Abstract
PURPOSE:
In view of the potential pharmacophoric nature of imidazole nucleus, two series of imidazole derivatives, 2,4-disubstituted-1 H-imidazoles (2a-m) and 1,2,4-trisubstituted-1 H-imidazoles (3a-m), were synthesized with an aim of obtaining dual acting compounds i.e., anti-inflammatory and antifungal agents.
MATERIALS AND METHODS:
The title compounds were synthesized from 4-methoxyphenyl glyoxal (1) following multistep synthesis, and their structures were established on the basis of modern analytical techniques (IR, NMR and MS). The synthesized imidazoles were tested for their in vivo anti-inflammatory activity. In addition to that, some compounds were also evaluated for their analgesic and ulcerogenic effects. The compounds were also evaluated for their in vitro antifungal activity.
RESULTS:
Di- and tri-substituted imidazole derivatives (2a-m and 3a-m) were successfully synthesized. In in vivo anti-inflammatory test, six compounds (2 h, 2 l, 3 g, 3 h, 3 l and 3 m) exhibited good anti-inflammatory activity (49.58 to 58.02% inhibition) with minimal GI irritation (severity index; 0.17 to 0.34). These compounds were also tested for their analgesic activity and showed appreciable protection (40.53 to 49.60% protection) against saline-induced writhing test. Indomethacin was used as standard drug for comparison. In antifungal test, two compounds (3 h and 3 l) displayed appreciable antifungal activity (MIC; 12.5 μg mL-1) against the fungal strains tested.
CONCLUSION:
Two compounds, 2-(4-nitrophenyl)-4-(4-methoxyphenyl)-1-phenyl-1H-imidazole (3 h) and 2,4-di-(4-methoxyphenyl)-1-phenyl-1H-imidazole (3 l), emerged as lead compounds having dual biological activities; good anti-inflammatory as well as antifungal effect with lesser GI irritation.
KEY WORDS: Analgesic, antifungal, anti-inflammatory, imidazole, ulcerogenicity
Polypharmacy or multiple drug therapy for the treatment of anti-inflammatory conditions associated with microbial infections is a common practice. Monotherapy of drug having dual inhibition i.e., both anti-inflammatory and antimicrobial effect, help a patient not only economically but also in the minimization of side effects.[1] The commonly used non-steroidal anti-inflammatory drugs (NSAIDs) are associated with GI side effects, thus there is still need for safer NSAIDs.[2]
Imidazoles are an important class of heterocyclic compounds due to their multiple therapeutic and pharmacological actions, such as anti-inflammatory,[3,4] antimicrobial,[5,6] anticonvulsant[7,8] and anticancer[9] activities. Many drugs have imidazole nucleus in their structure [Figure 1], like dacarbazine as anticancer, metronidazole as antifungal, cimetidine as antihistaminics and flumazenil as benzodiazepine antagonist.[3]
Figure 1.
Imidazole containing drugs
Imidazole-containing antifungal drug, ketoconazole, was also found to have anti-inflammatory activity in addition to its antifungal activity.[10] Flutrimazole, another imidazole-based wide spectrum antifungal agent, was found to be a good topical anti-inflammatory agent.[11] Therefore, imidazole nucleus seems to be an important pharmacophore for designing new drug candidates [Figure 2].
Figure 2.
Antifungal drugs having anti-inflammatory activity
Owing to the versatility of imidazole nucleus, in view of above-mentioned reports, in continuation to our work on imidazole derivatives[12] and in search of compounds as dual inhibitors,[13] we have synthesized twenty six new di-and tri-substituted imidazole derivatives that might act as dual anti-inflammatory-antifungal agents with minimal GI side effects.
Materials and Methods
All the chemicals and regents used in this study were purchased from Merck (India), Sigma Aldrich, S.D. fine and Sisco Research Laboratories Ltd. Melting points were determined in open capillary tubes and are uncorrected. Purity of the compounds was checked by thin layer chromatography (TLC) on silica gel G Plates (Merck No. 5544) using toluene:ethyl acetate:formic acid (5:4:1) and benzene:acetone (8:2) as solvent system and the spots were located either under ultra violet light or through exposure to iodine vapors.
The IR spectra were recorded using KBr pellets of the compounds on Bruker alpha-T spectrophotometer.[1] H-NMR spectra were recorded on DPX-300 NMR spectrometer or BRUKER-400 Ultra Shield™ spectrometer in DMSO-d6 with tetramethylsilane (TMS) as an internal standard; chemical shifts (δ) are reported in parts per million (ppm) downfield from TMS. Mass spectra were recorded on Jeol JMS-D 300 instrument. Elemental analyses were performed on Perkin-Elmer model 240 analyzer (C, H, N) and found within range of ± 0.4% of theoretical values. The compounds were prepared by reported method[12] with some modifications. The protocol for synthesis of title compounds is represented in [Scheme 1].
Scheme 1.
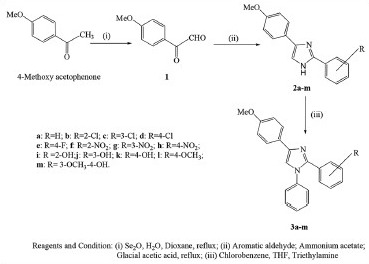
Schematic representation of synthesis of imidazoles
Chemistry
Synthesis of 4-methoxyphenyl glyoxal (1)
A mixture of pure selenium dioxide (0.5 mol), water (10 mL) and dioxane (300 mL) were heated in a round bottom flask at 50-55°C with stirring till clear solution was obtained. To the above solution, 4-methoxy-acetophenone (0.5 mol) was added in one lot. The mixture was further refluxed with stirring for 4 h, during refluxing small amount of selenium was precipitated. The reaction mixture was decanted to remove the precipitate. The clear solution so obtained was distilled to remove excess dioxane and water. A yellow liquid was found pure on TLC examination (toluene:ethyl acetate:formic acid, 5:4:1, v/v/v). Yield: 58%; mp 67-68°C; Rf: 0.45; 1H-NMR (δ, ppm): 3.86 (s, 3 H, OCH3), 6.98 and 7.46 (d, each, A2B2, p-anisyl ring), 9.73 (s, 1 H, CHO); Mass (m/z): 164 (M+).
