Abstract
Background:
To investigate the reasons for postvitrectomy diabetic vitreous hemorrhage (PDVH), and to analyze the time of PDVH onset, the treatment of PDVH, the visual outcome of the treatment, and factors that affect visual acuity after treatment.
Materials and Methods:
Overall, 292 eyes from 236 patients with proliferative diabetic retinopathy (PDR) underwent vitrectomy from 2006 to 2010. Fifty eyes out of 43 patients had severe postoperative vitreous hemorrhage. The average follow-up duration was 6.8 ± 3.8 months (range, 2–12 months).
Results:
Recurrent vitreous hemorrhage (VH) after primary vitrectomy occurred in 40 eyes (80%) with an average time of VH onset of 62.5 ± 32.8 days (range, 3–170 days). VH occurred after silicone oil removal occurred in 10 eyes (20%), with an average time of VH onset of 27.4 ± 20.3 days (range, 1–60 days). The reasons for PDVH included chronic errhysis from retinal neovessels (47.1% of the eyes), residual fibrous vascular membrane (12.8% of the eyes), fibrovascular ingrowth at sclerotomy sites (4.3% of the eyes), iris neovessels and neovascular glaucoma (4.3% of the eyes), retinal vein occlusion (2.8% of the eyes), retinal tears (8.1% of the eyes), retinotomy (1.4% of the eyes), epichoroidal bleeding (1.4% of the eyes), polycythemia rubra vera (1.4% of the eyes), hypoperfusional retinopathy (4.3% of the eyes), and unknown reasons (12.8% of the eyes). Visual acuity increased in 43 eyes (86%) after surgical or nonsurgical treatment. The improvement in visual acuity after treatment was not affected by age, sex, duration of diabetes, time of PDVH onset, frequency of surgery, or treatment methods.
Conclusion:
Postvitrectomy diabetic vitreous hemorrhage commonly occurs two months after vitrectomy. Residual epiretinal neovascularization is the most common cause of PDVH. Active surgical or nonsurgical treatment for severe vitreous hemorrhage can obviously improve the patients’ visual prognosis.
Keywords: Hemorrhage, proliferative diabetic retinopathy, vitrectomy
INTRODUCTION
Diabetes mellitus is a common chronic metabolic disease with an increasing prevalence worldwide.[1] Proliferative diabetic retinopathy (PDR), an end-stage diabetic eye disease, is the most common cause of severe visual impairment. The complications of PDR include tractional retinal detachment, rhegmatogenous retinal detachment, vitreous hemorrhage, and severe fibrovascular proliferation. Tractional retinal detachment and vitreous hemorrhage are the two complications of PDR that are good indicators of vitrectomy.[2,3] Since the introduction of par plana vitrectomy in the 1970s,[4] it has been used to treat these complications of PDR, with successful outcomes.[5] Vision improvement has been reported in about 75% of the PDR patients after diabetic vitrectomy.[6] However, the major complication associated with vitrectomy is recurrent vitreous hemorrhage, which causes visual impairment and reoperation.[7,8]
Postvitrectomy diabetic vitreous hemorrhage (PDVH) is the most common complication after vitrectomy in PDR. The incidence of PDVH in PDR was reported to be as high as 75% in the 1980 s, and has been reduced to be approximately 13 to 40% with recent advances in surgical techniques.[9–11] Intraocular tamponade with air, gas, and silicone oil is considered for postoperative hemostasis.[5] Laser photocoagulation, to eliminate neovascularization and cryotherapy to the peripheral retina and sclerotomy entry sites has been advocated, to reduce the likelihood of postoperative vitreous hemorrhage (VH).[5,11,12] Air-fluid exchange and vitreous lavage have also been used to restore visual acuity after VH.[5,8,13]
The causes of PDVH include fibrovascular ingrowth at the sclerotomy sites, residual or recurrent neovascular membrane formation on the retina, insufficient retinal photocoagulation, a residual or recurrent epiretinal proliferative membrane, retinal vein occlusion, postoperative low intraocular pressure, and ocular trauma.[11,14–16] The most common reasons of PDVH have been reported to be fibrovascular ingrowth at the sclerotomy sites, residual or recurrent neovascular membrane formation on the retina, and insufficient retinal photocoagulation.[14–16] These suggest that recurrent VH often arises as a result of fibrovascular tissue proliferation.[17] The incidence of PDVH as a result of different etiologies varies greatly between different studies. For example, Hershberger et al. has reported that 86% of eyes with PDVH have been caused by fibrovascular ingrowth at the sclerotomy sites,[15] compared to only 28% in the study by Yan et al.[14]
In this study, we reviewed our experience of patients with PDR who underwent vitrectomy at our hospital, and aimed to analyze the underlying etiology in each case. In addition, we also examined the duration of PDVH before treatment was received, the different treatment modalities, the visual outcomes after treatment, and factors that affected visual acuity after treatment.
