Abstract
Stress, both physical and psychological, is attracting increasing attention among neuroresearchers. In the last 20 decades, there has been a surge of interest in the research of stress-induced manifestations and this approach has resulted in the development of more appropriate animal models for stress-associated pathologies and its therapeutic management. These stress models are an easy and convenient method for inducing both psychological and physical stress. To understand the behavioral changes underlying major depression, molecular and cellular studies are required. Dysregulation of the stress system may lead to disturbances in growth and development, and may this may further lead to the development of various other psychiatric disorders. This article reviews the different types of stress and their neurobiology, including the different neurotransmitters affected. There are various complications associated with stress and their management through various pharmacological and non-pharmacological techniques. The use of herbs in the treatment of stress-related problems is practiced in both Indian and Western societies, and it has a vast market in terms of anti-stress medications and treatments. Non-pharmacological techniques such as meditation and yoga are nowadays becoming very popular as a stress-relieving therapy because of their greater effectiveness and no associated side effects. Therefore, this review highlights the changes under stress and stressor and their impact on different animal models in understanding the mechanisms of stress along with their effective and safe management.
KEY WORDS: Animal models, depression, manifestations, neurotransmitters, stress, therapeutic management
Stress is a common experience of daily life and all organisms have developed mechanisms to cope with it. Sustained stress can have numerous pathophysiological effects such as activation of neuro-endocrine (limbic-hypothalamic-pituitary adrenal system)[1] and hormonal (corticosterone release) functions.[2] Sustained and persistent stressful conditions can precipitate anxiety and affective disorders such as depression, which further leads to the excessive production of free radicals and oxidative burden.[3] In an organism, diverse stressors activate a wide spectrum of interacting hormonal and neuronal systems resulting in behavioral (anxiety disorders, decrease in food intake, decrease in sexual behavior, and loss of cognitive function) and physiological responses (activation of pituitary adrenal axis and release of glucocorticoids into the blood stream).[4] These stressors are stimulators of arousal and lead to autonomic (changes in body temperature and tachycardia) and behavioral changes; however, when arousal increases to stress-like levels, it results in psychiatric and physical disorders[5] [Figure 1]. Different animal models have been developed for chronic stress-induced neurological disorders such as the olfactory bulbectomy model, and the chronic unpredictable stress model. These animal models are used to screen various new chemical entities and to develop a better understanding of the underlying molecular pathway in chronic stress pathology. Stress responses are variable and there are individual differences both physiologically and behaviorally in how an organism perceives a perturbation and in the resulting adaptational/maladaptational processes.[6]
Figure 1.
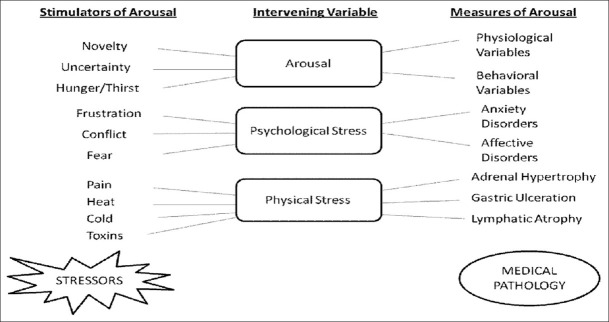
Relationship between arousal, psychological stress, physical stress and pathology
Stress and Stressors
Stress can be defined generally as responses to demands upon the body.[7] It is the body's reaction to a change that requires a physical, mental, or emotional adjustment or response[8]. It can come from any situation or thought that makes one feel frustrated, angry, nervous, or anxious. Conceptually, stress can be any threat, either real or perceived, to the well-being of an organism and it can be of two types as shown in Table 1.
Table 1.