General method for the preparation of 2,4-disubstituted-1 H-imidazole (2a-m)
A mixture of compound 1 (0.025 mol), aryl aldehyde (0.025 mol) and ammonium acetate (10 g) in glacial acetic acid (50 mL) was refluxed in a round bottom flask for 5 h. After refluxing, the mixture was cooled to room temperature and then poured into cold water (200 mL). A precipitate was separated out, which was filtered, washed, dried and crystallized from acetone. The compound was found pure on TLC (toluene:ethyl acetate: formic acid, 5:4:1, v/v/v) examination.
4-(4-methoxyphenyl)-2-Phenyl-1 H-imidazole (2a)
Yield: 46%; m.p. 161-162°C; Rf 0.59; IR (cm-1): 3462 (N-H), 3021 (C-H), 1590 (C = N), 1441 (C = C), 1328 (C-N); 1H-NMR (δ, ppm): 3.85 (s, 3 H, OCH3), 6.93 and 7.49 (d, each, A2B2, p-anisyl ring), 7.14-7.33 (m, 3 H, H-3,4,5, phenyl), 7.38-7.54 (m, 2 H, H-2,6, phenyl), 7.75 (d, 1 H, H-5, imidazole), 10.43 (bs, 1 H, NH); MS (m/z): 250 [M+]. Anal. Calcd. for C16H14N2O (%): C, 76.78; H, 5.64; N, 11.19. Found: C, 76.52; H, 5.65; N, 11.16.
2-(2-chlorophenyl)-4-(4-methoxyphenyl)-1 H-imidazole (2b)
Yield: 52%; m.p. 137-138°C; Rf 0.69; IR (cm-1): 3435 (N-H), 3018 (C-H), 1584 (C = N), 1436 (C = C), 1322 (C-N), 708 (C-Cl); 1H-NMR (δ, ppm): 3.87 (s, 3 H, OCH3), 6.97 and 7.53 (d, each, A2B2, p-anisyl ring), 7.23-7.42 (m, 2 H, H-4,5, phenyl), 7.65-7.78 (m, 3 H, H-3,6, phenyl + H-5, imidazole), 10.27 (bs, 1 H, NH); MS (m/z): 285 [M+]. Anal. Calcd. for C16H13ClN2O (%): C, 67.49; H, 4.60; N, 9.84. Found: C, 67.33; H, 4.59; N, 9.82.
2-(3-chlorophenyl)-4-(4-methoxyphenyl)-1 H-imidazole (2c)
Yield: 44%; m.p. 147-148°C; Rf 0.74; IR (cm-1): 3431 (N-H), 3022 (C-H), 1568 (C = N), 1435 (C = C), 1327 (C-N), 712 (C-Cl); 1H-NMR (δ, ppm): 3.86 (s, 3 H, OCH3), 6.95 and 7.50 (d, each, A2B2, p-anisyl ring), 7.21-7.85 (m, 5 H, H-2,4,5,6, phenyl + H-5, imidazole), 10.36 (bs, 1 H, NH); MS (m/z): 285 [M+]. Anal. Calcd. for C16H13ClN2O (%): C, 67.49; H, 4.60; N, 9.84. Found: C, 67.37; H, 4.60; N, 9.81.
2-(4-chlorophenyl)-4-(4-methoxyphenyl)-1 H-imidazole (2d)
Yield: 51%; m.p. 157-158°C; Rf 0.79; IR (cm-1): 3429 (N-H), 3014 (C-H), 1576 (C = N), 1429 (C = C), 1331 (C-N), 719 (C-Cl); 1H-NMR (δ, ppm): 3.87 (s, 3 H, OCH3), 6.99 and 7.48 (d, each, A2B2, p-anisyl ring), 7.42 and 7.57 (d, each, A2B2, p-substituted phenyl), 7.88 (d, 1 H, H-5, imidazole), 10.23 (bs, 1 H, NH); MS (m/z): 285 [M+]. Anal. Calcd. for C16H13ClN2O (%): C, 67.49; H, 4.60; N, 9.84. Found: C, 67.34; H, 4.60; N, 9.82.
2-(2-fluorophenyl)-4-(4-methoxyphenyl)-1 H-Imidazole (2e)
Yield: 52%; m.p. 109-10°C; Rf: 0.53; IR (cm-1): 3418 (NH), 3027 (CH), 1563 (C = N), 1424 (C = C), 1326 (C-N); 1H-NMR (δ, ppm): 3.85 (s, 3 H, OCH3), 7.01 and 7.54 (d, each, A2B2, p-anisyl ring), 7.38 (d, 2 H, H-2,6, phenyl), 7.69 (t, 2 H, H-3,5, phenyl), 7.83 (d, 1 H, H-5, imidazole), 10.38 (bs, 1 H, NH); Mass (m/z): 268 (M+). Anal. Calcd. for C16H13FN2O: C, 71.63; H, 4.88; N, 10.44. Found: C, 71.73; H, 4.87; N, 10.42.
2-(2-nitrophenyl)-4-(4-methoxyphenyl)-1 H-imidazole (2 f)
Yield: 63%; m.p. 161-162°C; Rf 0.59; IR (cm-1): 3424 (N-H), 3019 (C-H), 1571 (C = N), 1430 (C = C), 1318 (C-N); 1H-NMR (δ, ppm): 3.87 (s, 3 H, OCH3), 7.03 and 7.52 (d, each, A2B2, p-anisyl ring), 7.31-7.47 (m, 2 H, H-4,5, phenyl), 7.69-8.36 (m, 3 H, H-3,6 + H-5, imidazole), 10.28 (bs, 1 H, NH); MS (m/z): 295 [M+]. Anal. Calcd. for C16H13N3O3 (%): C, 65.08; H, 4.44; N, 10.23. Found: C, 65.23; H, 4.45; N, 10.25.