MATERIALS AND METHODS
Patients
A retrospective analysis was performed on 292 eyes of 236 patients who underwent vitrectomy for PDR between January, 2006 and December, 2010. This study included 50 eyes of 43 PDR patients who were hospitalized due to postoperative VH after primary vitrectomy. With the exception of two eyes in two patients, 48 eyes of the other 41 patients had undergone surgery at our hospital. Twenty-two patients (24 eyes) were male, and 21 patients were female (26 eyes), and their mean age was 55.5 ± 9.9 years (range, 24 to 75 years). The study was approved by the Medical Ethics Committee of our hospital (Research Project Number: R-0521), and all the patients gave their informed consent prior to their inclusion in the study.
The patients were diagnosed with diabetes according to the diagnostic criteria of the Chinese Diabetes Association,[18] as follows: one patient (two eyes) was diagnosed with type I diabetes and 42 patients (48 eyes) had type II diabetes. Thirty-two patients with type II diabetes were insulin-dependent. Their fasting blood glucose concentrations ranged from 7.8 to 11.2 mmol/L, and the blood pressure ranged from 100/70 to 190/110 mmHg. The average disease duration of diabetes was 16.5 ± 6.4 years (range, 2–26 years). According to the Chinese Diabetic Retinopathy Clinical Staging System published in 1985, the PDR was classified as either stage V(n = 14 eyes), or stage VI(n = 36 eyes).
Patient examination
Each patient underwent a complete ophthalmic examination, including visual acuity measurements using the Snellen chart and slit-lamp biomicroscopy. The intraocular pressure (IOP) was measured using non-contact tonometry (NT-200, Canon, Japan). The fundus was examined by indirect ophthalmoscopy. B-scan ultrasonography (Quentel Medical, France) was used to detect VH and to evaluate whether retinal detachment had occurred. Gonioscopy was used to assess whether neovascularization had occurred in the iridocorneal angle.
Definition and criteria of recurrent vitreous hemorrhage
According to previous reports,[14,19,20] recurrent VH after vitrectomy was defined as a VH that was large enough to cause decreased visual acuity and to prevent the clear visualization of retinal vessels under indirect ophthalmoscopy. This study included patients whose vitreous was clear at least one day after surgery. Patients with persistent VH that occurred within one day after surgery were excluded.
Surgical procedures
The indications for primary vitrectomy were severe PDR complicated with an unabsorbed VH, tractional retinal detachment, and rhegmatogenous retinal detachment.[21,22] All the patients underwent three-port pars plana vitrectomy (PPV) in combination with dissection and removal of the fibrovascular membranes. Vitreoretinal traction and extensive laser photocoagulation surgery was also performed as required. No intraocular tamponade was used if retinal breaks had not occurred. Retinal bleeding and retinal breaks were treated with silicone oil tamponade or gas tamponade. Retinal detachment and the failure to remove fibrovascular membranes were treated with the partial removal of the retina and silicone oil tamponade. Fluid-air exchange was performed to treat the border of the retinal breaks followed, by extensive laser photocoagulation.[23] Postoperative supplementary laser photocoagulation was performed if laser photocoagulation was not performed during surgery, due to viscous subretinal fluid and retinal edema. Phacoemulsification surgery or pars plana lensectomy by ultrasonic fragmentation were performed to remove the clouded lens that affected the vitrectomy, and an artificial lens was implanted when required. After surgery, patients with intraocular silicone oil tamponade and gas tamponade were maintained in a prone position for at least two weeks. Ophthalmological examinations were performed daily after surgery.
