Abstract
Background:
Pharmacovigilance assesses the safety profile of drugs. Its main aim is the increase of spontaneous reporting of adverse drug reactions (ADRs). The Italian Drug Agency (AIFA; Agenzia Italiana del Farmaco) is financing several projects to the aim of increasing reporting, and in Calabria a Pharmacovigilance Information Centre has been created.
Materials and Methods:
We analyzed the AIFA database relatively to Calabria in the year 2011 and we have analyzed ADRs using descriptive statistics. We have also collected a questionnaire-based interview in order to describe the background knowledge in the field.
Results:
Regarding the number of AIFA reported ADRs from Calabria, a 38% increase (138 vs. 100) in comparison to 2010 was evidenced. Hospital Doctors represent the main source of signaling (71.7 %). Ketoprofene and the combination amoxicillin/clavulanic acid represent the most frequently reported drugs causing ADRs. Our questionnaires indicated that despite the health professionals have met at least once an ADR only a small percentage of them was reported to the authorities (37%). There is a very good knowledge of the ADR concept and reporting system (90% of interviewed distinguish an ADR and knows how to report it), and there is a strong interest in participating to training courses in the field (95% are interested).
Conclusions:
Despite Calabria has had a positive increase in the number of reported ADRs, the total number is very low and the pharmacovigilance culture is far from being achieved in this region.
Keywords: Adverse drug reaction reporting systems, adverse effects, calabria, drug toxicity, italy, pharmacovigilance
INTRODUCTION
Pharmacovigilance is defined by the World Health Organization (WHO) as “The science and activities relating to the detection of assessment, understanding and prevention of adverse effects or any other drug problem” and guarantees physicians to have sufficient information in relation to drugs also increasing knowledge about drug safety.[1] The main aim of pharmacovigilance is the increase of spontaneous reporting of adverse drug reactions (ADRs). The study of adverse reactions associated with a drug is a continuous process, starting from the initial reports and represents the door to studies of drug-epidemiology and quantitative risk assessment,[2] this is very relevant for new drugs entering the market of which it is not possible to know all the adverse effects given the limitations of clinical trials.[3,4] Actually, clinical studies do not allow us to have enough reliable data because the sample sizes in most clinical trials are often too low for detection of rare ADRs and are not able to detect ADRs with long latency periods, they also often exclude fragile groups such as children and elderly; furthermore, in most cases the incidence and prevalence of an ADR is statistically different when considering the entire population in comparison to the clinical trial group.[3,4] Therefore, continuous monitoring of alerts is the only tool that allows timely detection of signals in order to ensure a favorable risk/benefit for the population and to ensure that all health care has a support for a correct and proper use of medications. Literature data show that factors associated with under-reporting include: i. Lack of knowledge (it is believed that only serious ADRs should be reported); ii. Absence of interest or time; iii. Indifference to the problem; iv. Uncertainty about the causal link between a drug and an ADR and; v. The mistaken belief that only safe drugs are marketed.[5]
In this context, the Italian Drug Agency (AIFA = Agenzia Italianodel Farmaco) has been promoting several projects in Italy in order to increase the “Pharmacovigilance Culture”. The Calabria region of south Italy by the end of 2010, at the “Mater Domini” University Hospital of Catanzaro, has started a project entitled “Regional Network of drug information: information, training and pharmacovigilance”, through an AIFA funding and an agreement with the regional administration, to increase the scientific skills of health workers and inform the community about the topic of pharmacovigilance.
This centre has implemented several strategies to increase the quality and quantity of reports of suspected adverse reactions to drugs and vaccines, working together with several training health workers, doctors and pharmacists. Financing activities and coordination of the AIFA have had a positive effect on the whole Italian system of spontaneous reporting. In all the best systems, regional pharmacovigilance centres have a key role; the funds allocated to projects for the AIFA pharmacovigilance have contributed to the improvement of spontaneous reporting. The situation of spontaneous reports of adverse reactions in Italy in 2010 showed a 39% increment in comparison to 2009. This is in line with the average annual increase of the last five years, which is around 30%.[6] The spontaneous reporting rate in our region for 2011 in comparison to 2010 amounted to an increase of 38%, whereas in 2010 a 25% increase only was recorded. In the present work, we report an analysis of the ADRs recorded in 2011 considering various aspects with the hope of stimulating interest in the pharmacovigilance field and suggest possible corrections to improve the system in Calabria. Furthermore, we report the results of a questionnaire-based interview relatively to the background knowledge in the field of pharmacovigilance.