Types of stress
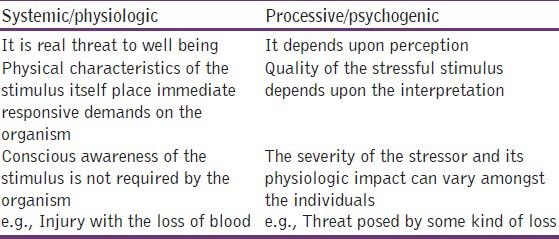
Stressor is a stimulus, either internal or external, that activates the hypothalamic pituitary adrenal (HPA) axis and the sympathetic nervous system resulting in physiological change.[9] Long-term exposure to stressors can cause depression,[10] post-traumatic stress disorder, and anxiety disorders. The degree of behavioral control that an individual has over a stressor often determines the consequence of that stressor and plays a key role in the development of pathological behaviors after a traumatic event.[11] The potency to cope with the stressors is a fundamental requirement for survival. Brain is the target for different stressors because of its high sensitivity to stress-induced degenerative conditions.[12] The brain tissue is made up of large amounts of polyunsaturated fatty acid, thus making it vulnerable to free radical attacks.[13]
Neurobiology of Stress
Hormonal involvement
In biological terms, stress has been defined by various physiologic changes including activation of the pituitary adrenal axis, which leads to the liberation of adrenal steroids triggered by the release of adrenocorticotrophic hormone (ACTH) from the pituitary.[1] This ACTH stimulation is controlled by the corticotropin releasing factor (CRF) present in the hypothalamus and released in response to various stressors. This over-activation can produce the psychopathology of anxiety such as disorders, depression, and even damage to body organs in chronic severe cases.[7] The stress-responsive systems are interconnected and the activation of the HPA axis is facilitated by stress-induced norepinephrine (NE) release in certain regions of the brain such as hippocampus and amygdala.[14] Any dysregulation of these systems can lead to various stress-related complications.[15] CRF is the principal activator of ACTH secretion by the anterior pituitary.[1]. Activation of this axis, also called the HPA axis, results in glucocorticoid release into systemic circulation. These glucocorticoids are in turn considered to be key players in an organism's response to stress.[16] Stress and glucocorticoids have specific effects on cognitive functions in humans and in animal models. Adrenal steroids and stressful events result in short-term and reversible deficits in episodic and spatial memory in both animal models and humans. Acute effects of stress are evident within a time span ranging from a few hours to a day are generally reversible and selective to a particular task or situation.[17]
Neurotransmitters and Stress
Gamma-aminobutyric acid
It is an important inhibitory neurotransmitter in the central nervous system. The role of GABA and benzodiazepine receptors has been well documented in stress disorders such as anxiety, epilepsy, insomnia, and convulsive disorders.[18] Stress has been reported to alter the content of the GABA neurotransmission, which suggests the involvement of GABA in stress-induced behavioral and biochemical alterations.[19] Melatonin (N-acetyl-5-methoxy-tryptamine) is a secretory product primarily synthesized in the pineal gland and released into the blood stream and cerebrospinal fluid. Study revealed the involvement of GABAergic mechanism in the hypnotic action of melatonin.[20] Stress induces the release of CRF and GABA from the amygdale and hypothalamus.[21] It down-regulates vesicular glutamate transporter and its coupling with GABA-synthesizing enzyme, glutamic acid decarboxylase (GAD65) in response to maternal separation could lead to decreased GABA levels in the hippocampus.[22]
Dopamine
Stress-induced changes in dopamine (DA) levels within terminal areas seem to involve mainly ventral tegmental area projecting cells. Findings from preclinical studies suggest an uneven response of DA in different stressful stimuli. Specifically, an acute and controllable/escapable physical stress was seen to cause an enhanced DA efflux in the ventral striatum, whereas chronic and uncontrollable/inescapable exposure to the same stress attenuated DA release. Parkinson's disease is an age-associated neurodegenerative disease, clinically characterized as a movement disorder arising due to selective degeneration of dopaminergic neurons in the substantia nigra of the ventral midbrain, thereby depleting the dopamine levels in the striatum.
Norepinephrine
Brain epinephrine serves globally as an alarm system that decreases neurovegetative functions, such as eating and sleeping, and this contributes to accompanying increase in autonomic and neuroendocrine responses to stress, including HPA axis activation.[23] NE also activates the amygdala, the principal brain locus for fear-related behaviors, and enhances the long-term storage of aversively charged emotional memories in sites such as the hippocampus and striatum. Monoaminergic systems regulate the activity of neurons in the amygdala.[24] Stress has been reported to increase the turnover of NE in many terminal projection areas of the locus ceruleus[25] and also increase extracellular NE in the hippocampus. There are substantial evidences suggesting that neurons in the brain containing and secreting noradrenaline and CRF are activated during stress.[26] Hence, it is quite evident that both noradrenaline and CRF are involved in behavioral responses to stress [Figure 2].[27,28]
Figure 2.
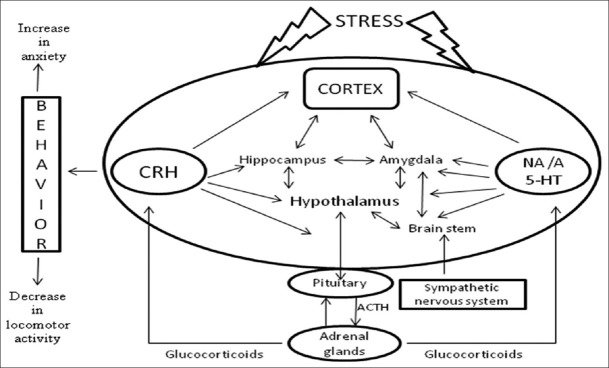
Schematic presentation of major components of limbic system, modulating neurotransmitters, and their interplay with two major neurotransmitter systems that mediate stress response
Serotonin
Previous reports have suggested that stress affects the activity of central dopaminergic and serotonergic neurons.[29,30] Interactions between serotonin and CRF have been demonstrated by various studies in different parts of the brain [Figure 2]. Studies have proved that significant reduction in serotonin level increases the responsiveness to stress.[31,32] Hippocampal serotonin concentrations are increased during psychosocial conflict in animals.[33] The 5-hydroxytryptamine receptor 1A (5-HT1A) receptors are down-regulated in distinct brain regions including the hippocampus and cortex following stress.[34]
Melatonin
It is synthesized from tryptophan within the pinealocytes. Most synthetic activity occurs during the dark phase, with a major increase (7-150 fold) in the activity of serotonin-N-acetyltransferase.[35] Melatonin, being an endogenous hormone, has also been known to improve the quality of sleep and reduce the formation of free radicals; it also allows the restoration of antioxidant enzymes.[36] Serotonin is the intermediate product in melatonin synthesis and in the presence of serotonin-N-transferase it gets converted into N-acetyl serotonin, which further with the help of hydroxyl indole o-methyl transferase gets converted into melatonin [Figure 3]. Thus, there is possibility of the involvement of serotonergic neurotransmission in the protective effects of melatonin.