Management of postvitrectomy diabetic vitreous hemorrhage
If the recurrent VH was not very large after vitrectomy, the eyes were patched and a semi-reclining position was adopted. The patients were treated with Chinese Traditional medicine (Yunnan Biayao) and iodized lecithin.[14] Elevated IOP was treated with mannitol and timololbrinzolamide as described below. If the VH was sufficiently large to prevent clear visualization of the retina under indirect ophthalmoscopy, or the VH was not absorbed after non-surgical treatment for a month, secondary surgery was performed. The reasons for recurrent hemorrhage were evaluated during the operation. Alternative appropriate management strategies were performed as necessary, including dissection or the cautery of neovascularization, the removal of any residual vascular membrane, the removal and cryocoagulation of fibrovascular ingrowth at the sclerotomy sites, silicone oil tamponade for subretinal fluid, retinal edema, breaks and detachment, and the supplementation of laser photocoagulation as required.[13,24,25] The same treatment was applied if VH occurred after the secondary surgery.
Management of complications
Elevated IOP was treated with an intravenous injection of 20% of mannitol (250 ml / day) and tropical timololbrinzolamide eye drops. Iris neovasularization was treated with trans-scleral cryocoagulation of the peripheral retina.[26] Neovascular glaucoma was treated with cryocoagulation of the peripheral retinal and the ciliary body.[27] The IOP after the treatment was controlled within the normal range, or decreased by more than 10 mmHg, compared to the pre-treatment levels.
Statistical analysis
Analyses were performed using SPSS 13.0 software (SPSS, Chicago, IL, USA). The Student’s t-test was used to compare the differences in the onset of PDVH. Categorical data were compared using the chi-squared test. Probability values less than 0.05 were considered statistically significant.
RESULTS
This study included 50 eyes of 43 PDR patients who had postoperative VH. Primary vitrectomy was performed with C3F8 tamponade in 23 eyes (46%), with silicone oil tamponade in 17 eyes (34%), gas tamponade in seven eyes (14%), and no tamponade in three eyes (6%). The visual acuity after PDVH, but before treatment, ranged from light perception to 0.2*. The first intraocular pressure (IOP) measurement after PDVH ranged from 7.3 to 54 mmHg. Secondary glaucoma occurred in eight eyes, with the IOP ranging from 21–54 mmHg. Epichoroidal bleeding occurred in one eye that had an IOP of 42 mmHg. Neovascular glaucoma occurred in three eyes with IOP values that ranged from 32.5–52.1 mmHg. The average (SD) follow-up duration was 6.8 (3.8) months (range, 2–12 months).
Postvitrectomy diabetic vitreous hemorrhage onset
Recurrent VH after primary vitrectomy occurred in 40 eyes (80%) with an average onset time of 62.5 ± 32.8 days (range, 3–170 days). The other 10 eyes, which were treated with silicone oil tamponade, did not have VH after primary vitrectomy, but VH occurred after the removal of the silicone oil. The average hemorrhage onset time in these 10 eyes was 27.4 ± 20.3 days (range, 1–60 days), which was significantly earlier than that in the 40 eyes in which VH occurred after primary vitrectomy (t test, P = 0.0032). The frequency of VH onset time was normally distributed [Figure 1]. The VH after primary vitrectomy occurred, most frequently, 60–70 days after surgery, and VH after silicone oil removal occurred, most frequently, 25–30 days postoperatively.
Figure 1.
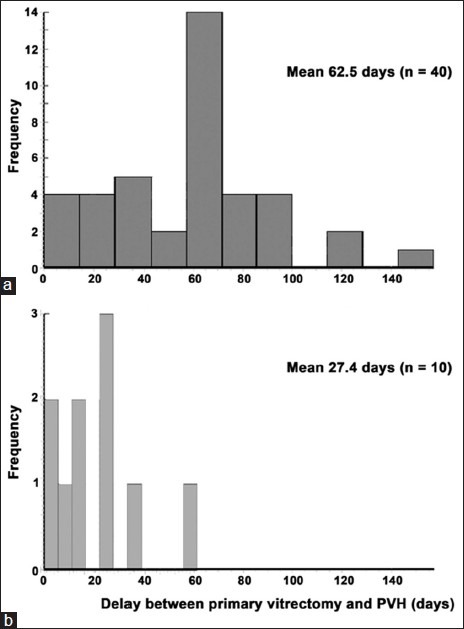
The frequency distribution of the onset time of vitreous hemorrhage after primary vitrectomy (a); and after silicone oil removal (b)
Reasons of postvitrectomy diabetic vitreous hemorrhage
The causes and treatment of PDVH are listed in Table 1. This study included 50 eyes with PDVH, but the pathology had more than one cause in several eyes, suggesting that there were 70 separate pathologies in total.
