Abstract
Background:
Sex-related differences in the severity of multiple sclerosis (MS) could be influenced by the sex hormones.
Materials and Methods:
This cohort (historical) study evaluated the sex hormone levels during menstrual cycle and their correlation with disease severity in MS.
Result:
Patients with MS had significantly lower testosterone, dehydroepiandrosterone sulfate and prolactin levels than controls in the follicular and luteal phase but lower estradiol levels only in the follicular phase. A positive correlation coefficient between follicle stimulating hormone and disease severity, and a reverse correlation with estradiol were found.
Conclusion:
The hormone-related modulation of disease severity supports the suggestion that sex hormones play a role in MS disease.
Keywords: Disease severity, estradiol, hormones, multiple sclerosis, progesterone, testosterone, women
INTRODUCTION
Multiple sclerosis (MS) is a progressive autoimmune disease accompanied by a degenerative damage to the myelin sheath of the central nervous system (CNS).[1]
Both prevalence and relapse of disease has noticeably raised in comparison to year 2007. This increase is even more threatening in Isfahan City in the whole Asia region;[2] it is reported to be two to three times more popular in women with late adolescence compared to men. The reason for this disparity among the two sexes is not clear. This could be due to sexual difference; so recently, hormone therapy for MS has been greatly evaluated.[3] A lot of studies have proven the correlation between sex hormones and the severity of disease. For example, MS exacerbation immediately before the menstruation cycle, when estrogen and progesterone levels are very low can be seen;[4] also, the relapse of disease symptoms lowers during the last three months of pregnancy and again increases right after delivery. These changes are said to be due to immunological and hormonal changes.[5] Although no definite treatment is known for MS, but modulators of immune system including sex hormones could be promising.[6] Considering all that, provoking the capacity of myelin to rebuild or regenerate itself is a goal and it seems that the role of sex hormones in disease progression is inevitable.[7] The aim of this study was the evaluation of sex hormone levels in reproductive age women with MS and their relationship with disease severity.
MATERIALS AND METHODS
This cohort (historical) study has been attempted on a group of patients who have been to Council of MS patients of Najaf-Abad in Isfahan City, Iran. The diagnosis was carried out by two experienced neurologists. Past medical history, clinical examination, magnetic resonance imaging (MRI), lumbar puncture (LP) and evoked potentials study were used (Poser criteria for definite MS).[8] The disability status was evaluated by using the Kurtzke Expanded Disability Status Scale (EDSS).[9] We included 16 relapsing remitting MS (RRMS) patients (16 patients for follicular phase and 16 patients for luteal phase) with an EDSS score of 2(1–5.5). Patients had no history of diseasemodifying treatments and during the past two months had not received any steroid treatment. All subjects were more than 15 and less than 35 years of age, had normal menstrual cycles and did not have a history of taking contraceptive pills or hormone replacement therapy. Thirty healthy age-matched subjects served as controls for every phase.
A blood sample in fasting condition was taken from all subjects, and the range of sex hormones including follicle stimulating hormone (FSH; mU/ml), luteinizing hormone (LH; mU/ml), progesterone (ng/ml), testosterone (ng/ ml), 17-β estradiol (pg/ml), prolactin (ng/ml), cortisol (ng/ ml), dehydroepiandrosterone sulfate (DHEA-S; μg/ dl), and dehydroepiandrosterone (DHEA; μg/dl) were measured during the follicular (day 3 to day 9) and luteal phases (day 21 to day 28)[10] of their menstrual cycle by radioimmunoassay.
Data were stated as means ± standard deviations, using an independent t-test for the comparison of group’s means and Spearman’s correlation coefficient for evaluating the relation between disease severity and sex hormone concentrations. A P-value less than 0.05 was considered as statistically significant. All analyses was done using SPSS (v.16).
RESULTS
The mean (SD) age of RRMS patients was 27.2 (4.6) years and that of healthy controls was 26.2 (4.1) years. The mean (SD) sex serum hormone levels in MS patients and controls are shown in Table 1.
Table 1.
Serum sex hormone concentrations in patients with multiple sclerosis and controls
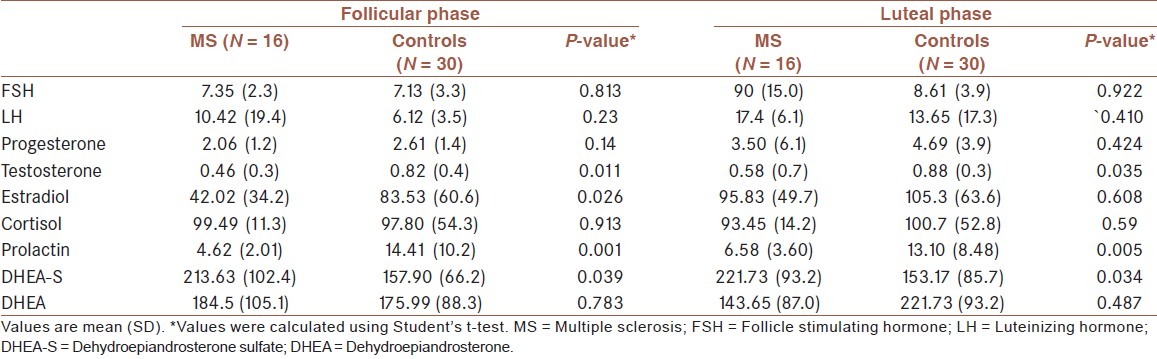
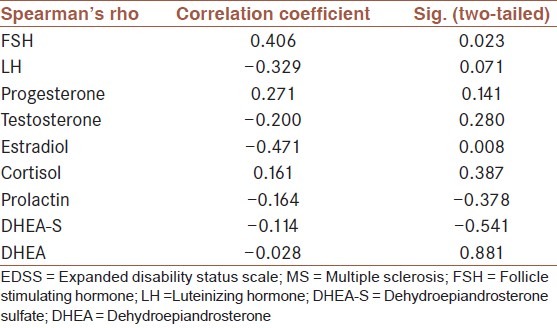
In female patients with RRMS during reproductive ages, levels of sex hormones including testosterone (P = 0.011, P = 0.035), DHEA-S (P = 0.039, P = 0.034), and prolactin (P = 0.001, P = 0.005) in the follicular as well as luteal phase, respectively, were significantly lower than those in the normal group but the estrogen level only in the follicular phase was significantly lower than that in healthy subjects (P = 0.026). Also, no significant difference was noted between other sex hormones in patients and controls. A positive correlation coefficient between FSH and EDSS scores (r = 0.406; P = 0.023) and a reverse correlation with estradiol (r = −0.471; P = 0.008) were found. No significant correlations were found between other sex hormone levels and EDSS score [Table 2].
