 100%. DCF-DA (%) was expressed as a percentage of the untreated control as follows: % DCF-DA = (fluorescence intensity of treated cells/fluorescence intensity of untreated cells) × 100.
Measurement of TNF-α concentrations in culture media
Quantitative determination of TNF–α production in the supernatants was performed by colorimetric enzyme-linked immunosorbent assay (ELISA) under the manufacturer's protocol. Supernatants of samples were added to ELISA plates to measure the cytokine content. The plates were analyzed at A450 nm. The TNF–α level in each sample was calculated with the following equation: TNF–α (pg/mL) = A450 nm/0.0019.
Assessment of cell viability
HIPN were seeded on a 96-well plate at a concentration of 2.66 × 103 cells per well and either exposed or not exposed to the PSAE for 24 h. The cells were then exposed to 10 μM Aβ (25-35) for 24 h. After 24 h incubation, a 10 μL Cell Counting Kit-8 (CCK-8; Dojindo Molecular Technologies, Inc., Kumamoto, Japan) solution was added and the culture was continued for 1 h under 5% CO2 at 37°C. A450 nm was measured. Cell viability (%) was expressed as a percentage of the untreated control as follows: % cell viability = (A450 nm of treated cells/A450 nm of untreated cells) × 100.
Statistical analysis
We present all data as mean ± SD. The statistical comparison between the groups was carried out using either analysis of variance (ANOVA) or Student's t-test. P < 0.05 were considered statistically significant.
Results and Discussion
TPC estimation
TPC was expressed as milligram of chlorogenic acid equivalents/gram of dry matter [Figure 1]. Significant differences were observed for TPC among the 15 plant seed varieties. TPC was in the range of 0.024-1.96 mg. A similar result was reported by Takeoka and Dao[15] with regard to the phenolic content of almond hull (Nonpareil variety) extract. They indicated that the chlorogenic acid concentration of almond hulls was 0.425 ± 0.045 mg/g of fresh weight. However, our results for all seed extracts except those of the common bean were higher than theirs. Among the studied plant seeds, kaiware radish showed the highest phenolic content (1.96 mg) while the lowest content was observed in common bean (0.024 mg). The considerable difference observed between the results of phenolic content may be due to the presence or absence of volatile compounds.[16] For example, two varieties of pumpkin seed are uniquely flavored and include different volatile compounds.[17] In addition, phenolic compounds are a ubiquitous group of polyphenolic compounds of variable chemical structure present in vegetables, seeds, fruit, and fruit products.[18] More than 4000 types of phenolic compounds have been isolated from various plants. There may be a difference in the phenolic content of every seed type due to differences in the phenolic compounds as explained above.
Figure 1.
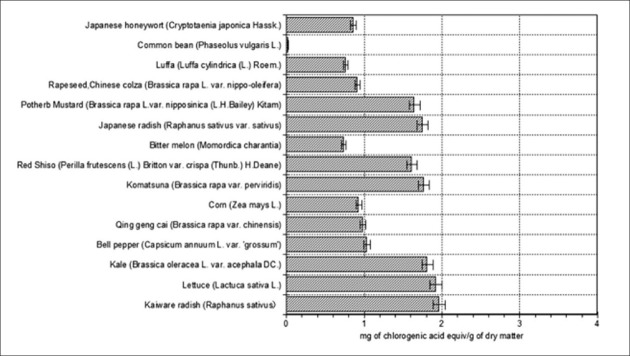
Total phenolic content of aqueous extracts from the seeds of 15 dried plants marketed in Japan. Each end of the vertical bar represents the standard error of multiple (3) samples
DPPH radical scavenging activity
The extracts of 15 PSAE were compared for their scavenging activities on DPPH radicals [Table 1]. With regard to IC50 value, the highest scavenging activity was found in the extracts of rapeseed, Chinese colza (6.76 mg/mL) and the lowest activity was found in lettuce (67.5 mg/mL). The order of the scavenging activity was 8.91, 12.05, and 12.10 mg/mL for Japanese honeywort, potherb mustard, and red shiso, respectively. However, the scavenging activity of all extracts was less than that of (±)-catechin. The activity of rapeseed, Chinese colza was approximately 10 times higher than that of lettuce. The presence of coumarin as a major component of Copaifera langsdorffii seeds was demonstrated by Stupp, et al.[19] Therefore, the higher scavenging activity of rapeseed, Chinese colza may be due to a high constituent of scavengers such as coumarins in the plant extracts.[19,20] The varied scavenging activity of PSAE may depend on the amount and type of antioxidants in seeds.