Figure 3.
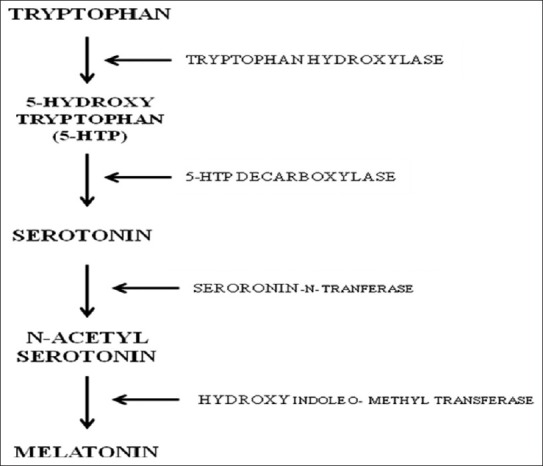
De novo synthesis pathway of melatonin
Glutamate
There is substantial evidence indicating that the Para ventricular nucleus (PVN) receives glutaminergic innervations from large brain areas involving the PVN itself and several other nuclei in and outside the hypothalamus. Among the neuroanatomical regions of glutamatergic afferents to the PVN, dorsomedial hypothalamic nucleus is the candidate locus for glutamatergic neurons that could be activated by immobilization stress. Microinjection of N-methyl-D-aspartate (NMDA) into the dorsomedial hypothalamic nucleus causes an increase in glutamate release in the PVN and results in cardiovascular response very similar to the one evoked by emotional stress.[37]
Consequences of Stress
Normal development and preservation of life and species depend on a normally functioning stress system. Maladaptive neuroendocrine responses, i.e., dysregulation of the stress system, may lead to disturbances in growth and development, and cause psychiatric, endocrine/metabolic, and/or autoimmune diseases or vulnerability to such diseases.
Stress and anxiety
According to previous reports stress induces anxiety-like behavior in both humans and animals.[38] In response to stress, there is an increase in CRF levels. The CRF level decreases when the stressor is no longer present. Lee, et al. reported that chronic stress increases the length and volume of expression of CRF in areas of the brain associated with fear and emotion, including the amygdala[39] [Figure 3]. Such chronic stress changes the body's response, and the resulting increased expression of CRF is believed to be the cause of health-related stress problems such as anxiety, depression, and infertility.[40] Exposure to stress represents an important factor for a number of neuropsychiatric disorders such as depression, post-traumatic stress disorder, and other anxiety disorders.[41] There are earlier reports of enhanced noradrenergic or HPA axis activity in many psychopathological states such as depression and anxiety disorders.[42,43]
Oxidative stress contributes toward neuronal degeneration in the central nervous system in the process of aging as well as neurodegenerative diseases.[44] The production of reactive oxygen species (ROS) is greatly increased under many conditions of toxic stress.[45] One of the reasons for stress-induced enhancement of free radicals may be the elevation of nitric oxide (NO) production.[46] This is further supported by the present determination of nitrite levels, which revealed significant increase in brain NO levels in stressed mice. The reactive nitrogen species along with ROS, working in concert with an inflammatory process, may play a substantial role in the pathogenesis of depression.[46] Stress has been shown to be responsible for the depletion of several free radical detoxifying enzymes such as glutathione peroxidase, catalase, and superoxide dismutase.[47] This results in oxidative burden, which has been implicated in stress as well as in the pathogenesis of several disease states. Since brain tissues consist of a high content of polyunsaturated fatty acids and one of the important consequences of oxidative stress is peroxidation of membrane lipids, this reaction produces marked damage to the structure and function of cell membranes in these tissues.[48] Therefore, lipid peroxidation was supposed as the major biochemical alteration and consequence of oxidant-induced cell injury. Thus, the important consequences of stress could be attributed to stress-induced lipid peroxidation.
Stress and memory loss
Memory impairment is a common and usual comorbidity associated with exposure to prolonged stress. Chronic stress has been found to induce cognitive dysfunction in psychiatric patients, which leads to the loss of synaptic connectivity and perhaps neuronal networks in limbic brain structures including the hippocampus and cortex. This further leads to loss of cholinergic neurons and results in a state of dementia.
