Abstract
Objectives:
There is an association between viral infection and development of diabetes mellitus. This study aimed to investigate the role of rubella virus, cytomegalovirus and coxsackievirus in patients with type 1 (T1D) and type 2 (T2D) diabetes mellitus in respect to the glycemic control and immune response presented by serum γ-interferon leveland antiviral antibodies.
Materials and Methods:
A total number of 160 (70 male and 90 female) T1D and 75 T2D (25 male and 50 female) patients allocated randomly from Martyr Layla Qasm center for diabetes mellitus in Erbil, Iraq, were enrolled in the study. Serum IgG antibody (I.U./mL) against rubella virus, cytomegalovirus coxsackievirus as well as serum interferon-g were determined.
Results:
Type-1 diabetic patients with positive anti-coxsakievirus antibody presented with significantly shorter duration of illness (4.822 ± 2.442 year) and poorer glycemic control (HbA1c %: 9.895 ± 1.272) This observation was not noticed with other viral infection as well as in T2D. Significant alterations in serum interferon-g (8.051 ± 13.371 pg/ml) were observed in T1D and related to coxasackievirus infection (13 patients had a level higher than 10.975 pg/ml; the upper limit of 95% C.I of control, and 34 had a level less than 4.457 pg/ml; the lower limit of 95% C.I of control).
Conclusions:
Subjects with type 1 diabetes and Coxsackie infections seem to have a different immunological and clinical profile. This needs further study.
Keywords: Diabetes, glycemic control, virus
INTRODUCTION
Viral infections such as mumps, rubella, enteroviruses, cytomegalovirus, rotavirus, and parvovirus have all been associated with human type-1 diabetes (T1D).[1,2] An association between diabetes and virus infection was first made in 1864 in a patient who developed the disease following mumps infection. It is now known that the ssRNA enveloped mumps virus is capable of infecting islet and pancreatic cells in vitro and in vivo, respectively, and mediating direct beta cell cytolysis.[3–5] Similarly, rubella virus was first associated with human T1D in 1969. Additionally, cytomegalovirus (CMV) infection was linked to the development of T1D in 1979.
The mechanisms by which viruses implicated in pathogenesis of T1D include: first, direct infection of beta cells which resulted in beta cell lysis and release of self-antigens which are picked up by antigen presenting cells (APCs) that in turn activate self-reactive lymphocytes that mediate beta cell destruction, leading to the expression of hyperglycemia.[1,3] Second, viral infection of APCs may cause an increased expression of cytokines that activate self reactive lymphocytes, or directly mediate beta cell cytolysis.[3] Third, viral antigens with homology to self-epitopes cross react, leading to the activation of self-reactive lymphocytes that mediate beta cell destruction i.e. ‘molecular mimicry’.[6] Finally, in experimental animal models, viral infections may cause a transient lymphopenia that disturbs the equilibrium between selfreactive lymphocytes and regulatory T lymphocytes, tipping the immune balance toward an autoimmune environment.[7] There are increasing reports of association between hepatitis C and type-2 diabetes (T2D),[8,9] but there is no evidence of association between rubella, cytomegalovirus or coxsacki B viral infection and T2D. This study is aimed to compare the sero-positive T2D and T1D patients toward rubella virus, cytomegalovirus and coxsackievirus in respect to the glycemic control and g-interferon in a small sample of patients lived in the Kurdistan, north of Iraq.
MATERIALS AND METHODS
This cross-sectional study was conducted in Martyr Layla Qasm center for diabetes mellitus in Erbil, Iraq during the period of 1st of August 2008 to 30 December 2009.
The study was approved by the local scientific committee of college of Pharmacy, Hawler Medical University. A consent form was obtained from each participant prior to the study. A total number of 160 (70 male and 90 female) T1D and 75 T2D (25 male and 50 female) patients allocated randomly (using randomized tables) from patients attended the diabetic center over the period of sixteen months were enrolled in the study. Fasting venous blood samples were obtained from participants and the sera were separated for determination of glucose, glycosylated hemoglobin (HbA1c %). ELISA-based determination of serum IgG antibody (I.U./mL) against rubella virus, cytomegalovirus coxsacki virus were used. The concentration of antibodies at the cut-off absorbance were: 15 I.U./mL (absorbance 2 at λ 450nm), 1.2 I.U./mL (absorbance 1.2 at λ 450nm) and 100 I.U./mL (absorbance 1.5 at λ 405nm) against rubella virus, cytomegalovirus and coxsacki virus respectively. The serum antibody concentration was calculated according to the following equation.[10]:

Also the serum immunoglobulin M(mg/dl) is determined by ELISA
Interferone-γ was determined in serum using enzyme linked immunosorbent assay (ELISA) technique. In brief, serum samples were added into the wells, incubated with shaking at 37°C for 2 h, then washed and biotinylated antibody and streptavidin-HRP conjugate were added in consequence. After 30 min incubation, the wells were washed and the substrate was added, incubated with shaking at room temperature for 20 min followed by adding stopping solution and then the absorbance was read at wavelength 450 nm.