Table 1.
DPPH radical-scavenging activity in aqueous extracts from the seeds of 15 dried plants marketed in Japan
PSAE effects on intracellular ROS in Aβ (25-35)-induced HIPN
Some plant extracts have been reported to prevent ROS-induced apoptosis in cultured neuronal cells.[21] We indicated that the dried plant seed extracts have antioxidant activities.[12] We measured the levels of cellular oxidative stress using a DCF-DA assay. To find a protectant against Aβ-induced oxidative stress, we screened various plant seeds extracts using this assay. Exposure of HIPN to 10 μM Aβ for only 24 h resulted in a significant increase of ROS levels. On the other hand, intracellular ROS accumulation resulting from Aβ treatment was significantly reduced when the cells were treated with some plant seeds extracts as compared to those treated with Aβ only. Many plant seed extracts (9/15 PSAE) had high scavenging activities, and the highest protective activity was observed in bell pepper followed by kale, bitter melon, and corn [Figure 2]. ROS scavenging activity of all extracts except komatsuna and Qing geng cai was higher than that of (±)-catechin. They protected HIPN from oxidative stress in vitro. These results indicate that nine PSAE can inhibit Aβ-mediated ROS production in the neurons. Because constituents of some plant seeds have antioxidant properties, it is suggested that inhibition of Aβ-induced ROS generation by the extracts may be due to the neutralization of ROS. For example, Vitex negundo Linn. (Verbenaceae) seeds include diterpenes. Rosmadial and carnosol, which are diterpenes, decreased the number and/or mobility of water molecules located at the polar head group region of the membrane phospholipids. Carnosol also strongly enhanced lipid order at the hydrophobic core of the membrane. These effects throughout the bilayer correlated to the stronger antioxidant capacity of carnosol to inhibit lipid peroxidation. Pérez-Fons, et al. suggested that these effects may contribute to membrane stabilization and hindrance of radical propagation, which may cooperate with the electron donor ability of rosemary diterpenes in protecting the membranes against oxidative damage.[22] Our work may suggest a mechanism similar to the one they discuss.
Figure 2.

Effect of PSAE on ROS generation in Aβ(25-35)-induced neurons. The ROS production of control and PSAE is indicated by unshaded and shaded columns, respectively. Values are the mean ± SD of three measurements. **P < 0.01, *P < 0.05 compared with the controls
Effects of PSAE on TNF-α secretions in Aβ (25-35)-induced HIPN
Figure 3 shows the effects of 15 PSAE on the secretion of pro-inflammatory cytokine TNF-α in Aβ-induced neurons. Kale, bitter melon, kaiware radish, red shiso, and corn treatments, significantly (P < 0.05) inhibited TNF-α secretions in the Aβ-stimulated HIPN. In addition, komatsuna, rapeseed, Chinese colza, significantly inhibited TNF-α secretions. However, quite half of the remaining (7/15) PSAE did not significantly affect TNF-α secretions. The results suggest that kale, bitter melon, kaiware radish, red shiso, and corn have strong anti-inflammatory potential on Aβ-induced inflammation by decreasing TNF-α. However, different PSAE seem to have different effects on anti-inflammation in the presence of Aβ. On the basis of changes in TNF-α levels, kale treatment demonstrated the strongest anti-inflammation potential. In an in vitro study, the roots of Brassica rapa which belonged to the Brassicaceae same as kale were reported to have anti-inflammatory potential.[23] Therefore, it may be that a compound similar to B. rapa is present in the kale seed. Among the 15 PSAE, bitter melon treatment exhibited the second greatest potential for anti-inflammation. Bitter melon is also reported to have anti-inflammatory potential via ferulic acid, which is the component of bitter melon, in splenocytes.[24] Our results may also indicate a ferulic acid effect. Although our results are promising, the anti-inflammation mechanisms of kale and bitter melon require further clarification.
Figure 3.