Stress and its metabolic consequences
Activation of the stress system leads to behavioral and hormonal changes that improve the ability of the organism to adjust to homeostasis and increase its chances of survival.[49] Hypothalamic corticotrophin-releasing harmone (CRH) plays a major role in inhibiting gonadotropin-releasing hormone secretion during stress, while via somatostatin it also inhibits growth hormone, thyrotropin-releasing hormone, and thyrotropin secretion, thus suppressing reproduction, growth, and thyroid function. Chronic activation of the stress system would be expected to increase visceral adiposity, decrease lean body (muscle and bone) mass, and suppress osteoblastic activity.[23] Glucocorticoids directly inhibit pituitary gonadotropin, growth hormone, and thyrotropin secretion and make the target tissues of sex steroids and growth factors resistant to these substances.[50] Glucocorticoids also have direct effects on the bone, inhibiting osteoblastic activity and causing osteoporosis.[49] Obese subjects with psychiatric manifestations ranging from those of melancholic depression to anxiety with perception of ‘uncontrollable’ stress frequently have mild hypercortisolism. Stress-induced hypercortisolism and visceral obesity and their cardiovascular as well as other sequel increase the mortality risk of affected subjects by 2-3fold and curtail their life expectancy by several years.[23]
Stress and immunological changes
Stress has been associated with impaired immune function and increased susceptibility to infectious diseases.[51] It is now believed that the nervous, endocrine, and immune systems are so intimately connected that they should be regarded as a single network rather than as three separate systems.[51] It is widely accepted that psychological stress and psychiatric illness can compromise immune function,[52] and soluble mediators released by immune cells can affect the central nervous system, thus producing alterations in behavior. Exposure to stressful life events such as academic examinations and divorce was reported to cause impairments in various aspects of cellular immune function.[53] There are also reports of immune activation,[53] in addition to immunosuppression in both the depressed and subjects exposed to stressful life events.
Animal Models for Acute and Chronic Stress
Animal models are the basic tools to understand the pathophysiology of the disorders and for the development of newer therapeutics. The model should be simple and reproducible in other animal species. Stress can be acute or chronic based on the duration of exposure to the stressors [Table 2]. Failure to terminate acute stress response can lead to persistent changes that are characteristic of chronic stress state. An interesting characteristic of the endocrine response to an acute stressor is that it is facilitated in animals exposed to chronic or repeated stress[54]
Table 2.
Types of stressors used in animal studies

Therapeutic Strategies Involved in the Management of Stress
Pharmacological approaches
Benzodiazepines
One of the important consequences of stress is anxiety. Benzodiazepines (e.g., flurazepam, diazepam, chlordiazepoxide) are effective in the rapid treatment of anxiety disorder. Benzodiazepines are often associated with the risk of dependence, which is a major drawback associated with this class of drugs.[55]
5-HT1A receptor agonists
The 5-HT1A receptor is related to emotion and 5-HT1A receptor agonists can induce anxiolytic and antidepressant effects in both humans and animals.[56] 5-HT1A receptor agonists are effective in stress-induced behavioral symptoms. Buspirone is a non-benzodiazepine anxiolytic agent and is a partial 5-HT1A receptor agonist.[57] The 5-HT1A receptor partial agonist, tandospirone, is an azapirone derivative similar to buspirone and has anxiolytic effects.[58] Diazepam is also a widely used anxiolytic and inhibits various stress-induced changes such as activation of the HPA axis.
Cyclooxygenase-inhibitors
COXis the rate-limiting enzyme that converts arachidonic acid into prostaglandins and has been suggested to play an important role in various central nervous system-related disorders. Moreover, prostaglandins play an important role in mediating the HPA responses to immune insults.[59] Naproxen (non-selective cyclooxygenase (COX) inhibitor) and rofecoxib (selective COX-2 inhibitor) are known to attenuate oxidative stress by inhibiting COX and thereby prostaglandin release.
Antioxidants
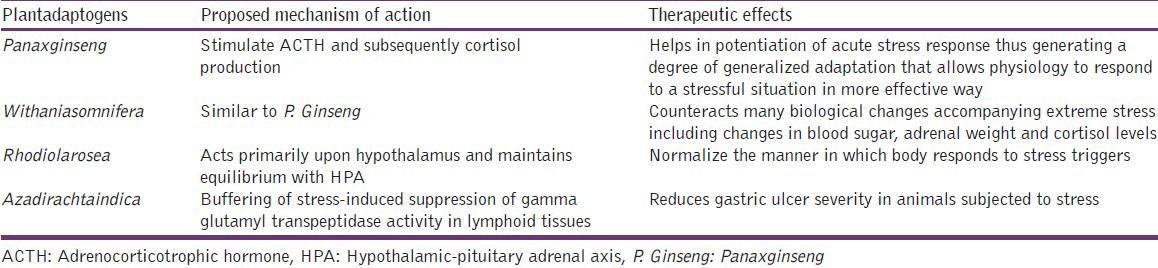
Antioxidants are known to have great importance in human disease pathology because of their possible action against free radicals.[60] Dietary macronutrients contribute to the antioxidant defense system. These include β-carotene, vitamin C, and vitamin E. In view of the vital role of oxidative stress in the pathogenesis of Alzheimer's disease (AD), the potential role of these antioxidant supplements to prevent AD has gained much interest.[61] A complete list of herbal antioxidants in management of stress along with their proposed mechanism of action is shown in Table 3.