2-(3-nitrophenyl)-4-(4-methoxyphenyl)-1 H-imidazole (2 g)
Yield: 51%; m.p. 127-128°C; Rf 0.55; IR (cm-1): 3430 (N-H), 3021 (C-H), 1566 (C = N), 1433 (C = C), 1329 (C-N); 1H-NMR (δ, ppm): 3.87 (s, 3 H, OCH3), 7.05 and 7.55 (d, each, A2B2, p-anisyl ring), 7.28-8.33 (m, 5 H, H-2,4,5,6, phenyl + H-5, imidazole), 10.35 (bs, 1 H, NH); MS (m/z): 295 [M+]. Anal. Calcd. for C16H13N3O3 (%): C, 65.08; H, 4.44; N, 10.23. Found: C, 65.10; H, 4.42; N, 10.28.
2-(4-nitrophenyl)-4-(4-methoxyphenyl)-1 H-imidazole (2 h)
Yield: 58%; m.p. 131-132°C; Rf 0.71; IR (cm-1): 3427 (N-H), 3016 (C-H), 1568 (C = N), 1426 (C = C), 1330 (C-N); 1H-NMR (δ, ppm): 3.88 (s, 3 H, OCH3), 6.98 and 7.53 (d, each, A2B2, p-anisyl ring), 7.46 and 8.27 (d, each, A2B2, p-substituted phenyl), 7.93 (d, 1 H, H-5, imidazole), 10.31 (bs, 1 H, NH); MS (m/z): 295 [M+]. Anal. Calcd. for C16H13N3O3 (%): C, 65.08; H, 4.44; N, 10.23. Found: C, 65.18; H, 4.45; N, 10.26.
2-(2-hydroxyphenyl)-4-(4-methoxyphenyl)-1 H-imidazole (2i)
Yield: 46%; m.p. 139°C; Rf 0.63; IR (cm-1): 3432 (N-H), 3314 (O-H), 3016 (C-H), 1578 (C = N), 1431 (C = C), 1318 (C-N); 1H-NMR (δ, ppm): 3.85 (s, 3 H, OCH3), 6.87 and 7.45 (d, each, A2B2, p-anisyl ring), 7.16-7.32 (m, 2 H, H-4,5, phenyl), 7.51-7.83 (m, 2 H, H-3,6, phenyl), 7.91 (d, 1 H, H-5, imidazole), 10.28 (bs, 1 H, NH); MS (m/z): 266 [M+]. Anal. Calcd. for C16H14N2O2 (%): C, 72.17; H, 5.30; N, 10.52. Found: C, 72.33; H, 5.29; N, 10.55.
2-(3-hydroxyphenyl)-4-(4-methoxyphenyl)-1 H-imidazole (2j)
Yield: 40%; m.p. 128-130°C; Rf 0.71; IR (cm-1): 3425 (N-H), 3309 (O-H), 3007 (C-H), 1572 (C = N), 1426 (C = C), 1311 (C-N); 1H-NMR (δ, ppm): 3.83 (s, 3 H, OCH3), 6.85 and 7.42 (d, each, A2B2, p-anisyl ring), 7.12-7.98 (m, 5 H, phenyl + H-5, imidazole), 10.25 (bs, 1 H, NH); MS (m/z): 266 [M+]. Anal. Calcd. for C16H14N2O2 (%): C, 72.17; H, 5.30; N, 10.52. Found: C, 72.32; H, 5.25; N, 10.44.
2-(4-hydroxyphenyl)-4-(4-methoxyphenyl)-1 H-imidazole (2k)
Yield: 56%; m.p. 150°C; Rf 0.61; IR (cm-1): 3428 (N-H), 3312 (O-H), 3015 (C-H), 1566 (C = N), 1431 (C = C), 1323 (C-N); 1H-NMR (δ, ppm): 3.86 (s, 3 H, OCH3), 6.82 and 7.46 (d, each, A2B2, p-anisyl ring), 7.43 and 7.88 (d, each, A2B2, p-substituted phenyl), 7.92 (d, 1 H, H-5, imidazole), 10.32 (bs, 1 H, NH); MS (m/z): 266 [M+]. Anal. Calcd. for C16H14N2O2 (%): C, 72.17; H, 5.30; N, 10.52. Found: C, 72.32; H, 5.28; N, 10.56.
2,4-Di-(4-methoxyphenyl)-1 H-imidazole (2 l)
Yield: 49%; m.p. 183-184°C; Rf 0.69; IR (cm-1): 3422 (N-H), 3010 (C-H), 1571 (C = N), 1428 (C = C), 1331 (C-N); 1H-NMR (δ, ppm): 3.84 and 3.91 (s, each, 2 × OCH3), 6.86, 7.12, 7.44 and 7.63 (d, each, 2 × A2B2, 2x p-anisyl ring), 7.86 (d, 1 H, H-5, imidazole), 10.29 (bs, 1 H, NH); MS (m/z): 280 [M+]. Anal. Calcd. for C17H16N2O2 (%): C, 72.84; H, 5.75; N, 9.99. Found: C, 72.57; H, 5.56; N, 9.84.
2-(4-hydroxy-3-methoxyphenyl)-4-(4-methoxyphenyl)-1 H-imidazole (2 m)
Yield: 53%; m.p. 144°C; Rf 0.78; IR (cm-1): 3431 (N-H), 3018 (C-H), 1563 (C = N), 1433 (C = C), 1326 (C-N); 1H-NMR (δ, ppm): 3.87 and 3.95 (s, each, 2 × OCH3), 6.89 and 7.45 (d, each, A2B2, p-anisyl ring), 7.03 (d, 1 H, H-5, phenyl), 7.17 (d, 1 H, H-2, phenyl), 7.32 (dd, 1 H, H-6, phenyl), 7.82 (d, 1 H, H-5, imidazole), 10.26(bs, 1 H, NH); MS (m/z): 296 [M+]. Anal. Calcd. for C17H16N2O3: C, 68.91; H, 5.44; N, 9.45. Found: C, 68.88; H, 5.46; N, 9.17.
General method for the preparation of 1,2,4-trisubstituted-1 H-imidazoles (3a-m)
Compound 2a-m (0.01 mol) was suspended in tetrahydrofuran (20 mL) and was refluxed with chlorobenzene (2 mL) in the presence of 2-3 drops of triethylamine for 8 h. The completion of reaction was determined by TLC. After refluxing, acetone was added to the reaction mixture and it was kept at room temperature overnight. Later a precipitate formed which was filtered and recrystallized from ethanol. The compound was found pure on TLC examination (benzene: acetone, 8:2, v/v).