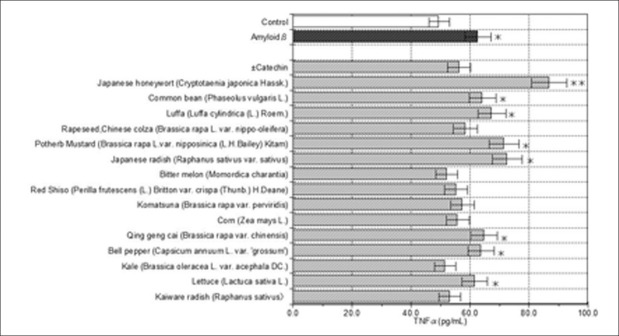
Effects of PSAE on TNFαin Aβ(25-35)-induced neurons. The TNFαlevels of control and PSAE are indicated by unshaded and shaded columns, respectively. Values are the mean ± SD of three measurements. **P < 0.01, *P < 0.05 compared with the controls
Effects of PSAE on cell viability in Aβ (25-35)-induced HIPN
The neuroprotective effects of the 15 plant seed extracts were evaluated by CCK-8 assay, measuring the cell viability in Aβ-induced HIPN [Figure 4]. Following induction of Aβ for 24 h, cell survival was reduced to 75.3% compared to the control. Of the 15 PSAE that were used to treat Aβ-induced neurons, all samples except Japanese honeywort showed enhancement of cell survival compared to the Aβ treatment. Luffa, komatsuna, rapeseed, Chinese colza, common bean, kaiware radish, bitter melon, and Japanese radish all showed enhancement of cell survival compared to the control level, amounting to 100% blockade of Aβ-induced cell death. The degrees of cell survival ranged from 102.5% (of the control level) to 159.7%. These were higher than the level of cell protection by 50 μM kaempferol and apigenin (Aβ-induced cell death decreased by ca. 81.5% and 49.2%, respectively).[25]
Figure 4.

Effect of PSAE on Aβ(25-35)-induced cell death. Cell viability was assessed by CCK-8 assay. The cell viability of control and PSAE are indicated by unshaded and shaded columns, respectively. *P < 0.05, **P < 0.01 compared with the controls
The nature of Aβ (25-35)-induced cell death is not yet clear. Kwon et al. have reported that danthron, a component of Rumex japonicus, senna, and aloe, attenuates Aβ-induced neurotoxicity in a murine cortical culture.[26] Danthron reduced the neuronal injury induced by Aβ (25-35). Also, the authors suggest danthron treatment may, in part, reduce neurotoxicity related to Aβ by both dominant inhibitory effects on membrane lipid peroxidation and glutathione deprivation. The effect of 14 PSAE may employ a similar mechanism.
We examined the cytotoxicity and TNF–α secretion effects of 15 PASE in Aβ-induced neurons. The results show that all of them except Japanese honeywort protected against cell death, and that seven PSAE had additional anti-inflammatory effects. Kim et al. investigated the effect of water-soluble chitosan (WSC) on cytotoxicity and production of pro-inflammatory cytokines on human astrocytoma cell stimulated with Aβ (25-35).[27] Although an anti-inflammatory effect of WSC may have been observed, WSC had no effect on cell viability. Though they prove an anti-inflammatory effect of WSC, our results indicate an anti-inflammatory effect by a mechanism unlike that of WSC, and we think that PSAE inhibits cell death. The neuroprotective effects of the 7 PSAE on Aβ (25-35) toxicity are probably based on both the protective effects on Aβ-induced neuron toxicity and pro-inflammatory effects. Anti-inflammatory effects are thought to play a particularly important role. Further studies are needed to clarify the neuroprotective effect of the samples in vivo.
Assessment of the relationship between TPC, antioxidant activity, inflammation, and cell viability
There was not a high correlation between the phenolic content, antioxidant activity, inhibition of inflammation, and improvement in cell viability. However, TPC was related with DPPH radical scavenging activity (r = 0.53, P = 0.01), cell viability (r = 0.27, P = 0.05), and TNFα level (r = 0.21, P = 0.05).
Some plant seeds include cysteine,[28] glutathione,[29] tocopherol,[30] and phenolic compounds (flavanol, proanthocyanidins, anthocyanins, ellagic acid, ellagitannins, and flavonol glycosides).[31] These compounds might be extracted from the seed which we used for this study. Previous study indicated that cysteine, glutathione, tocopherol, and phenolic compounds reduce and decolorize DPPH through their hydrogen donating ability.[32] Thus, PSAE may possess a similar group of compounds with hydrogen donating ability which reduced DPPH [Table 1]. In other words, the reason why there was not the correlation that was very high between DPPH radical scavenging activity and TPC might be influence of the compounds except the phenolic compounds (r = 0.53).