Table 1.
Data regarding the different causes, onset, and treatment options for postvitrectomy diabetic vitreous hemorrhage in this cohort of patients
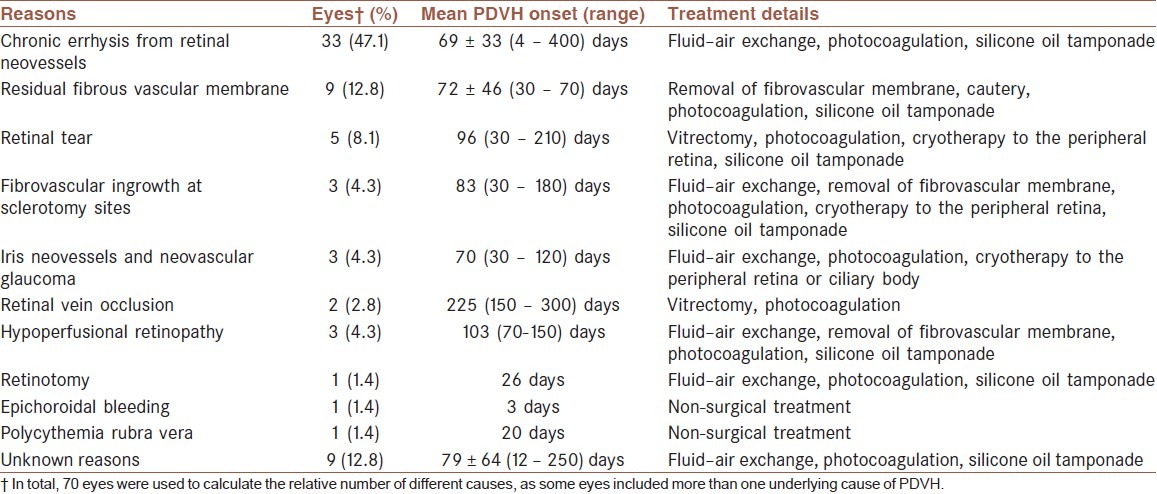
Treatment of postvitrectomy diabetic vitreous hemorrhage
Vitreous Hemorrhage occurred in 36 eyes (72%) after primary surgery, in 10 eyes (20%) after secondary surgery, and in four eyes (8%) after more than two surgeries. The treatment of PDVH included non-surgical treatment (n = 4 eyes) and surgical treatment (n = 46 eyes). The surgical strategies for the treatment of PDVH caused by all etiologies are listed in Table 1. Table 2 summarizes the surgical treatment of PDVH.
Table 2.
Summary of the surgical treatment of postvitrectomy diabetic vitreous hemorrhage

Visual acuity
Visual acuity scores are listed in the Table 3. Before treatment, visual acuity was < 0.01*01 in 49/50 eyes (98%), and between 0.01* and 0.04* in the remaining eye (2%). The visual acuity after treatment was 0.01* or better in over half of the eyes (28 56%), and was > 0.1* in 19 eyes (38%), which was significantly better than before treatment (P = 0.0005, Table 3). Visual acuity increased in 43 eyes (86%), and decreased in four eyes (8%) after the treatment.
Table 3.