4-(4-methoxyphenyl)-1,2-diphenyl-1 H-imidazole (3a)
Yield: 47%; m.p. 176-178°C; Rf 0.63; IR (cm-1): 3455 (N-H), 3024 (C-H), 1579 (C = N), 1440 (C = C), 1333 (C-N); 1H-NMR (δ, ppm): 3.83 (s, 3 H, OCH3), 6.87 and 7.42 (d, each, A2B2, p-anisyl ring), 7.08-7.71 (m, 10 H, 2 × phenyl), 7.95 (s, 1 H, H-5, imidazole), 10.41 (bs, 1 H, NH); MS (m/z): 326 [M+]. Anal. Calcd. for C22H18N2O (%): C, 80.96; H, 5.56; N, 8.58. Found: C, 80.74; H, 5.48; N, 8.37.
2-(2-Chlorophenyl)-4-(4-methoxyphenyl)-1-phenyl-1 H-imidazole (3b) Yield: 54%; m.p. 156°C; Rf 0.71; IR (cm-1): 3013 (C-H), 1581 (C = N), 1443 (C = C), 1325 (C-N), 708 (C-Cl); 1H-NMR (δ, ppm): 3.85 (s, 3 H, OCH3), 6.81-7.56 (m, 13 H, 3x phenyl ring), 7.93 (s, 1 H, H-5, imidazole); MS (m/z): 361 [M+]. Anal. Calcd. for C22H17ClN2O (%): C, 73.23; H, 4.75; N, 7.76. Found: C, 73.35; H, 4.68; N, 7.54.
2-(3-chlorophenyl)-4-(4-methoxyphenyl)-1-phenyl-1 H-imidazole (3c)
Yield: 43%; m.p. 135-36°C; Rf 0.81; IR (cm-1): 3008 (C-H), 1574 (C = N), 1437 (C = C), 1329 (C-N), 715 (C-Cl); 1H-NMR (δ, ppm): 3.84 (s, 3 H, OCH3), 6.87-7.55 (m, 13 H, 3x phenyl ring), 7.95 (s, 1 H, H-5, imidazole); MS (m/z): 361 [M+]. Anal. Calcd. for C22H17ClN2O (%): C, 73.23; H, 4.75; N, 7.76. Found: C, 73.16; H, 4.72; N, 7.79.
2-(4-chlorophenyl)-4-(4-methoxyphenyl)-1-phenyl-1 H-imidazole (3d)
Yield: 41%; m.p. 140°C; Rf 0.86; IR (cm-1): 3010 (C-H), 1578 (C = N), 1439 (C = C), 1328 (C-N), 708 (C-Cl); 1H-NMR (δ, ppm): 3.85 (s, 3 H, OCH3), 6.84-7.51 (m, 13 H, 3x phenyl ring), 7.92 (s, 1 H, H-5, imidazole); MS (m/z): 361 [M+]. Anal. Calcd. for C22H17ClN2O (%): C, 73.23; H, 4.75; N, 7.76. Found: C, 73.17; H, 4.68; N, 7.81.
2-(4-fluorophenyl)-4-(4-methoxyphenyl)-1-phenyl-1 H-imidazole (3e)
Yield: 65%; m.p. 146-148°C; Rf : 0.62; IR (cm-1): 3016 (C-H), 1566 (C = N), 1441 (C = C), 1332 (C-N); 1H-NMR (δ, ppm): 3.81 (s, 3 H, OCH3), 6.82-7.38 (m, 13 H, 3x phenyl ring), 7.83 (s, 1 H, H-5, imidazole); MS (m/z): 344 [M+]. Anal. Calcd. for C22H17FN2O (%): C, 76.73; H, 4.98; N, 8.13. Found: C, 76.56; H, 5.02; N, 8.05.
2-(2-nitrophenyl)-4-(4-methoxyphenyl)-1-phenyl-1 H-imidazole (3 f)
Yield: 48%; m.p. 178°C; Rf 0.61; IR (cm-1): 3020 (C-H), 1572 (C = N), 1438 (C = C), 1329 (C-N); 1H-NMR (δ, ppm): 3.84 (s, 3 H, OCH3), 6.92-7.55 (m, 13 H, 3x phenyl ring), 7.95 (s, 1 H, H-5, imidazole); MS (m/z): 371 [M+]. Anal. Calcd. for C22H17N3O3 (%): C, 71.15; H, 4.61; N, 11.31. Found: C, 71.32; H, 4.52; N, 11.29.
2-(3-nitrophenyl)-4-(4-methoxyphenyl)-1-Phenyl-1 H-imidazole (3 g)
Yield: 43%; m.p. 161-162°C; Rf 0.68; IR (cm-1): 3015 (C-H), 1575 (C = N), 1433 (C = C), 1326 (C-N); 1H-NMR (δ, ppm): 3.86 (s, 3 H, OCH3), 6.90-7.52 (m, 13 H, 3x phenyl ring), 7.97 (s, 1 H, H-5, imidazole); MS (m/z): 371 [M+]. Anal. Calcd. for C22H17N3O3 (%): C, 71.15; H, 4.61; N, 11.31. Found: C, 71.08; H, 4.56; N, 11.32.
2-(4-nitrophenyl)-4-(4-methoxyphenyl)-1-Phenyl-1 H-imidazole (3 h)
Yield: 50%; m.p. 157-58°C; Rf 0.78; IR (cm-1): 3023 (C-H), 1582 (C = N), 1440 (C = C), 1333 (C-N); 1H-NMR (δ, ppm): 3.83 (s, 3 H, OCH3), 6.87-7.47 (m, 13 H, 3x phenyl ring), 7.92 (s, 1 H, H-5, imidazole); MS (m/z): 371 [M+]. Anal. Calcd. for C22H17N3O3 (%): C, 71.15; H, 4.61; N, 11.31. Found: C, 71.11; H, 4.58; N, 11.36.