In addition, previous studies showed that there is a potential relationship between antioxidant and inflammation inhibition in AD; this topic was reviewed recently.[33] It is reported that polyphenols seem to be promising therapeutic tools for inhibiting ROS formation and arresting cytokine-mediated neuroinflammation in AD. This relationship has been studied in curcumin mainly. We assessed the correlation between TNFα level and TPC. The correlation that we observed was a weak relationship between TPC and TNFα level (r = 0.21). That is why phenolic compounds may inhibit inflammation, but probably may depend on the influence of the compound except phenolic compounds because the correlation between them is not so high.
We were surprised about the lack of correlation between intracellular ROS level and TNFα level or cell viability. We do not understand the reason why a high correlation was not seen between intracellular ROS level and TNFα level. However, it is reported that grape seeds promote production of TNFα.[34] Some PSAE might have a similar effect. On the other hand, one plausible explanation is that intracellular ROS level controlled by some PSAE may be the optimal concentration to work as the enhancement of cell viability. Indeed, although intracellular ROS level often has been used to assess cytotoxicity in Aβ treated cells,[35] previous studies reveal that ROS act as primary or secondary messengers to promote cell growth,[36] and therefore may not be a consistently reliable indicator of cellular damage.
In summary, our results suggest that some PSAE offer protection against Aβ-mediated cell death by (1) reducing the generation of ROS and/or (2) inhibiting TNF–α secretions, and (3) attenuating other cellular damage. This effect of aqueous extracts could be due to the active compounds present in plant seeds, which may increase the capacity of endogenous antioxidant defenses and may modulate the cellular redox state. Some plant seeds may therefore reduce Aβ-mediated cytotoxicity, neuronal loss, and the risk of developing AD.
Footnotes
Source of Support: This study was supported by the research funds from Aichi Prefectural University
Conflict of Interest: None declared.
References
- 1.Querfurth HW, LaFerla FM. Alzheimer's disease. N Engl J Med. 2010;362:329–44. doi: 10.1056/NEJMra0909142. [DOI] [PubMed] [Google Scholar]
- 2.Zheng L, Terman A, Hallbeck M, Dehvari N, Cowburn RF, Benedikz E, et al. Macroautophagy-generated increase of lysosomal amyloid β-protein mediates oxidant-induced apoptosis of cultured neuroblastoma cells. Autophagy. 2011;7:1528–45. doi: 10.4161/auto.7.12.18051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cai Z, Zhao B, Ratka A. Oxidative stress and β-amyloid protein in Alzheimer's disease. Neuromolecular Med. 2011;13:223–50. doi: 10.1007/s12017-011-8155-9. [DOI] [PubMed] [Google Scholar]
- 4.Zampagni M, Wright D, Cascella R, D’Adamio G, Casamenti F, Evangelisti E, et al. Novel S-acyl glutathione derivatives prevent amyloid oxidative stress and cholinergic dysfunction in Alzheimer disease models. Free Radic Biol Med. 2012;52:1362–71. doi: 10.1016/j.freeradbiomed.2012.01.012. [DOI] [PubMed] [Google Scholar]
- 5.Kuang X, Du JR, Chen YS, Wang J, Wang YN. Protective effect of Z-ligustilide against amyloid beta-induced neurotoxicity is associated with decreased pro-inflammatory markers in rat brains. Pharmacol Biochem Behav. 2009;92:635–41. doi: 10.1016/j.pbb.2009.03.007. [DOI] [PubMed] [Google Scholar]
- 6.Schini-Kerth VB, Auger C, Kim JH, Etienne-Selloum N, Chataigneau T. Nutritional improvement of the endothelial control of vascular tone by polyphenols: Role of NO and EDHF. Pflugers Arch. 2010;459:853–62. doi: 10.1007/s00424-010-0806-4. [DOI] [PubMed] [Google Scholar]