Visual acuity before and after treatment in postvitrectomy diabetic vitreous hemorrhage patients

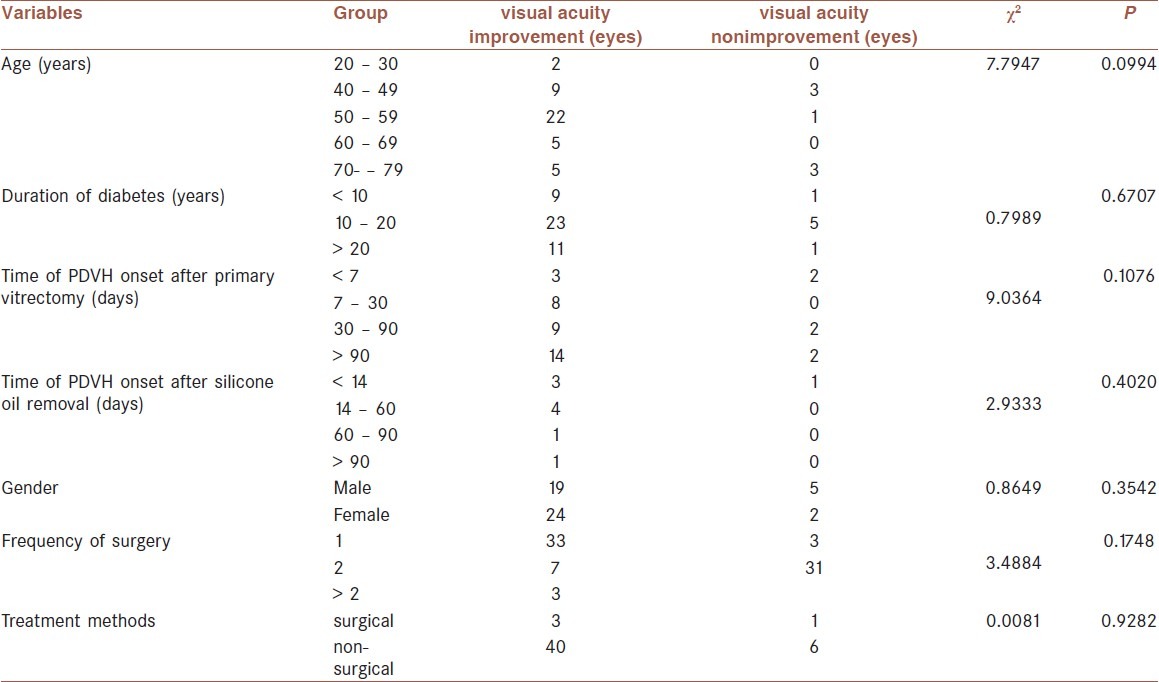
We further investigated whether the improvement of visual acuity after the treatment was related to age, sex, duration of diabetes, time of PDVH onset, frequency of surgery, or the different treatment methods [Table 4]. These results suggested that the improvement in visual acuity after treatment was not affected by any of the tested variables.
Table 4.
Chi-squared test analysis of the relationship among the improvement in the visual acuity after treatment and the tested variables
Complications
Elevated IOP after primary vitrectomy occurred in eight eyes, including five eyes with silicone oil tamponade and three eyes with C3F8 tamponade. The IOP ranged from 25 to 32 mmHg after vitrectomy, and decreased to 18–21 mmHg after the intravenous injection of mannitol and tropical timololbrinzolamide eye drops. Iris neovasularization occurred in three eyes, which was treated with trans-scleral retinal cryocoagulation. Neovascular glaucoma occurred in three eyes, and was treated with cryocoagulation of the peripheral retina and ciliary body. The IOP level was then controlled within the range of 18–22 mmHg.
Par plana vitrectomy has been used to treat the complications of PDR, with an improvement in visual acuity.[5] However, PDVH is a common cause of visual impairment and often requires further surgery after diabetic vitrectomy.[7,8] In this study, we reviewed patients with PDR, who underwent a vitrectomy at our hospital, to identify the reasons associated with the occurrence of PDVH. The incidence of PDVH was 16.2% in this study, which was similar to the incidence (13%) reported by Sima et al.,[10] but was lower than the incidence (37.5%) reported by Yeh et al.[11] These difference in PDVH incidence may have been largely due to different ages, disease duration of the diabetes, and severity of eye lesions of the patients in these studies.
In this study, we found that VH after primary vitrectomy occurred, most frequently, 60–70 days postoperatively. In contrast, VH after silicone oil removal occurred most frequently at 25–30 days. This finding agreed with the previous results in the literature, which recorded that most cases of PDVH occurred within three months of the vitrectomy.[5] In addition, we also determined that PDVH after primary vitrectomy had an average time of onset of 62.5 days, which was significantly later than that after silicone oil removal (27.4 days). The early onset of VH after silicone oil removal could be a consequence of the removal of the effect of silicone oil tamponade on the retinal vessels, thus promoting rapid re-bleeding throughout the retina.