2-(2-hydroxyphenyl)-4-(4-methoxyphenyl)-1-Phenyl-1 H-imidazole (3i)
Yield: 58%; m.p. 168°C; Rf 0.78; IR (cm-1): 3319 (O-H), 3015 (C-H), 1561 (C = N), 1433 (C = C), 1324 (C-N); 1H-NMR (δ, ppm): 3.85 (s, 3 H, OCH3), 6.81-7.49 (m, 13 H, 3x phenyl ring), 7.95 (s, 1 H, H-5, imidazole); MS (m/z): 342 [M+]. Anal. Calcd. for C22H18N2O2 (%): C, 77.17; H, 5.30; N, 8.18. Found: C, 77.04; H, 5.32; N, 8.21.
2-(3-hydroxyphenyl)-4-(4-methoxyphenyl)-1-Phenyl-1 H-imidazole (3j)
Yield: 54%; m.p. 157-58°C; Rf 0.79; IR (cm-1): 3313 (O-H), 3016 (C-H), 1555 (C = N), 1431 (C = C), 1326 (C-N); 1H-NMR (δ, ppm): 3.86 (s, 3 H, OCH3), 6.85-7.51 (m, 13 H, 3x phenyl ring), 8.02 (s, 1 H, H-5, imidazole); MS (m/z): 342 [M+]. Anal. Calcd. for C22H18N2O2 (%): C, 77.17; H, 5.30; N, 8.18. Found: C, 77.03; H, 5.26; N, 8.20.
2-(4-Hydroxyphenyl)-4-(4-Methoxyphenyl)-1-Phenyl-1 H-Imidazole (3k)
Yield: 41%; m.p. 175°C; Rf 0.64; IR (cm-1): 3316 (O-H), 3013 (C-H), 1553 (C = N), 1438 (C = C), 1317 (C-N); 1H-NMR (δ, ppm): 3.84 (s, 3 H, OCH3), 6.78-7.64 (m, 13 H, 3 × phenyl ring), 8.11 (s, 1 H, H-5, imidazole); MS (m/z): 342 [M+]. Anal. Calcd. for C22H18N2O2 (%): C, 77.17; H, 5.30; N, 8.18. Found: C, 77.28; H, 5.31; N, 8.14.
2,4-Di-(4-methoxyphenyl)-1-phenyl-1 H-imidazole (3 l)
Yield: 52%; m.p. 163-64°C; Rf 0.71; IR (cm-1): 3009 (C-H), 1546 (C = N), 1432 (C = C), 1318 (C-N); 1H-NMR (δ, ppm): 3.82 and 3.86 (s, each, 2x OCH3), 6.89-7.68 (m, 13 H, 3 × phenyl ring), 7.98 (s, 1, H-5, imidazole); MS (m/z): 356 [M+]. Anal. Calcd. for C23H20N2O2 (%): C, 77.51; H, 5.66; N, 7.86. Found: C, 77.33; H, 5.61; N, 7.82.
2-(4-Hydroxy-3-methoxyphenyl)-4-(4-methoxyphenyl)-1-phenyl-1 H-imidazole (3 m) Yield: 58%; m.p. 172°C; Rf 0.63; IR (cm-1): 3322 (O-H), 3014 (C-H), 1553 (C = N), 1427 (C = C), 1324 (C-N); 1H-NMR (δ, ppm): 3.87 (s, 3 H, OCH3), 6.88-7.72 (m, 13 H, 3 × phenyl ring), 8.15 (s, 1H, H-5, imidazole); MS (m/z): 372 [M+]. Anal. Calcd. for C23H20N2O3 (%): C, 74.18; H, 5.41; N, 7.52. Found: C, 74.02; H, 5.43; N, 7.45.
Pharmacology
All the animals used in the present study were kept in the animal house in accordance with the University Animal Ethical Committee of Jamia Hamdard, which applies the guidelines and rules laid down by the Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA), Ministry of Social Justice and Empowerment, Government of India. Albino rats of Wistar strain of either sex, weighing 180-200 g and Albino mice of either sex weighing 22-25 g, were used for pharmacological activities. The animals were housed in groups of six and acclimatized to room conditions for at least 2 days before the experiments. Food and water were freely available up to the time of experiments except during the short time they were removed from the cages for testing.
Anti-inflammatory activity
The synthesized compounds were evaluated for their in vivo anti-inflammatory activity using carrageenan-induced rat paw edema method[14] using Indomethacin as standard. The paw volume was measured by using digital Plethysmometer (Panlab).
The rats were randomly divided into groups of six. One group was kept as control, and received only 0.5% carboxymethyl cellulose (CMC) solution. Another group was kept as standard and received Indomethacin (10 mgkg-1 p.o.), other groups received test compounds (10 mgkg-1 p.o.). Carrageenan solution (0.1% in sterile 0.9% NaCl solution) in a volume of 0.1 mL was injected subcutaneously into the sub-plantar region of the right hind paw of each rat, 1 h after the administration of the test compounds and standard drugs. The paw volume was measured by saline displacement shown on screen of digital Plethysmometer (Panlab) at 2 and 3 h after carrageenan injection. Thus the edema volume in control group (Vc) and edema volume in groups treated with test compounds (Vt) was measured and the percentage inhibition of edema was calculated using the formula:
Anti-inflammatory activity (% inhibition) = {(Vc - Vt)/Vc} × 100
Analgesic activity
The compounds which showed anti-inflammatory activity above 70% of that of the Indomethacin were screened for analgesic activity. Analgesic activity was evaluated by acetic acid induced writhing method.[15]
Swiss albino mice (25-30 g) of either sex were divided into group of six in each. A 1% aqueous sodium chloride solution (i.p. injection in a volume of 0.1 mL) was used as writhing induced agent. Mice were kept individually in the test cage, before acetic acid injection and habituated for 30 min. Screening of analgesic activity was performed after p.o. administration of test drugs at a dose of 10 mgkg-1. Group I was taken as control and received CMC suspension only, group II received reference drug Indomethacin and rest of the groups were treated with test drugs suspended in 1.0% CMC. After 1 h of drug administration 0.10 mL of 1% acetic acid solution was given to mice intraperitoneally. Stretching movements consisting of arching of the back, elongation of body and extension of hind limbs were counted for 5-15 min of sodium chloride injection. The analgesic activity was expressed in terms of percentage protection.