MATERIALS AND METHODS
All reports of ADRs were obtained from the AIFA database relatively to the Calabria region of Italy; these collected in the year 2011 were studied and analyzed from a statistical descriptive point of view. A total of 138 ADRs were present in the database in the period 1st January 2011-31st December 2011.
Two questionnaire based interview forms were prepared (see supplementary file), one specific for physicians and the second for pharmacists, according to previously published results.[7,8] The physician questionnaire was based on 3 sections: I.) General information about the physician such as age, year of graduation, specialty; II.) 6 items relatively to the relationship between physician and ADR, and III.) 2 items on the difference between adverse event and side effect. The pharmacists questionnaire was also based on 3 sections with the first and last similar to the one present in the other questionnaire and section II with 15 items relatively to the role of the pharmacist in pharmacovigilance and their knowledge of the field. Even if several physicians and pharmacists were contacted by e-mail or personally (about 1000) only 270 questionnaires have been completed.
RESULTS
ADRs analysis
The reporting forms of suspected adverse reactions included in the National Network of Pharmacovigilance (RNF) coveringthe region Calabria between January 1and December 31, 2011 were 138, an increase of 58.62% (88) comparedto 2009 and 38% (100) compared to 2010.
The general trend of reports by age group shows a higher frequency in the range 41–65 years, followed by range “over 65”, which is justified by the frequent polytherapy [Figure 1]. In particular, in the age group 41–65 most of there actions are categorized as not serious for the 48.71% while in 43.58% of the cases the reaction was severe [Figure 1].
Figure 1.

Frequency of ADRs in different age range. Y = Number of reported ADR in 2011; X = age range groups
Considering the gender, no differences were observed in the number of ADRs. However, in women, the age group most affected by serious ADRs appears to be over 65, while in males it was 19–40. In general, considering the totality of ADRs, the higher occurence in both sexes was in the age range 41–65.
The largest source of reporting (71.7%) in 2011, was hospital doctors. The main other sources (General Practitioners (GPs), vaccine medical services, Pediatricians and Community Pediatricians, specialists) have made a contribution of 25% and among them the major markers were medical specialists and physicians (12.31% and 11.6% of the total, respectively). As for the remaining sources, the number of reports produced by pharmacists, nurses and citizen (0.7%) is unfortunately still very low. Moreover, GPs and Hospital Doctors reported the most serious ADRs.
Of the 138 reports ofADRs in 2011, 55% (corresponding to 76 reports) involved serious events, 35.5% (49) non-serious events and 9.42% (13) was not defined. Furthermore, 38% had complete resolution, whereas in 2 cases the outcome was fatal.
Considering the drugs’ class, the largest number of ADRs derives from antimicrobial (24.6%), followed by anti-inflammatory drugs (19%) [Figure 2]. However, the drug with the highest number of signals was ketoprofene with 15 alerts followed by preparations combining amoxicillin/clavulanic acid with 11 ADRs. Vaccines’ related ADRs had a total number of 10 (0.7% only of total reports).
Figure 2.