There are multiple potential underlying etiologies for PDVH, including fibrovascular ingrowth at the sclerotomy sites, residual or recurrent neovascular membrane on the retina, insufficient retinal photocoagulation, residual and recurrent epiretinal proliferative membrane, retinal vein occlusion, postoperative low intraocular pressure, and ocular trauma.[11,14–16] We determined numerous pathologies [Table 1], which were in accordance with the literature. In addition, we determined a case of polycythemia rubra vera that affected one eye, and hypoperfusional retinopathy caused by ocular hypotension and ocular artery occlusion after the use of ciliary body photocoagulation and cryotherapy in three eyes, which had not been reported previously in the literature.
The most common reported cause of PDVH was fibrovascular ingrowth at the sclerotomy sites,[11,14,15] with an incidence that ranged from 86% of the affected eyes[15] to 28%.[14] In our study, we only found that 4.3% of the eyes with PDVH were caused by fibrovascular ingrowth at the sclerotomy sites. These differences could be largely due to the different populations of patients, varied surgical techniques, and different surgeons who performed the surgery. In addition, our patients underwent a complete vitrectomy that included the vitreous base and ciliary epithelia. These structures were reported to play a role in the formation of fibrovascular ingrowth.[28] The residual vitreous formed a good scaffold for fibrovascular proliferation,[29] and therefore, a complete vitrectomy was likely to be associated with the low incidence of PDVH caused by fibrovascular ingrowth at the sclerotomy sites, as observed in our study.
We have also demonstrated that PDVH is caused by chronic errhysis from retinal neovessels, and occurs in almost 50% of the eyes. The proliferation of new blood vessels on the retina is a common phenomenon in patients with PDR, and new vessels are structurally similar to those seen in the diabetic retina.[17] Therefore, the effective removal of retinal neovessels is critical for preventing VH. If the vascular membrane is not effectively removed during the operation, the residual vascular membrane may not cause intraoperative hemorrhage, due to elevated intraocular pressure. However, a recurrent VH can occur after the normal IOP is re-established, if the vascular membrane is not completely excised or cautery of the new vessels is not completely performed. Therefore, the residual vascular membrane on the retina may have been the major source of recurrent VH in our study. Laser photocoagulation has been reported to be an effective method to prevent VH in such cases.[30,31]
Retinal photocoagulation insufficiency has been reported to be one of the major causes of PDVH.[14] We performed intraoperative panretinal photocoagulation on all patients. Supplementary laser photocoagulation was performed on patients who did not receive laser photocoagulation during the surgery due to viscous subretinal fluid and retinal edema. Laser photocoagulation inhibited the production of angiogenic factors and reduced the proliferation of the fibrovascular membrane.[24,25] Yan et al.[14] reported that PDVH caused by insufficient retinal photocoagulation occurred in seven (22%) out of 32 eyes that did not receive supplementary retinal photocoagulation. However, in this study, no case of PDVH was found to be caused by insufficient retinal photocoagulation, which was most likely to be due to retinal photocoagulation during surgery, and supplement retinal photocoagulation was sufficient to prevent VH.