Statistical analysis
The results are expressed as number, percent and mean ± SD. The data had normal distribution and were analyzed using two tailed unpaired Student’s “t” test, and 95% confidence intervals (95% C.I.) test taking P≤ 0.05 as the lowest limit of significance.
RESULTS
Table 1 shows that the age of T1D patients presented with antibody against coxsackievirus is significantly less than corresponding age of T1D patients with negative anti-coxsackie virus antibody. Such observation is not detected in patients with T2D who had anticoxackie virus antibody [Table 2]. Type -1 diabetic patients with positive anti-coxsakievirus antibody presented with significant short duration of illness (4.822 ± 2.442 years, P< 0.01) while those with anti-rubella or anti cytomegalovirus antibody did not show significant difference from corresponding patients without positive antibody [Table 1]. Such observation again not demonstrated in patients type-2 diabetes [Table 2]. Although the fasting serum glucose of T1D patients was higher than corresponding T2D which amounted approximately 1.5 fold but the difference in fasting serum glucose in each type of diabetes did not show significant differences regarding the presence of antibody against any studied virus [Tables 1 and 2]. The HbA1c % as indicator of glycemic control was significantly higher (which indicated poor glycemic control) in T1D patients with positive antibody against coxsackie virus (9.895 ± 1.272%) [Table 1]. In T2D patients with positive anti-coxsackie virus antibody, the HbA1c % is non significantly lower than corresponding patients without antibody [Table 2]. The serum level of interferon-g shows variations in both T1D and T2D patients which reflected in non significant differences [Tables 1 and 2]. Further statistical analysis using 95% C.I. revealed that all T1D patients who have positive antibody against virus, having either significant low or high antibody level [Table 1]. More than 70% of T1D patients with positive anti-coxsackie or anti-cytomegalovirus antibody have significant low serum interferon-γ[Table 1]. [Table 2] shows that T2D patients with positive anti-viral antibody required less doses of insulin compared with those with negative anti-viral antibody. There are no significant differences between serum level of IgM between patients with T1D and T2D (181.1 ± 58.8 vs 183.8 ± 54.1 mg/dl respectively) and these values are less than reference value of healthy subjects in the laboratory (219 ± 44 mg/dl).
Table 1.
Characteristics of type-1 diabetes (T1D) patients
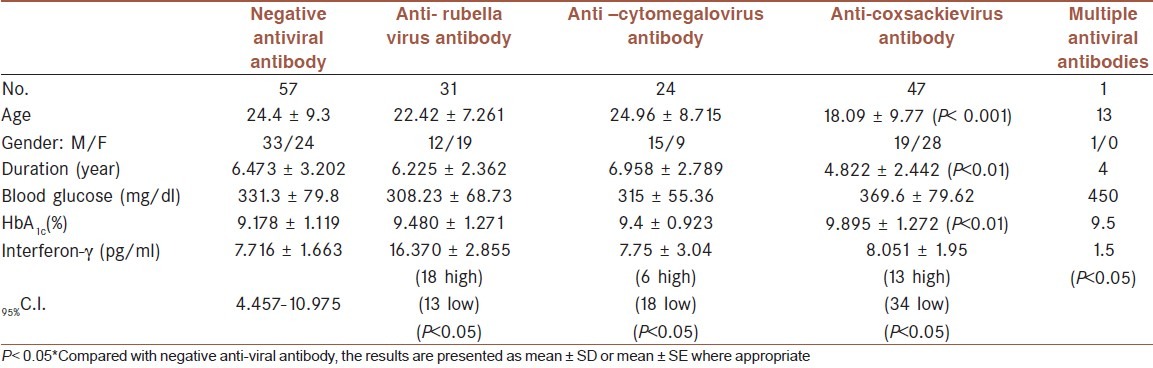
Table 2.