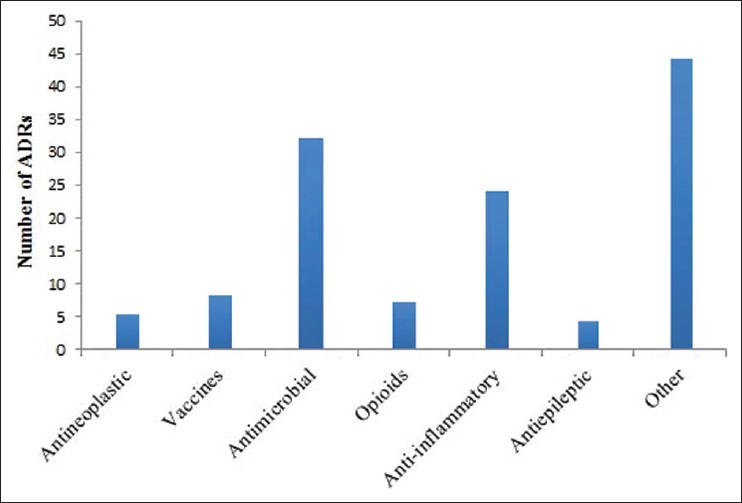
Number of ADRs in relation to the classes of drugs. Other = Antihypertensive (4); Radiocontrast agents (3); Antiparkinsonian (3); Antiulcer (3); Antipsychotics (2); Antiaggregant (2); Antimycotics (2); Antidiabetics (2). Others with only 1 report. Number represent the number of reports
Questionnaires analysis
We have received 270 questionnaires out of 1048 sent. 198 were from pharmacists and 72 from doctors. Only the 17% of them has never followed a course on pharmacovigilance [Figure 3] and most of them (95%) were very interested in following a new course or seminars. Their major problems (84%) for reporting an ADR were difficulties or uncertainty about the identification and correlation of an ADR with a drug, even if, they identified as major criteria for the recognition and association of ADRs to a given drug both the time and the consultation of databases and scientific literature [Figure 4].
Figure 3.

Frequency pharmacovigilance’s course. Ranges in the legend represent the number of courses followed, relative percentages are reported in the graph
Figure 4.

Criteria used for the recognition of ADRs
Despite the fact that the 90% of interviewed knows how to report an ADR and 79% declare that at least once in their profession has come across an ADR, only the 37% of them has made a report whereas, the 50% has never reported an ADR.
DISCUSSION
Under-reporting of ADRs is a central issue also in Western countries where the pharmacovigilance system is well organized.[9] Indeed, as confirmed by the results obtained from our questionnaires, although most of health system professionist know the procedures for reporting and have come across at least an ADR, very few of them have ever communicated a full official report. The ability to prevent ADRs could be facilitated by the creation of standardized approaches and active reporting of suspected ADRs by all healthcare.[10] The main reasons for under reporting in our population seem to be the certain correlation between the selected drug and the ADR itself, above all in patients under politherapy; also the time needed to compile the form seems to play a role in the lack of communication.
About 2 million people live in Calabria, therefore, as indicated by OMS,[11] the number of ADRs/year should be about 600. In the year 2011, they were only 138 and therefore, well below the international standard. Most of reports were received from hospital doctors; also medical specialists and general practitioners have made a modest contribution and unfortunately, the number of reports made by pharmacists and other health personnel is still very low. Antibiotics followed by anti-inflammatory drugs represent the main classes of drugs. These data reflect those reported from other Italian regions, so it seems that the common problem is the lack of cooperation of various professionals such as general practitioners, pharmacists, dentists and nurses. Undoubtedly the main reason for under-reporting are the time needed to complete the form and the certainty in the correlation between the observed phenomenon and the drug treatment. In Italy in 2011, the signals were around 21.473 an increase of 32% compared to 2009 but only 6% in comparison to 2010. The increase has been steady over the past five years, with an average annual increase of 30%. Italy is now reporting 356/million ADRs, therefore it is in line with standards set by WHO. However, the number of reports is not homogenous in the country and our region is one of the last in this special ladder. The observed pattern of spontaneous reporting in our region for 2011 confirms a growth rate with an increase in the number of signals equal to 38% thus allowing the achievement of most targets set by the World Health Organization for the creation of a system of pharmacovigilance quality capable of generating timely warning signals. However, the actual number remains very low. Lombardia is the main for ADRs reports (about 850 reports in 2010 and 934 in 2011) and together with Toscana (788 in 2011), Basilicata (388) and Molise (341) are the only regions reaching the OMS gold standard. Important increases were observed in Lazio (+73%), Toscana (+63%) and Campania (+57%) where are active pharmacovigilance centres while Veneto had a significant decrease (-53%) and this has coincided with the end of the project financing the regional centre. This data support a crucial role for the funded projects by AIFA and indicate that this system can be implemented following appropriate politics of intervention.[11]
The ADRs have covered a substantial proportion of serious events, with a small percentage of events with not defined gravity. Still very low is the numberof physicians making at least one report of suspected ADRs. Nevertheless, the increase of reports of drugs should be interpreted as a positive signal. While the data of recent years high light the effectiveness of national and regional initiatives to encourage voluntary reporting, on the hand, it emphasizes the need for greater efforts to ensure that there is a widespread diffusion of the culture of pharmacovigilance essential tools for continuous monitoring of drugs’ safety. The questionnaires-based interviews also indicated a strong desire from health professionals to participate to new training courses in the field of pharmaco vigilance. To this purpose, the Regional Centre, in order to educate as many health professionals about the importance of pharmaco vigilance for themselves and their patients continue the initiative of organizing several scientific events in the future further continuing on its support.