In conclusion, both surgical and non-surgical treatments were used for patients with PDVH, to improve their visual prognosis. Most of the patients showed an improvement in visual acuity on day 2 postoperatively, and they exhibited complete blood re-absorption three months after surgery. Visual acuity increased in 86% of the eyes after treatment, which suggested that the treatment was effective in reducing the hemorrhage volume. In addition, we also analyzed several factors that could potentially affect the improvement of visual acuity after treatment, including age, sex, duration of diabetes, time of PDVH onset, frequency of surgery, and treatment methods. None of these factors were associated with the improvement in visual acuity after treatment, which further suggested that the treatment choices in our study were effective for improving visual acuity. Therefore, we believe that the surgical strategies for the treatment of PDVH, including vitrectomy with fluid-air exchange, removal of fibrovascular membrane, cautery of the neovascularization, photocoagulation, cryotherapy to the peripheral retina or ciliary body, and silicone oil tamponade can result in a better visual outcome.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Shaw JE, Sicree RA, Zimmet PZ. Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes Res Clin Pract. 2010;87:4–14. doi: 10.1016/j.diabres.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 2.Lewis H, Abrams GW, Blumenkranz MS, Campo RV. Vitrectomy for diabetic macular traction and edema associated with posterior hyaloidal traction. Ophthalmology. 1992;99:753–9. doi: 10.1016/s0161-6420(92)31901-3. [DOI] [PubMed] [Google Scholar]
- 3.Fine SL, Patz A. Ten years after the Diabetic Retinopathy Study. Ophthalmology. 1987;94:739–40. doi: 10.1016/s0161-6420(87)33523-7. [DOI] [PubMed] [Google Scholar]
- 4.Machemer R, Buettner H, Norton EW, Parel JM. Vitrectomy: A pars plana approach. Trans Am Acad Ophthalmol Otolaryngol. 1971;75:813–20. [PubMed] [Google Scholar]
- 5.Newman DK. Surgical management of the late complications of proliferative diabetic retinopathy. Eye (Lond) 2010;24:441–9. doi: 10.1038/eye.2009.325. [DOI] [PubMed] [Google Scholar]
- 6.Yorston D, Wickham L, Benson S, Bunce C, Sheard R, Charteris D. Predictive clinical features and outcomes of vitrectomy for proliferative diabetic retinopathy. Br J Ophthalmol. 2008;92:365–8. doi: 10.1136/bjo.2007.124495. [DOI] [PubMed] [Google Scholar]
- 7.Yang CM. Surgical treatment for diabetic retinopathy: 5-year experience. J Formos Med Assoc. 1998;97:477–84. [PubMed] [Google Scholar]
- 8.Brown GC, Tasman WS, Benson WE, McNamara JA, Eagle RC., Jr Reoperation following diabetic vitrectomy. Arch Ophthalmol. 1992;110:506–10. doi: 10.1001/archopht.1992.01080160084037. [DOI] [PubMed] [Google Scholar]
- 9.Mason JO, 3rd, Colagross CT, Vail R. Diabetic vitrectomy: Risks, prognosis, future trends. Curr Opin Ophthalmol. 2006;17:281–5. doi: 10.1097/01.icu.0000193098.28798.18. [DOI] [PubMed] [Google Scholar]
- 10.Sima P, Zoran T. Long-term results of vitreous surgery for proliferative diabetic retinopathy. Doc Ophthalmol. 1994;87:223–32. doi: 10.1007/BF01203852. [DOI] [PubMed] [Google Scholar]
- 11.Yeh PT, Yang CM, Yang CH, Huang JS. Cryotherapy of the anterior retina and sclerotomy sites in diabetic vitrectomy to prevent recurrent vitreous hemorrhage: An ultrasound biomicroscopy study. Ophthalmology. 2005;112:2095–102. doi: 10.1016/j.ophtha.2005.07.010. [DOI] [PubMed] [Google Scholar]
- 12.di Lauro R, De Ruggiero P, di Lauro MT, Romano MR. Intravitreal bevacizumab for surgical treatment of severe proliferative diabetic retinopathy. Graefes Arch Clin Exp Ophthalmol. 2010;248:785–91. doi: 10.1007/s00417-010-1303-3. [DOI] [PubMed] [Google Scholar]
- 13.West JF, Gregor ZJ. Fibrovascular ingrowth and recurrent haemorrhage following diabetic vitrectomy. Br J Ophthalmol. 2000;84:822–5. doi: 10.1136/bjo.84.8.822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yan H, Cui J, Lu Y, Yu J, Chen S, Xu Y. Reasons for and management of postvitrectomy vitreous hemorrhage in proliferative diabetic retinopathy. Curr Eye Res. 2010;35:308–13. doi: 10.3109/02713680903572491. [DOI] [PubMed] [Google Scholar]