Characteristics of type-2 diabetes (T2D) patients
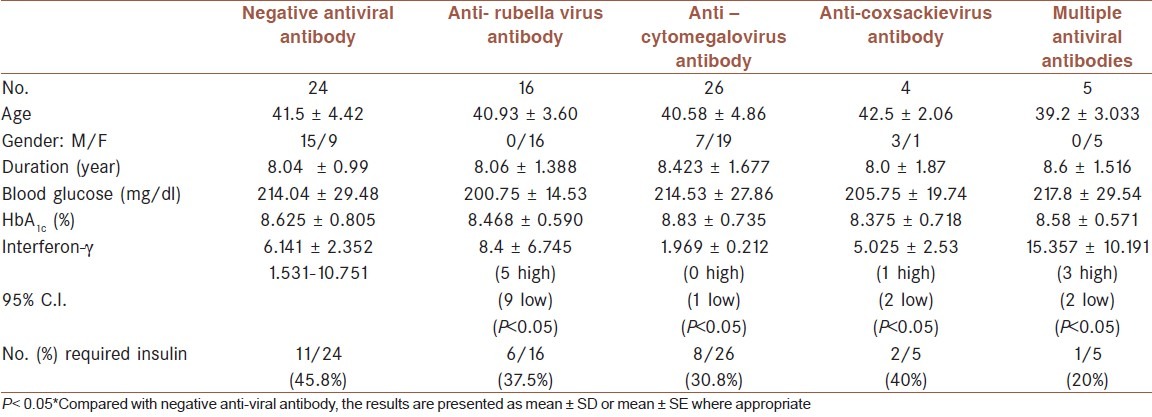
DISCUSSION
The results reported here show that certain Coxsackievirus is one of the many factors that involved in the glycemic control and in the immune response (presented with serum interferon-γ in T1D and not in T2D). It is well known that T1D occurs in patients with congenital rubella.[11] However, most of these patients have the HLA and immune markers characteristic of type 1 diabetes. In addition, Coxsackievirus B, cytomegalovirus, adenovirus, and mumps have been implicated in inducing certain cases of the disease. It is generally believed that the environmental agents trigger disease development in genetically susceptible individuals.[12–14] In this study, the viruses, though they may not have been directly shown to be implicated in inducing T1D have been probably linked to an alteration in the glycemic control as evidenced by the short duration of illness and high percent of HbA1c (%). This observation is not found in T2D and this supports the previous suggestion that viruses may trigger the disease in susceptible individuals. The significant short duration of illness in T1D is in agreement with the Horwitz et al. study who reported experimentally that a Coxsackievirus B4 infection accelerates diabetes development in transgenic mice.[15] Recently, it has been observed that interferon-γ production is critical for the mechanism by which a coxasackivirus B4 infection accelerates the progression to overt diabetes in transgenic mice and this explain the significant alteration in serum interferon-γ of T1D patient.[16] Further evidence about the interaction of interferon-g with Coxasackievirus infection was reported by the one who found that Coxsakievirus B infection triggers the production of interferon-γ.[17] This study adds a further observation that the changes in interferon-γ are associated with significant poor glycemic control. In T2D patients, only 4 cases out of 75 patients have positive anti-coxasackievirus antibody and this factor among many factors explains the non significant changes in duration of illness and glycemic control in T2D. This study points out the role of Coxasackievirus in poor glycemic control in T1D while its role in T2D is negligible. In fact T2D patients with Coxasackievirus infection required less recommended dose to control their glycemia than corresponding patients without viral infection. One of the limitations of the study is determination of the proinflamatory markers that indicate an association between viral infection and diabetes. The other limitation of the study is related to the many factors involved in using high doses of insulin in patients with positive antiviral antibodies; therefore logestic regression could be a useful model to avoid this problem, but the sample size is small. It may be concluded that the clinical profile of Coxsackievirus antibody associated type 1 diabetes is an area where future research should be carried out, and this may have implications for the better management of these patients.