Table 3.
Herbal management of stress
Tricyclic anti depressants
Tricyclic antidepressants such as trazodone, mirtapazine, doxepin, and amitriptyline having a stronger sedative effect are also prescribed for insomnia in doses sub-threshold for the treatment of depression. These drugs may have a partial protective role against sleep deprivation-induced anxiety-like behavior and altered locomotor activity.
Non-pharmacological approaches
These strategies include exercises, relaxation techniques, and physical exercise programs including laughter and motivation. These may include:
Exercising
Exercising is the most effective way to becoming stress-free. One should exercise daily. This will help to relax and keep mind off things that cause stress. There are a few simple exercises that can help release stress. Walking, light aerobics, jogging, and riding a cycle or bike are some of the simplest ways out of de-stressing. Playing games is also effective in releasing stress.
Relaxation techniques
Relaxation techniques can help relieve stress and put the mind at ease. With some daily schedule, stress can be reduced. Soft music can be therapeutic for a stressful mind. Yoga and meditation can also be used as relaxation techniques to relive stress. Meditation can help one get rid of negative feelings, such as anger. Deep breathing can help relax and relieve stress.
Laughter
Humor in one's life decreases stress. Laughter is a good medicine for everyone. Laughter in life can increase the positive energy and mitigate any negative vibes. Laughter releases the anger and frustration bottled up inside from being stressed.
Motivation techniques
Motivation provides an impetus to get up and do something. Stress can be used as a motivator to change the way of thinking in life. One should learn to be motivated in life and make things better.
Conclusions
Although stress has been intensely studied over the past half-century, the mechanism of stress is still unknown because of a wide array of cellular activities. However, investigations into the mechanisms regarding these roles are still yielding new surprises and twists. This review describes stressor and neurobiology of stress in relation to their animal model. It also discusses the pathological and clinical symptomatic management of stress. However, further research investigations arerequired to reveal the unknown mechanisms that would help in developing a mechanism that would help manage stress.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Bonfiglio JJ, Inda C, Refojo D, Holsboer F, Arzt E, Silberstein S. The corticotropin-releasing hormone network and the hypothalamic-pituitary-adrenal axis: Molecular and cellular mechanisms involved. Neuroendocrinology. 2011;94:12–20. doi: 10.1159/000328226. [DOI] [PubMed] [Google Scholar]
- 2.Fuchs E, Flügge G. Stress, glucocorticoids and structural plasticity of the hippocampus. Neurosci Biobehav Rev. 1998;23:295–300. doi: 10.1016/s0149-7634(98)00031-1. [DOI] [PubMed] [Google Scholar]
- 3.Maes M, Kubera M, Obuchowiczwa E, Goehler L, Brzeszcz J. Depression's multiple comorbidities explained by (neuro) inflammatory and oxidative and nitrosative stress pathways. Neuro Endocrinol Lett. 2011;32:7–24. [PubMed] [Google Scholar]
- 4.Henry JP, Stephens PM. Berlin: Springer-Verlag; 1977. Stress, health, and the social environment: A sociobiologic approach to medicine; pp. 245–63. [Google Scholar]
- 5.Hennessy JW, Levine S. Stress arousal, and the pituitary adrenal system: A psychoendocrine hypothesis. In: Sprague JM, Epstein AN, editors. Progress in psychobiology and physiological psychology. New York, NY: Academic Press; 1979. pp. 133–78. [Google Scholar]
- 6.Weiner H. Chicago: University of Chicago Press; 1992. Perturbing the Organism: The biology of stressful experience. [Google Scholar]
- 7.Koob GF. Corticotropin-releasing factor, norepinephrine, and stress. Biol Psychiatry. 1999;46:1167–80. doi: 10.1016/s0006-3223(99)00164-x. [DOI] [PubMed] [Google Scholar]
- 8.Selye H. A syndrome produced by diverse noxious agents. Nature. 1936;32:138. doi: 10.1176/jnp.10.2.230a. [DOI] [PubMed] [Google Scholar]
- 9.Maier SF, Watkins LR. Cytokines for psychologists: Implications of bidirectional immune-to-brain communication for understanding behavior, mood, and cognition. Psychol Rev. 1998;105:83–107. doi: 10.1037/0033-295x.105.1.83. [DOI] [PubMed] [Google Scholar]