Analgesic activity (% protection) = {(n-n’)/n} × 100
where, n = mean number of writhes of control group, n’ = mean number of writhes of test group.
Ulcerogenic activity
Acute ulcerogenesis test was done according to the reported method.[2] Albino rats (150-200 g) were divided into different groups consisting of six animals in each group. Ulcerogenic activity evaluated after p.o. administration of test compounds or Indomethacin at the dose of 30 mg kg-1. Control rats received p.o. administration of vehicle (suspension of 1% methyl cellulose). Food but not water was removed 24 h before administration of the test compounds. After the drug treatment the rats were fed with normal diet for 17 h and then sacrificed. The stomach was removed and opened along the greater curvature, washed with distilled water and cleaned gently by dipping in normal saline. The mucosal damage was examined by means of a magnifying glass. For each stomach the mucosal damage was assessed according to the following scoring system: 0.5: redness, 1.0: spot ulcers, 1.5: hemorrhagic streaks, 2.0: ulcers >3 but <5, 3.0: ulcers >5. The mean score of each treated group minus the mean score of control group was regarded as severity index of gastric mucosal damage.
Antifungal activity
The newly prepared compounds were screened for their antifungal activity against Candida albicans, Rhizopus oryza, Penicillium citrum and Aspergillus niger fungal strains at a concentration of 100 μgmL-1 by cup-plate method[16] using ketoconazole as standard. Compounds inhibiting growth of one or more of the above microorganisms were further tested for minimum inhibitory concentration (MIC).[17] Sabouraud agar media was prepared by dissolving peptone (1 g), D-glucose (4 g) and agar (2 g) in distilled water (100 mL) and adjusting pH to 5.7. Normal saline was used to make a suspension of spore of fungal strain for lawning. A loopful of particular fungal strain was transferred to 3 mL saline to get a suspension of corresponding species. Agar media (20 mL) was poured into each petridish. Excess of suspension was decanted and the plates were dried by placing in an incubator at 37°C for 1 h. Wells were made using an agar punch and, each well was labeled accordingly. A control was also prepared in triplicate and maintained at 37°C for 3-4 days. The antifungal activity of the compounds was compared with the standard drug; ketoconazole. The nutrient broth, which contained logarithmic serially two fold diluted amount of test compound and controls, was inoculated with approximately 1.6 × 104-6 × 104 c.f.u. mL-1. The cultures were incubated for 48 h at 37°C and the growth was monitored. The lowest concentration (highest dilution) required to inhibit the growth of fungus was regarded as MIC.
Results and Discussion
Chemistry
Synthesis of the intermediates and target compounds (2a-m and 3a-m) was accomplished as outlined in Scheme I. The tri-substituted imidazoles, 1,2,4-trisubstituted-1 H-imidazoles (3a-m), were synthesized by refluxing 2,4-disubstituted-1 H-imidazoles (2a-m) with chlorobenzene in tetrahydrofuran (THF) using triethylamine as catalyst. 2,4-Disubstituted-1 H-imidazoles (2a-m) were synthesized by refluxing 4-methoxyphenyl glyoxal (1) with aryl aldehyde in glacial acetic acid using ammonium acetate as catalyst. The required intermediate compound 4-methoxyphenyl glyoxal (1) was synthesized from 4-methoxy acetophenone using selenium dioxide. The structures were established on the basis of modern analytical techniques (IR, NMR and MS).
In general, the IR spectra of the compounds showed characteristic band around 3430 cm-1 for N-H of di-substituted imidazoles (2a-m). Disappearance of this band from the spectra of tri-substituted imidazoles (3a-m) indicated the substitution of hydrogen of NH of the di-substituted imidazole ring by phenyl to give tri-substituted imidazoles. Other bands were observed at appropriate places corresponding to the structure. In[1] H-NMR spectral data, all the compounds showed characteristic peaks at appropriate δ-values. Presence of a broad singlet around δ 10.35 (NH) and a doublet around δ 7.75 (imidazole, H-5) indicated the formation of di-substituted imidazole ring. Similarly, disappearance of broad singlet of NH of imidazole confirmed the conversion of di-substituted imidazoles (2a-m) to tri-substituted imidazoles (3a-m). The structures of the compounds were further supported by mass spectral data. The synthesized compounds gave M+ peak in reasonable intensities. In case of compounds having phenyl rings with chloro-substituents (2b, 2c, 2d, 3b, 3c and 3d) the molecular ion peak or their fragments having chloro-group appeared as cluster of peaks.
Pharmacology
The synthesized compounds were evaluated for anti-inflammatory activity along with analgesic and ulcerogenic effect. The selected compounds which showed good anti-inflammatory effect (70% to that of the standard drug) were also tested for their antifungal effect.
In vivo anti-inflammatory activity of the title compounds was determined by carrageenan-induced rat paw edema method[14] at the dose level of 10 mg kg-1 of body weight. Six of the twenty tested compounds showed statistically significant anti-inflammatory activity with respect to standard drug indomethacin. Compounds, 2-(4-nitrophenyl) -4-(4-methoxyphenyl)-1-phenyl-1 H-imidazole (3 h) and 2,4-di-(4-methoxyphenyl)-1-phenyl-1 H-imidazole (3 l) having p-nitro and p-methoxy substitution, were found to be the most active compounds with 58.02 and 56.17% inhibition, respectively. In addition, four more compounds, 2-(4-nitrophenyl)-4-(4-methoxyphenyl)-1 H-imidazole (2 h) 2,4-di-(4-methoxyphenyl)-1 H-imidazole (2L), 2-(3-nitrophenyl)-4-(4-methoxyphenyl)-1-phenyl-1 H-imidazole (3 g) and 2-(4-hydroxy-3-methoxyphenyl)-4-(4-methoxyphenyl)-1-phenyl-1 H-imidazole (3 m) also showed appreciable activity with 51.02, 49.79, 49.58 and 52.88% inhibition, respectively. The standard drug indomethacin exhibited 70.78% inhibition in rat paw edema [Table 1]. After analyzing the results, following remarks could be made regarding the SAR:
Table 1.