- 15.Hershberger VS, Augsburger JJ, Hutchins RK, Raymond LA, Krug S. Fibrovascular ingrowth at sclerotomy sites in vitrectomized diabetic eyes with recurrent vitreous hemorrhage: Ultrasound biomicroscopy findings. Ophthalmology. 2004;111:1215–21. doi: 10.1016/j.ophtha.2003.08.043. [DOI] [PubMed] [Google Scholar]
- 16.Zaninetti M, Petropoulos IK, Pournaras CJ. Proliferative diabetic retinopathy: Vitreo-retinal complications are often related to insufficient retinal photocoagulation. J Fr Ophtalmol. 2005;28:381–4. doi: 10.1016/s0181-5512(05)81068-x. [DOI] [PubMed] [Google Scholar]
- 17.Sawa H, Ikeda T, Matsumoto Y, Niiya A, Kinoshita S. Neovascularization from scleral wound as cause of vitreous rebleeding after vitrectomy for proliferative diabetic retinopathy. Jpn J Ophthalmol. 2000;44:154–60. doi: 10.1016/s0021-5155(99)00194-x. [DOI] [PubMed] [Google Scholar]
- 18.Zhao X, Zhong J, Mo Y, Chen X, Chen Y, Yang D. Association of biochemical hyperandrogenism with type 2 diabetes and obesity in Chinese women with polycystic ovary syndrome. Int J Gynaecol Obstet. 2010;108:148–51. doi: 10.1016/j.ijgo.2009.09.021. [DOI] [PubMed] [Google Scholar]
- 19.Asensio Sanchez VM, Perez Flandez FJ, Carlos Bejarano J, Fernandez Concellon L. Vitreous hemorrhage after vitrectomy in diabetic retinopathy and tissue plasminogen activator. Arch Soc Esp Oftalmol. 2002;77:7–12. [PubMed] [Google Scholar]
- 20.Wu WC, Chang SM, Chen JY, Chang CW. Management of postvitrectomy diabetic vitreous hemorrhage with tissue plasminogen activator (t-PA) and volume homeostatic fluid-fluid exchanger. J Ocul Pharmacol Ther. 2001;17:363–71. doi: 10.1089/108076801753162771. [DOI] [PubMed] [Google Scholar]
- 21.Helbig H, Sutter FK. Surgical treatment of diabetic retinopathy. Graefes Arch Clin Exp Ophthalmol. 2004;242:704–9. doi: 10.1007/s00417-004-0977-9. [DOI] [PubMed] [Google Scholar]
- 22.Helbig H. Surgery for diabetic retinopathy. Ophthalmologica. 2007;221:103–11. doi: 10.1159/000098255. [DOI] [PubMed] [Google Scholar]
- 23.Tan HS, Mura M, Bijl HM. Early vitrectomy for vitreous hemorrhage associated with retinal tears. Am J Ophthalmol. 2010;150:529–33. doi: 10.1016/j.ajo.2010.04.005. [DOI] [PubMed] [Google Scholar]
- 24.Tran AT, Bula DV, Kovacs KD, Savageau J, Arroyo JG. Apoptosis in diabetic fibrovascular membranes after panretinal photocoagulation. Ophthalmic Surg Lasers Imaging. 2010;41 doi: 10.3928/15428877-20100625-06. Online doi:103928/15428877-20100625-06. [DOI] [PubMed] [Google Scholar]
- 25.Simo R, Carrasco E, Garcia-Ramirez M, Hernandez C. Angiogenic and antiangiogenic factors in proliferative diabetic retinopathy. Curr Diabetes Rev. 2006;2:71–98. doi: 10.2174/157339906775473671. [DOI] [PubMed] [Google Scholar]
- 26.Patel J, Turaka K, Shields CL. Resolution of iris neovascularization following chemoreduction of retinoblastoma. J Pediatr Ophthalmol Strabismus. 2010;47:1–3. doi: 10.3928/01913913-20101018-06. [DOI] [PubMed] [Google Scholar]
- 27.A Janiæijeviæ-Petroviæ M, Sarenac T, Petroviæ M, Vuloviæ D, Janiæijeviæ K. Cyclocryotherapy in neovascular glaucoma treatment. Med Glas Ljek komore Zenicko-doboj kantona. 2012;9:106–9. [PubMed] [Google Scholar]
- 28.Koch FH, Kreiger AE, Spitznas M, Glasgow B, Foos RY, Yoshizumi MO. Pars plana incisions of four patients: Histopathology and electron microscopy. Br J Ophthalmol. 1995;79:486–93. doi: 10.1136/bjo.79.5.486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McLeod D. Entry site neovascularisation after diabetic vitrectomy. Br J Ophthalmol. 2000;84:810–1. doi: 10.1136/bjo.84.8.810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tinley CG, Gray RH. Routine, single session, indirect laser for proliferative diabetic retinopathy. Eye (Lond) 2009;23:1819–23. doi: 10.1038/eye.2008.394. [DOI] [PubMed] [Google Scholar]
- 31.Kollias AN, Ulbig MW. Diabetic retinopathy: Early diagnosis and effective treatment. Dtsch Arztebl Int. 2010;107:75–83. doi: 10.3238/arztebl.2010.0075. quiz 4. [DOI] [PMC free article] [PubMed] [Google Scholar]