In conclusion, despite Italy might have reached the standard set by OMS, the 6% increase in the last year raises some concerns. Furthermore, Calabria together with most of the other Italian regions, remains much below this standard. The positive trend is encouraging and Regional Centre work is finally satisfactory, many courses have been organized and this has lead to about 180 reports in 2012 up to end of June, which is already more than the previous year. More generally, information and support seems to be the main need in the field; to this aim, we feel that in the next forthcoming years much work by information centres will be needed in many Italian areas until the pharmacovigilance culture will not take root in the health system.
ACKNOWLEDGMENTS
Every physician and pharmacist that contributed to the questionnaire is kindly acknowledged. We thank the pharmaceutical society section of Catanzaro Ordine dei Farmacisti della Provincia di Catanzaro for its support.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Jeetu G, Anusha G. Pharmacovigilance: A worldwide master key for drug safety monitoring. J Young Pharm. 2010;2:315–20. doi: 10.4103/0975-1483.66802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schjøtt J, Reppe LA, Roland PD, Westergren T. A question-answer pair (QAP) database integrated with websites to answer complex questions submitted to the Regional Medicines Information and Pharmacovigilance Centres in Norway (RELIS): A descriptive study. BMJ Open. 2012;2:e000642. doi: 10.1136/bmjopen-2011-000642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Benkirane RR, Abouqal R, Haimeur CC, S EchCherif El Kettani SS, Azzouzi AA, MdaghriAlaoui AA, et al. Incidence of adverse drug events and medication errors in intensive care units: A prospective multicenter study. J Patient Saf. 2009;5:16–22. doi: 10.1097/PTS.0b013e3181990d51. [DOI] [PubMed] [Google Scholar]
- 4.Napoleone E. Children and ADRs (Adverse Drug Reactions) Ital J Pediatr. 2010;36:4. doi: 10.1186/1824-7288-36-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lopez-Gonzalez E, Herdeiro MT, Figueiras A. Determinants of under-reporting of adverse drug reactions: A systematic review. Drug Saf. 2009;32:19–31. doi: 10.2165/00002018-200932010-00002. [DOI] [PubMed] [Google Scholar]
- 6.Conforti A, Opri S, D’Incau P, Sottosanti L, Moretti U, Ferrazin F, et al. Adverse drug reaction reporting by nurses: Analysis of Italian pharmacovigilance database. Pharmacoepidemiol Drug Saf. 2012;21:597–602. doi: 10.1002/pds.3225. [DOI] [PubMed] [Google Scholar]
- 7.Desai CK, Iyer G, Panchal J, Shah S, Dikshit RK. An evaluation of knowledge, attitude, and practice of adverse drug reaction reporting among prescribers at a tertiary care hospital. Perspect Clin Res. 2011;2:129–36. doi: 10.4103/2229-3485.86883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Su C, Ji H, Su Y. Hospital pharmacists’ knowledge and opinions regarding adverse drug reaction reporting in Northern China. Pharmacoepidemiol Drug Saf. 2010;19:217–22. doi: 10.1002/pds.1792. [DOI] [PubMed] [Google Scholar]
- 9.Kimura T, Matsushita Y, Yang YH, Choi NK, Park BJ. Pharmacovigilance systems and databases in Korea, Japan, and Taiwan. Pharmacoepidemiol Drug Saf. 2011;20:1237–45. doi: 10.1002/pds.2244. [DOI] [PubMed] [Google Scholar]
- 10.Sriram S, Ghasemi A, Ramasamy R, Devi M, Balasubramanian R, Ravi TK, et al. Prevalence of adverse drug reactions at a private tertiary care hospital in south India. J Res Med Sci. 2011;16:16–25. [PMC free article] [PubMed] [Google Scholar]
- 11. [Last accessed on 20 June 2012]. Available from: http://www.agenziafarmaco.gov.it/