Anti-inflammatory activity of the title compounds
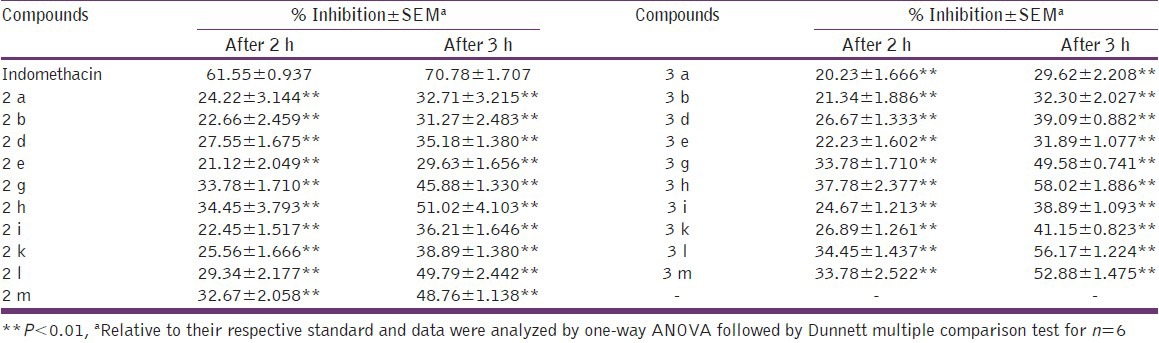
The anti-inflammatory activity was found to be better in tri-substituted imidazoles (3a-m) as compared to that of the di-substituted imidazoles (2a-m).
Compounds with electropositive groups like methoxy, nitro, etc., (2 h, 2 l, 3 h and 3 l) were found to be more active than the compounds with electronegative group like chloro, fluoro, etc., (2d, 2e, 3d and 3e).
p-Substituted derivatives were found to be better in their anti-inflammatory action than o- or m-substituted derivatives (h was more active than g; k was more active than i).
The compounds (2 h, 2 l, 3 g, 3 h, 3 l and 3 m) that exhibited above 70% of anti-inflammatory activity of indomethacin were further evaluated for their analgesic effects using sodium chloride induced writhing method[15] and ulcerogenic effect by reported method.[2]
The results of analgesic activity [Table 2] indicated that compounds 3 h and 3 l showed 49.99 and 47.14% protection, respectively, against sodium chloride induced writhings test. The standard drug indomethacin was found to have 59.60% protection at the same dose level, 10 mgkg-1 p.o. Compounds 2 h, 2 l, 3 g and 3 m also showed appreciable analgesic activity with 40.53, 42.74, 41.79 and 44.83% protection, respectively.
Table 2.
Analgesic effects along with ulcerogenic effect of some selected imidazole derivatives
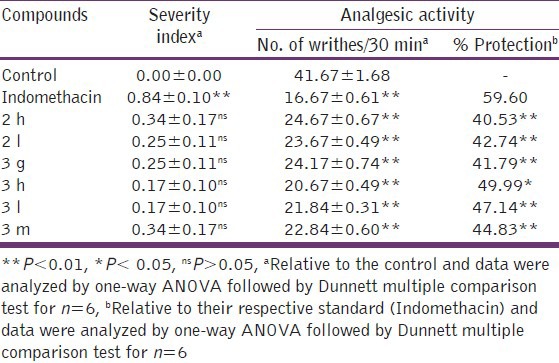
The results indicated that the compounds with p-nitro substitution and p-methoxy substitution were found to be a good anti-inflammatory agent with high analgesic activity. The activity was found to increase by the replacement of N-proton of imidazole ring by phenyl group.
The selected compounds were also screened for their acute ulcerogenic activity and found to be low in ulcerogenic action; severity index ranging from 0.17 to 0.34 whereas the standard drug indomethacin showed high severity index of 0.84. 2-(4-Nitrophenyl)-4-(4-methoxyphenyl)-1-phenyl-1 H-imidazole (3 h) and 2,4-di-(4-methoxyphenyl)-1-phenyl-1 H-imidazole (3 l) showed maximum reduction in ulcerogenic activity (severity index = 0.17). The other tested compounds also exhibited better GI safety profile as compared to that of the standard drug indomethacin.
The results of pharmacological activities indicate that the synthesized compounds not only have good anti-inflammatory-analgesic activity but also have less GI irritation. The results are presented in Tables 1 and 2.
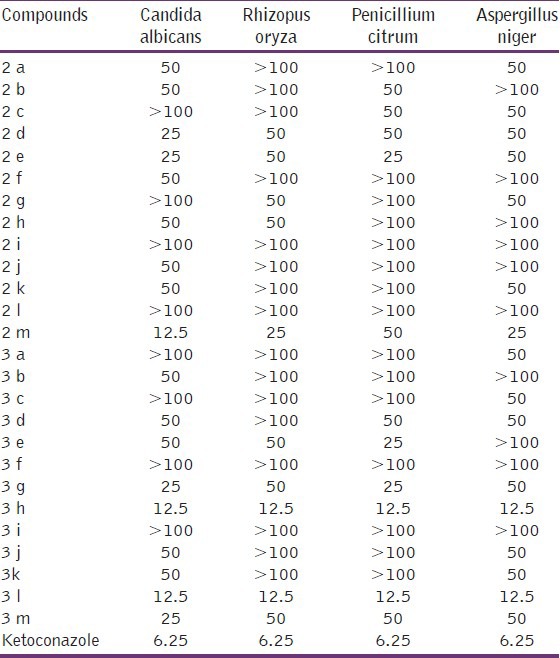
All the synthesized compounds (2a-m and 3a-m) were tested for their antifungal effect initially at 100 μg mL-1 by cup-plate method and the compounds inhibiting the growth of one or more strains were further tested for their MIC [Table 3]. Two compounds, 2-(4-nitrophenyl)-4-(4-methoxyphenyl)-1-phenyl-1 H-imidazole (3 h) and 2,4-di-(4-methoxyphenyl)-1-phenyl-1 H-imidazole (3 l), were found to be the most active among all the tested twenty compounds with MIC of 12.5 μgmL-1 against all the tested four fungal strains. Two more compounds, 2-(4-hydroxy-3-methoxyphenyl)-4-(4-methoxyphenyl)-1 H-imidazole (2 m) and 2-(4-hydroxy-3-methoxyphenyl)-4-(4-methoxyphenyl)-1-phenyl-1H-imidazole (3 m), were also good in their antifungal action. Rests of the compounds were moderate to low in their action.