- 10.Nirmal J, Babu CS, Harisudhan T, Ramanathan M. Evaluation of behavioural and antioxidant activity of Cytisus scoparius Link in rats exposed to chronic unpredictable mild stress. BMC Complement Altern Med. 2008;8:15. doi: 10.1186/1472-6882-8-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Christianson JP, Thompson BM, Watkins LR, Maier SF. Medial prefrontal cortical activation modulates the impact of controllable and uncontrollable stressor exposure on a social exploration test of anxiety in the rat. Stress. 2009;12:445–50. doi: 10.1080/10253890802510302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sahin E, Gümüşlü S. Immobilization stress in rat tissues: Alterations in protein oxidation, lipid peroxidation and antioxidant defense system. Comp Biochem Physiol C Toxicol Pharmacol. 2007;144:342–7. doi: 10.1016/j.cbpc.2006.10.009. [DOI] [PubMed] [Google Scholar]
- 13.Gutteridge JM. Lipid peroxidation and antioxidants as biomarkers of tissue damage. Clin Chem. 1995;41:1819–28. [PubMed] [Google Scholar]
- 14.Plotsky PM. Facilitation of immunoreactive corticotropin-releasing factor secretion into the hypophysial-portal circulation after activation of catecholaminergic pathways or central norepinephrine injection. Endocrinology. 1987;121:924–30. doi: 10.1210/endo-121-3-924. [DOI] [PubMed] [Google Scholar]
- 15.Johnson EO, Kamilaris TC, Chrousos GP, Gold PW. Mechanisms of stress: A dynamic overview of hormonal and behavioral homeostasis. Neurosci Biobehav Rev. 1992;16:115–30. doi: 10.1016/s0149-7634(05)80175-7. [DOI] [PubMed] [Google Scholar]
- 16.Herman JP, Prewitt CM, Cullinan WE. Neuronal circuit regulation of the hypothalamo-pituitary-adrenocortical stress axis. Crit Rev Neurobiol. 1996;10:371–94. doi: 10.1615/critrevneurobiol.v10.i3-4.50. [DOI] [PubMed] [Google Scholar]
- 17.Lupien SJ, McEwen BS. The acute effects of corticosteroids on cognition: Integration of animal and human model studies. Brain Res Brain Res Rev. 1997;24:1–27. doi: 10.1016/s0165-0173(97)00004-0. [DOI] [PubMed] [Google Scholar]
- 18.Nikolaus S, Antke C, Beu M, Müller HW. Cortical GABA, striatal dopamine and midbrain serotonin as the key players in compulsive and anxiety disorders – Results from in vivo imaging studies. Rev Neurosci. 2010;21:119–39. doi: 10.1515/revneuro.2010.21.2.119. [DOI] [PubMed] [Google Scholar]
- 19.Quintero L, Cardenas R, Suarez-Roca H. Stress-induced hyperalgesia is associated with a reduced and delayed GABA inhibitory control that enhances post-synaptic NMDA receptor activation in the spinal cord. Pain. 2011;152:1909–22. doi: 10.1016/j.pain.2011.04.017. [DOI] [PubMed] [Google Scholar]
- 20.Buscemi N, Witmans M. What is the role of melatonin in the management of sleep disorders in children? Paediatr Child Health. 2006;11:517–19. doi: 10.1093/pch/11.8.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bagosi Z, Csabafi K, Jászberényi M, Telegdy G. The effects of corticotropin-releasing factor and the urocortins on hypothalamic gamma-amino butyric acid release – The impacts on the hypothalamic-pituitary-adrenal axis. Neurochem Int. 2012;60:350–4. doi: 10.1016/j.neuint.2012.01.007. [DOI] [PubMed] [Google Scholar]
- 22.Martisova E, Solas M, Horrillo I, Ortega JE, Meana JJ, Tordera RM, et al. Long lasting effects of early-life stress on glutamatergic/GABAergic circuitry in the rat hippocampus. Neuropharmacology. 2012;62:1944–53. doi: 10.1016/j.neuropharm.2011.12.019. [DOI] [PubMed] [Google Scholar]
- 23.Tsigos C, Chrousos GP. Hypothalamic-pituitary-adrenal axis, neuroendocrine factors and stress. J Psychosom Res. 2002;53:865–71. doi: 10.1016/s0022-3999(02)00429-4. [DOI] [PubMed] [Google Scholar]
- 24.McGaugh JL, Roozendaal B. Role of adrenal stress hormones in forming lasting memories in the brain. Curr Opin Neurobiol. 2002;12:205–10. doi: 10.1016/s0959-4388(02)00306-9. [DOI] [PubMed] [Google Scholar]
- 25.Korf J, Aghajanian GK, Roth RH. Increased turnover of norepinephrine in the rat cerebral cortex during stress: Role of the locus coeruleus. Neuropharmacology. 1973;12:933–8. doi: 10.1016/0028-3908(73)90024-5. [DOI] [PubMed] [Google Scholar]
- 26.Owens MJ, Edwards E, Nemeroff CB. Effects of 5-HT1A receptor agonists on hypothalamo-pituitary-adrenal axis activity and corticotropin-releasing factor containing neurons in the rat brain. Eur J Pharmacol. 1990;190:113–22. doi: 10.1016/0014-2999(90)94118-h. [DOI] [PubMed] [Google Scholar]