- 7.Khattak KF. Nutrient composition: Phenolic content and free radical scavenging activity of some uncommon vegetables of Pakistan. Pak J Pharm Sci. 2011;24:277–83. [PubMed] [Google Scholar]
- 8.Qin XY, Cheng Y, Yu LC. Potential protection of green tea polyphenols against intracellular amyloid beta-induced toxicity on primary cultured prefrontal cortical neurons of rats. Neurosci Lett. 2012;513:170–3. doi: 10.1016/j.neulet.2012.02.029. [DOI] [PubMed] [Google Scholar]
- 9.Bakowska-Barczak AM, Schieber A, Kolodziejczyk P. Characterization of Canadian black currant (Ribes nigrum L.) seed oils and residues. J Agric Food Chem. 2009;57:11528–36. doi: 10.1021/jf902161k. [DOI] [PubMed] [Google Scholar]
- 10.Ghanbari R, Anwar F, Alkharfy KM, Gilani AH, Saari N. Valuable nutrients and functional bioactives in different parts of olive (Olea europaea L: A review. Int J Mol Sci. 2012;13:3291–340. doi: 10.3390/ijms13033291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hwang HJ, Lee HJ, Kim CJ, Shim I, Hahm DH. Inhibitory effect of amygdalin on lipopolysaccharide-inducible TNF-α and IL-1β mRNA expression and carrageenan-induced rat arthritis. J Microbiol Biotechnol. 2008;18:1641–7. [PubMed] [Google Scholar]
- 12.Okada Y, Okada M, Sagesaka Y. Screening of dried plant seed extracts for adiponectin production activity and tumor necrosis factor-alpha inhibitory activity on 3T3-L1 adipocytes. Plant Foods Hum Nutr. 2010;65:225–32. doi: 10.1007/s11130-010-0184-2. [DOI] [PubMed] [Google Scholar]
- 13.Swain T, Hills WE. The phenolic constituents of Prunus domestica L.: The quantitative analysis of phenolic constituents. J Sci Food Agric. 1959;10:63–81. [Google Scholar]
- 14.Negro C. Phenolic compounds and antioxidant activity from red grape marc extracts. Bioresour Technol. 2003;87:41–4. doi: 10.1016/s0960-8524(02)00202-x. [DOI] [PubMed] [Google Scholar]
- 15.Takeoka GR, Dao LT. Antioxidant constituents of almond [Prunus dulcis (Mill.) D.A. Webb] hulls. J Agric Food Chem. 2003;51:496–501. doi: 10.1021/jf020660i. [DOI] [PubMed] [Google Scholar]
- 16.Jana S, Shekhawat GS. Anethum graveolens: An Indian traditional medicinal herb and spice. Pharmacogn Rev. 2010;4:179–84. doi: 10.4103/0973-7847.70915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bowman T, Barringer S. Analysis of factors affecting volatile compound formation in roasted pumpkin seeds with selected ion flow tube-mass spectrometry (SIFT-MS) and sensory analysis. J Food Sci. 2012;77:C51–60. doi: 10.1111/j.1750-3841.2011.02465.x. [DOI] [PubMed] [Google Scholar]
- 18.Kandaswami C, Middleton E., Jr Free radical scavenging and antioxidant activity of plant flavonoids. Adv Exp Med Biol. 1994;336:351–76. doi: 10.1007/978-1-4615-1833-4_25. [DOI] [PubMed] [Google Scholar]
- 19.Stupp T, de Freitas RA, Sierakowski MR, Deschamps FC, Wisniewski A, Jr, Biavatti MW. Characterization and potential uses of Copaifera langsdorfii seeds and seed oil. Bioresour Technol. 2008;99:2659–63. doi: 10.1016/j.biortech.2007.04.051. [DOI] [PubMed] [Google Scholar]
- 20.Ma XM, Liu Y, Shi YP. Phenolic derivatives with free-radical-scavenging activities from Ixeridium gracile (DC.) Shih. Chem Biodivers. 2007;4:2172–81. doi: 10.1002/cbdv.200790174. [DOI] [PubMed] [Google Scholar]
- 21.Ban JY, Jeon SY, Bae K, Song KS, Seong YH. Catechin and epicatechin from Smilacis chinae rhizome protect cultured rat cortical neurons against amyloid beta protein (25-35)-induced neurotoxicity through inhibition of cytosolic calcium elevation. Life Sci. 2006;79:2251–9. doi: 10.1016/j.lfs.2006.07.021. [DOI] [PubMed] [Google Scholar]