ACKNOWLEDGMENTS
I thank my fellows, collaborators, and the physicians at Martyr Layla Qasm center for diabetes mellitus in Erbil who help me in this study. I thank Prof. Dr. Marwan S. M. Al-Nimer for his excellently revising the manuscript.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Van der WN, Kroese FG, Rozing J, Hillebrands JL. Viral infections as potential triggers of type 1 diabetes. Diabetes Metab Res Rev. 2007;23:169–83. doi: 10.1002/dmrr.695. [DOI] [PubMed] [Google Scholar]
- 2.Honeyman M. How robust is the evidence for viruses in the induction of type 1 diabetes? CurrOpinImmunol. 2005;17:616–23. doi: 10.1016/j.coi.2005.09.007. [DOI] [PubMed] [Google Scholar]
- 3.Coppieters KT, Wiberg A, Tracy SM, vonHerrath MG. Immunology in the clinic review series: Focus on type 1 diabetes and viruses: The role of viruses in type 1 diabetes: A difficult dilemma. ClinExpImmunol. 2012;168:5–11. doi: 10.1111/j.1365-2249.2011.04554.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sané F, Moumna I, Hober D. Group B coxsackieviruses and autoimmunity: Focus on Type 1 diabetes. Expert Rev ClinImmunol. 2011;7:357–66. doi: 10.1586/eci.11.11. [DOI] [PubMed] [Google Scholar]
- 5.Haverkos HW, Battula N, Drotman DP, Rennert OM. Enteroviruses and type 1 diabetes mellitus. Biomed Pharmacother. 2003;57:379–85. doi: 10.1016/j.biopha.2003.03.001. [DOI] [PubMed] [Google Scholar]
- 6.Robles DT, Eisenbarth GS. Type 1A diabetes induced by infection and immunization. J Autoimmun. 2001;16:355–62. doi: 10.1006/jaut.2000.0483. [DOI] [PubMed] [Google Scholar]
- 7.Rossini AA. Autoimmune diabetes and the circle of tolerance. Diabetes. 2004;53:267–75. doi: 10.2337/diabetes.53.2.267. [DOI] [PubMed] [Google Scholar]
- 8.Liu JL, Chen JY, Chen CT, Wang JH, Lin CY, Chen PF, et al. A community-based cross-sectional study: The association of lipids with hepatitis C seropositivity and diabetes mellitus. J GastroenterolHepatol. 2012 doi: 10.1111/j.1440-1746.2012.07212.x. [DOI] [PubMed] [Google Scholar]
- 9.Balogun WO, Adeleye JO, Akinlade KS, Kuti M, Otegbayo JA. Low prevalence of hepatitis-C viral seropositivity among patients with type-2 diabetes mellitus in a tertiary hospital. J Natl Med Assoc. 2006;98:1805–8. [PMC free article] [PubMed] [Google Scholar]
- 10.SERION ELISA classic. CoxsackievirusIgA/IgG/IgM. Available from: http://www.virion-serion.de/uploads/mit_download/Coxsackievirus .
- 11.Lammi N, Karvonen M, Tuomilehto J. Do microbes have a causal role in type 1 diabetes? Med SciMonit. 2005;11:63–9. [PubMed] [Google Scholar]
- 12.Li Q, Xing H, Zhou Y, Qiu LL, Zhang ZW, Liao L. Association of coxsackievirus infection and T lymphocyte subset changes with type 1 diabetes. Nan Fang Yi Ke Da XueXueBao. 2010;30:2699–701. [PubMed] [Google Scholar]
- 13.Karjalainen J, Knip M, Hyoty H, Linikki P, Ilonen J, Akerblom HK, et al. Relationship between serum insulin antibodies, islet cell antibodies and Coxsackie-B4 and mumps virus-specific antibodies at the clinical manifestation of type 1 (insulin-dependent) diabetes. Diabetologia. 1988;31:146–52. doi: 10.1007/BF00276847. [DOI] [PubMed] [Google Scholar]
- 14.Akatsuka H, Yano Y, Gabazza EC, Morser J, Sasaki R, Suzuki T, et al. A case of fulminant type 1 diabetes with coxsackieB4 virus infection diagnosed by elevated serum levels of neutralizing antibody. Diabetes Res ClinPract. 2009;84:e50–2. doi: 10.1016/j.diabres.2009.03.009. [DOI] [PubMed] [Google Scholar]
- 15.Horwitz MS, Bradley LM, Harbertson J, Krahl T, Lee J, Sarvetnick N. Diabetes induced by coxsackie virus: Initiation by bystander damage and not molecular mimicry. Nat Med. 1998;4:781–5. doi: 10.1038/nm0798-781. [DOI] [PubMed] [Google Scholar]
- 16.Serreze DV, Wasserfall C, Ottendorfer EW, Stalvey M, Pierce MA, Gauntt C, et al. Diabetes acceleration or prevention by a Coxsackievirus B4 infection: Critical requirements for both interleukin-4 and gamma interferon. JVirol. 2005;79:1045–52. doi: 10.1128/JVI.79.2.1045-1052.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Filippi CM, Estes EA, Oldham JE, von Herrath MG. Immunoregulatory mechanisms triggered by viral infections protect from type 1 diabetes in mice. J Clin Invest. 2009;119:1515–23. doi: 10.1172/JCI38503. [DOI] [PMC free article] [PubMed] [Google Scholar]