- 27.Dunn AJ, Swiergiel AH. The role of corticotropin-releasing factor and noradrenaline in stress-related responses, and the inter-relationships between the two systems. Eur J Pharmacol. 2008;583:186–93. doi: 10.1016/j.ejphar.2007.11.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kvetnansky R, Ukropec J, Laukova M, Manz B, Pacak K, Vargovic P. Stress stimulates production of catecholamines in rat adipocytes. Cell Mol Neurobiol. 2012;32:801–13. doi: 10.1007/s10571-012-9822-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Torres IL, Gamaro GD, Vasconcellos AP, Silveira R, Dalmaz C. Effects of chronic restraint stress on feeding behavior and on monoamine levels in different brain structures in rats. Neurochem Res. 2002;27:519–25. doi: 10.1023/a:1019856821430. [DOI] [PubMed] [Google Scholar]
- 30.Nankova B, Kvetnanský R, McMahon A, Viskupic E, Hiremagalur B, Frankle G, et al. Induction of tyrosine hydroxylase gene expression by a nonneuronal nonpituitary-mediated mechanism in immobilization stress. Proc Natl Acad Sci U S A. 1994;91:5937–41. doi: 10.1073/pnas.91.13.5937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Temel Y, Helmy A, Pinnock S, Herbert J. Effect of serotonin depletion on the neuronal, endocrine and behavioural responses to corticotropin-releasing factor in the rat. Neurosci Lett. 2003;338:139–42. doi: 10.1016/s0304-3940(02)01392-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Texel SJ, Camandola S, Ladenheim B, Rothman SM, Mughal MR, Unger EL, et al. Ceruloplasmin deficiency results in an anxiety phenotype involving deficits in hippocampal iron, serotonin, and BDNF. J Neurochem. 2012;120:125–34. doi: 10.1111/j.1471-4159.2011.07554.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Blanchard DC, Sakai RR, McEwen B, Weiss SM, Blanchard RJ. Subordination stress: Behavioral, brain, and neuroendocrine correlates. Behav Brain Res. 1993;58:113–21. doi: 10.1016/0166-4328(93)90096-9. [DOI] [PubMed] [Google Scholar]
- 34.Flügge G. Dynamics of central nervous 5-HT1A-receptors under psychosocial stress. J Neurosci. 1995;15:7132–40. doi: 10.1523/JNEUROSCI.15-11-07132.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tan DX, Manchester LC, Hardeland R, Lopez-Burillo S, Mayo JC, Sainz RM, et al. Melatonin: A hormone, a tissue factor, an autocoid, a paracoid, and an antioxidant vitamin. J Pineal Res. 2003;34:75–8. doi: 10.1034/j.1600-079x.2003.02111.x. [DOI] [PubMed] [Google Scholar]
- 36.Barón V, Muriel P. Role of glutathione, lipid peroxidation and antioxidants on acute bile-duct obstruction in the rat. Biochim Biophys Acta. 1999;1472:173–80. doi: 10.1016/s0304-4165(99)00118-x. [DOI] [PubMed] [Google Scholar]
- 37.Gören MZ, Yananli HR, Berkman K, Onat F, Aker R. The influence of dorsomedial hypothalamic nucleus on contralateral paraventricular nucleus in NMDA-mediated cardiovascular responses. Brain Res. 2003;968:219–26. doi: 10.1016/s0006-8993(03)02241-8. [DOI] [PubMed] [Google Scholar]
- 38.Liezmann C, Klapp B, Peters EM. Stress, atopy and allergy: A re-evaluation from a psychoneuroimmunologic persepective. Dermatoendocrinol. 2011;3:37–40. doi: 10.4161/derm.3.1.14618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lee Y, Fitz S, Johnson PL, Shekhar A. Repeated Stimulation of CRF Receptors in the BNST of Rats Selectively Induces Social but not Panic-Like Anxiety. Neuropsychopharmacol. 2008;33:2586–94. doi: 10.1038/sj.npp.1301674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kimura M, Müller-Preuss P, Lu A, Wiesner E, Flachskamm C, Wurst W, et al. Conditional corticotropin-releasing hormone overexpression in the mouse forebrain enhances rapid eye movement sleep. Mol Psychiatry. 2010;15:154–65. doi: 10.1038/mp.2009.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Horstmann S, Binder EB. Glucocorticoids as predictors of treatment response in depression. Harv Rev Psychiatry. 2011;19:125–43. doi: 10.3109/10673229.2011.586550. [DOI] [PubMed] [Google Scholar]
- 42.Boyer P. Do anxiety and depression have a common pathophysiological mechanism? Acta Psychiatr Scand. 2000;406:24–9. [PubMed] [Google Scholar]