- 22.Pérez-Fons L, Garzón MT, Micol V. Relationship between the antioxidant capacity and effect of rosemary (Rosmarinus officinalis L.) polyphenols on membrane phospholipid order. J Agric Food Chem. 2010;58:161–71. doi: 10.1021/jf9026487. [DOI] [PubMed] [Google Scholar]
- 23.Shin JS, Yun CH, Cho YW, Baek NI, Choi MS, Jeong TS, et al. Indole-containing fractions of Brassica rapa inhibit inducible nitric oxide synthase and pro-inflammatory cytokine expression by inactivating nuclear factor-κB. J Med Food. 2011;14:1527–37. doi: 10.1089/jmf.2011.1611. [DOI] [PubMed] [Google Scholar]
- 24.Ou L, Kong LY, Zhang XM, Niwa M. Oxidation of ferulic acid by Momordica charantia peroxidase and related anti-inflammation activity changes. Biol Pharm Bull. 2003;26:1511–6. doi: 10.1248/bpb.26.1511. [DOI] [PubMed] [Google Scholar]
- 25.Wang CN, Chi CW, Lin YL, Chen CF, Shiao YJ. The neuroprotective effects of phytoestrogens on amyloid beta protein-induced toxicity are mediated by abrogating the activation of caspase cascade in rat cortical neurons. J Biol Chem. 2001;276:5287–95. doi: 10.1074/jbc.M006406200. [DOI] [PubMed] [Google Scholar]
- 26.Kwon YS, Koh JY, Song DK, Kim HC, Kwon MS, Choi YS, et al. Danthron inhibits the neurotoxicity induced by various compounds causing oxidative damages including beta-amyloid (25-35) in primary cortical cultures. Biol Pharm Bull. 2004;27:723–6. doi: 10.1248/bpb.27.723. [DOI] [PubMed] [Google Scholar]
- 27.Kim MS, Sung MJ, Seo SB, Yoo SJ, Lim WK, Kim HM. Water-soluble chitosan inhibits the production of pro-inflammatory cytokine in human astrocytoma cells activated by amyloid beta peptide and interleukin-1beta. Neurosci Lett. 2002;321:105–9. doi: 10.1016/s0304-3940(02)00066-6. [DOI] [PubMed] [Google Scholar]
- 28.Newkirk RW, Ram JI, Hucl P, Patterson CA, Classen HL. A study of nutrient digestibility and growth performance of broiler chicks fed hairy and hairless canary seed (Phalaris canariensis L.) products. Poult Sci. 2011;90:2782–9. doi: 10.3382/ps.2011-01408. [DOI] [PubMed] [Google Scholar]
- 29.Yi H, Ravilious GE, Galant A, Krishnan HB, Jez JM. From sulfur to homoglutathione: Thiol metabolism in soybean. Amino Acids. 2010;39:963–78. doi: 10.1007/s00726-010-0572-9. [DOI] [PubMed] [Google Scholar]
- 30.El-Mallah MH, El-Shami SM. Investigation of liquid wax components of Egyptian jojoba seeds. J Oleo Sci. 2009;58:543–8. doi: 10.5650/jos.58.543. [DOI] [PubMed] [Google Scholar]
- 31.Furuuchi R, Yokoyama T, Watanabe Y, Hirayama M. Identification and quantification of short oligomeric proanthocyanidins and other polyphenols in boysenberry seeds and juice. J Agric Food Chem. 2011;59:3738–46. doi: 10.1021/jf104976n. [DOI] [PubMed] [Google Scholar]
- 32.Abdille MH, Singh RP, Jayaprakasha GK, Jena BS. Antioxidant activity of the extracts from Dillenia fruits. J Food Chem. 2005;90:891–6. [Google Scholar]
- 33.Magrone T, Marzulli G, Jirillo E. Immunopathogenesis of neurodegenerative diseases: Current therapeutic models of neuroprotection with special reference to natural products. Curr Pharm Des. 2012;18:34–42. doi: 10.2174/138161212798919057. [DOI] [PubMed] [Google Scholar]
- 34.Tong H, Song X, Sun X, Sun G, Du F. Immunomodulatory and antitumor activities of grape seed proanthocyanidins. J Agric Food Chem. 2011;59:11543–7. doi: 10.1021/jf203170k. [DOI] [PubMed] [Google Scholar]
- 35.Hong WK, Han EH, Kim DG, Ahn JY, Park JS, Han BG. Amyloid-beta-peptide reduces the expression level of mitochondrial cytochrome oxidase subunits. Neurochem Res. 2007;32:1483–8. doi: 10.1007/s11064-007-9336-7. [DOI] [PubMed] [Google Scholar]
- 36.Dennery PA. Effects of oxidative stress on embryonic development. Birth Defects Res C Embryo Today. 2007;81:155–62. doi: 10.1002/bdrc.20098. [DOI] [PubMed] [Google Scholar]