Table 3.
Antifungal activity (minimum inhibitory concentration; μgmL-1) of some selected imidazole derivatives
Conclusion
To sum up, two compounds among the synthesized twenty imidazole derivatives, 2-(4-nitrophenyl)-4-(4-methoxyphenyl)-1-phenyl-1 H-imidazole (3 h) and 2,4-di-(4-methoxyphenyl)-1-phenyl-1 H-imidazole (3 l), emerged as lead compounds with good anti-inflammatory as well as antifungal actions. The synthesized compounds were also found to have less GI irritation. Further studies on these compounds may result in development of compounds with dual inhibition of inflammation and fungal infections.
Acknowledgements
The authors are thankful to UGC, Govt. of India, New Delhi for providing financial assistance.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Bekhit AA, Aziem TA. Design, synthesis and biological evaluation of some pyrazole derivatives as anti-inflammatory-antimicrobial agents. Bioorg Med Chem. 2004;12:1935–45. doi: 10.1016/j.bmc.2004.01.037. [DOI] [PubMed] [Google Scholar]
- 2.Cioli V, Putzolu S, Rossi V, Sorza Barcellona P, Corradino C. The role of direct tissue contact in the production of gastro-intestinal ulcer by anti-inflammatory drugs in rats. Toxicol Appl Pharmacol. 1979;50:283–9. doi: 10.1016/0041-008x(79)90153-4. [DOI] [PubMed] [Google Scholar]
- 3.Puratchikody A, Doble M. Antinociceptive and anti-inflammatory activities and QSAR studies on 2-substituted-4,5-diphenyl-1 H-imidazoles. Bioorg Med Chem. 2007;15:1083–90. doi: 10.1016/j.bmc.2006.10.025. [DOI] [PubMed] [Google Scholar]
- 4.Frank PV, Girish KS, Kalluraya B. Solvent-free microwave-assisted synthesis of oxadiazoles containing imidazole moiety. J Chem Sci. 2007;119:41–6. [Google Scholar]
- 5.Ramachandran R, Rani M, Senthan S, Jeong YT, Kabilan S. Synthesis, spectral, crystal structure and in vitro antimicrobial evaluation of imidazole/benzotriazole substituted piperidin-4-one derivatives. Eur J Med Chem. 2011;46:1926–34. doi: 10.1016/j.ejmech.2011.02.036. [DOI] [PubMed] [Google Scholar]
- 6.Fang B, Zhou CH, Rao XC. Synthesis and biological activities of novel amine-derived bis-azoles as potential anti-bacterial and anti-fungal agents. Eur J Med Chem. 2010;45:4388–98. doi: 10.1016/j.ejmech.2010.06.012. [DOI] [PubMed] [Google Scholar]
- 7.Hack S, Worlein B, Hofner G, Pabel J, Wanner KT. Development of imidazole alkanoic acids as mGAT3 selective GABA uptake inhibitors. Eur J Med Chem. 2011;46:1483–98. doi: 10.1016/j.ejmech.2011.01.042. [DOI] [PubMed] [Google Scholar]
- 8.Zuliani V, Fantini M, Nigam A, Stables JP, Patel MK, Rivara M. Anticonvulsant activity of 2,4(1H)-diarylimidazoles in mice and rats acute seizure models. Bioorg Med Chem. 2010;18:7957–65. doi: 10.1016/j.bmc.2010.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu J, Lee H, Huesca M, Young A, Allen C. Liposome formulation of a novel hydrophobic aryl-imidazole compound for anti-cancer therapy. Cancer Chemother Pharmacol. 2006;58:306–18. doi: 10.1007/s00280-005-0161-x. [DOI] [PubMed] [Google Scholar]
- 10.Steel HC, Tintinger GR, Anderson R. Comparison of the anti-inflammatory activities of imidazole antimycotics in relation to molecular structure. Chem Biol Drug Des. 2008;72:225–8. doi: 10.1111/j.1747-0285.2008.00694.x. [DOI] [PubMed] [Google Scholar]
- 11.Merlos M, Vericat ML, García-Rafanell J, Forn J. Topical anti-inflammatory properties of flutrimazole, a new imidazole antifungal agent. Inflamm Res. 1996;45:20–5. doi: 10.1007/BF02263500. [DOI] [PubMed] [Google Scholar]
- 12.Husain A, Drabu S, Kumar N. Synthesis and biological screening of substituted Imidazoles. Acta Pol Pharm. 2009;66:243–8. [PubMed] [Google Scholar]
- 13.Hasan SM, Alam MM, Husain A, Khanna S, Akhtar M, Zaman MS. Synthesis of 6-aminomethyl derivatives of benzopyran-4-one with dual biological properties: Antiinflammatory-analgesic and antimicrobial. Eur J Med Chem. 2009;44:4896–903. doi: 10.1016/j.ejmech.2009.08.001. [DOI] [PubMed] [Google Scholar]
- 14.Winter CA, Risley EA, Nuss GW. Carrageenin-induced edema in hind paw of the rat as an assay for anti-iflammatory drugs. Proc Soc Exp Biol Med. 1962;111:544–47. doi: 10.3181/00379727-111-27849. [DOI] [PubMed] [Google Scholar]
- 15.Fukawa K, Kawano O, Hibi M, Misaki N, Ohba S, Hatanaka Y. A method for evaluating analgesic agents in rats. J Pharmacol Methods. 1980;4:251–9. doi: 10.1016/0160-5402(80)90017-0. [DOI] [PubMed] [Google Scholar]
- 16.Cruickshank R, Dugid JP, Marmion DP, Swain RH. Vol. 2. Edinburge, London: Churchill-Livingstone; 1975. Medical Microbiology; pp. 196–202. [Google Scholar]
- 17.Varma RS, editor. Lucknow, India: National Academy of Chemistry and Biology; 1998. Antifungal Agents: Past, Present and Future Prospects; pp. 55–128. [Google Scholar]