- 43.Kendler KS. Major depression and generalised anxiety disorder. Same genes, (partly) different environments – Revisited. Br J Psychiatry. 1996:68–75. [PubMed] [Google Scholar]
- 44.Hovatta I, Juhila J, Donner J. Oxidative stress in anxiety and comorbid disorders. Neurosci Res. 2010;68:261–75. doi: 10.1016/j.neures.2010.08.007. [DOI] [PubMed] [Google Scholar]
- 45.Liu Y, Schubert DR. The specificity of neuroprotection by antioxidants. J Biomed Sci. 2009;16:98. doi: 10.1186/1423-0127-16-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Matsumoto K, Yobimoto K, Huong NT, Abdel-Fattah M, Van Hien T, Watanabe H. Psychological stress-induced enhancement of brain lipid peroxidation via nitric oxide systems and its modulation by anxiolytic and anxiogenic drugs in mice. Brain Res. 1999;839:74–84. doi: 10.1016/s0006-8993(99)01715-1. [DOI] [PubMed] [Google Scholar]
- 47.Zaidi SM, Banu N. Antioxidant potential of vitamins A, E and C in modulating oxidative stress in rat brain. Clin Chim Acta. 2004;340:229–33. doi: 10.1016/j.cccn.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 48.Jain A, Mårtensson J, Stole E, Auld PA, Meister A. Glutathione deficiency leads to mitochondrial damage in brain. Proc Natl Acad Sci U S A. 1991;88:1913–7. doi: 10.1073/pnas.88.5.1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chrousos GP. The role of stress and the hypothalamic-pituitary-adrenal axis in the pathogenesis of the metabolic syndrome: Neuro-endocrine and target tissue-related causes. Int J Obes Relat Metab Disord. 2000;24 2:S50–5. doi: 10.1038/sj.ijo.0801278. [DOI] [PubMed] [Google Scholar]
- 50.Chrousos GP, Gold PW. The concepts of stress and stress system disorders. Overview of physical and behavioural homeostasis. JAMA. 1992;267:1244–52. [PubMed] [Google Scholar]
- 51.Connor TJ, Leonard BE. Depression, stress and immunological activation: The role of cytokines in depressive disorders. Life Sci. 1998;62:583–606. doi: 10.1016/s0024-3205(97)00990-9. [DOI] [PubMed] [Google Scholar]
- 52.Leonard BE. Stress, the immune system and psychiatry. In: Leonard BE, Miller K, editors. Oxford: John Wiley and Sons Ltd; 1995. pp. 114–36. [Google Scholar]
- 53.Bartrop RW, Luckhurst E, Lazarus L, Kiloh LG, Penny R. Depressed lymphocyte function after bereavement. Lancet. 1977;1:834–36. doi: 10.1016/s0140-6736(77)92780-5. [DOI] [PubMed] [Google Scholar]
- 54.Curtis AL, Pavcovich LA, Grigoriadis DE, Valentino RJ. Previous stress alters corticotropin-releasing factor neurotransmission in the locus coeruleus. Neuroscience. 1995;65:541–50. doi: 10.1016/0306-4522(94)00496-r. [DOI] [PubMed] [Google Scholar]
- 55.Chen KW, Berger CC, Forde DP, D’Adamo C, Weintraub E, Gandhi D. Benzodiazepine use and misuse among patients in a methadone program. BMC Psychiatry. 2011;11:90. doi: 10.1186/1471-244X-11-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sugimoto Y, Yamada J, Noma T. Effects of anxiolytics, diazepam and tandospirone, on immobilization stress-induced hyperglycemia in mice. Life Sci. 1998;63:1221–6. doi: 10.1016/s0024-3205(98)00384-1. [DOI] [PubMed] [Google Scholar]
- 57.Mandrioli R, Mercolini L, Raggi MA. Metabolism of benzodiazepine and non-benzodiazepine anxiolytic-hypnotic drugs: An analytical point of view. Curr Drug Metab. 2010;11:815–29. doi: 10.2174/138920010794328887. [DOI] [PubMed] [Google Scholar]
- 58.Ettenberg A, Bernardi RE. Anxiolytic-like actions of buspirone in a runway model of intravenous cocaine self-administration. Pharmacol Biochem Behav. 2006;85:393–9. doi: 10.1016/j.pbb.2006.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dhir A, Padi SS, Naidu PS, Kulkarni SK. Protective effect of naproxen (non-selective COX-inhibitor) or rofecoxib (selective COX-2 inhibitor) on immobilization stress-induced behavioral and biochemical alterations in mice. Eur J Pharmacol. 2006;535:192–8. doi: 10.1016/j.ejphar.2006.01.064. [DOI] [PubMed] [Google Scholar]
- 60.Joshi R, Kumar MS, Satyamoorthy K, Unnikrisnan MK, Mukherjee T. Free radical reactions and antioxidant activities of sesamol: Pulse radiolytic and biochemical studies. J Agric Food Chem. 2005;53:2696–703. doi: 10.1021/jf0489769. [DOI] [PubMed] [Google Scholar]
- 61.Li FJ, Shen L, Ji HF. Dietary intakes of vitamin E, vitamin C, and β-carotene and risk of Alzheimer's disease: A meta-analysis. J Alzheimers Dis. 2012;31:253–8. doi: 10.3233/JAD-2012-120349. [DOI] [PubMed] [Google Scholar]