Table 2.
Relation between sex hormone levels and EDSS score in MS patients
DISCUSSION
The cooperation between immune, endocrine, neural and genetic systems of the body could be a possible reason for the influence of sex hormones in MS.[11]
Our results showed that testosterone, DHEA-S, and prolactin levels in MS patients were lower than those in normal subjects both in the follicular and luteal phase, but the level of estradiol only decreased in the follicular phase. In one study, in accordance with our results, they showed that testosterone levels in MS patients in both the follicular and luteal phase were lower than those in the normal group.[10] They concluded that testosterone levels in MS and other autoimmune diseases, especially during the active phase of the disease decreased, and this reduction was consistent with severity of brain lesions; in addition, MRI results confirmed these data.[10] Testosterone also plays an important role in repairing brain lesions and protective effects of this hormone have been shown in patients and animal models of MS.[12] Wei and Lightman reported an overactivation of the hypothalamic-pituitary-adrenal axis occurred in parallel with inflammation,[13] resulting in a decreased production of testosterone in MS patients.[14] Lower testosterone levels could also describe lower levels of estradiol during the luteal phase of the menstrual phase because estradiol is produced from testosterone in the granulose cells.[10] In addition, testosterone and its metabolites such as DHEA directly or through conversion to estrogen by the aromatase enzyme could affect the estrogen receptor.[15] The decreased level of estrogen has been seen in MS patients.[16] Tomasini showed that levels of estrogen only in the luteal phase were lower than those in controls. This discrepancy in our study could be explained with fewer patients in the control group compared to patients in the Tomasini study.[10]
DHEA can also express anti-inflammatory effects. In animal studies, DHEA regulates the immune response and influences the production of interleukin-2.[17] Roberts and Fauble showed that MS patients have a low level of this hormone and the administration of this hormone could help in treatment management.[18]
In our studies, a positive correlation coefficient between FSH and EDSS score and a reverse correlation with estradiol were found, but in one research, women having the lowest testosterone concentrations had more brain lesions detected by MRI.[10] In some studies any correlation between abnormal hormone balance and disease severity was not observed.[16] In an animal model of MS, in female rats, no correlation was observed between the estradiol level and clinical symptoms.[19] We assumed that, according to the chronically activated hypothalamic-pituitary-adrenal axis in MS patients,[10] a peripheral resistance to gonadotropins combined with an abnormal central regulation induces the increased pituitary secretion of FSH.[16]
However, there is evidence that testosterone and estradiol downregulate reactive gliosis and astrocyte proliferation,[20] the major problems to axonal regeneration in the mammalian CNS.[21]
In accordance with our study, Tomasini showed that the level of the progesterone hormone in patients and controls was similar.[10] In a small series of studies, a significant relationship between the increase in the lesion size and low levels of progesterone was found, together with high levels of estradiol, suggesting that progesterone has an important role in treatment.[22,23] It is established that progesterone also helps in myelin formation by oligodendrocytes in the CNS both in vivo[24,25] and in vitro.[26]
In one study, 30% of MS patients had mild to moderate high prolactin levels which were suspected to be associated with hypothalamic lesions.[27] This hormone is produced by activated lymphocytes, as an immunoregulatory co-factor participating in the immunopathogenic mechanism of MS.[28]
Heidbrink et al. determined cortisol and DEHA levels in serum and cerebrospinal fluid samples from 34 MS patients;[29] the cortisol level was normal in MS patients which was in agreement with our study but this level was high in the cerebrospinal fluid during acute relapse of disease due to the increased activity of the adrenal-pituitary-hypothalamic pathway.[29,30]
Low levels of the mentioned hormones in MS patients could be explained by a probable mechanism for disease severity and therefore hormone therapy could be considered as an effective method for MS treatment. There is possibly a limitation of our study, it was better to calculate the progression index (PI) of patients and then obtain correlation between PI and sex hormone levels, but the duration of disease was not available for all patients.
It is concluded that the hormone-related modulation of disease severity supports the suggestion that sex hormones play a role in the inflammation and repair mechanisms.
ACKNOWLEDGMENTS
This study was funded by a grant no. 51501881216012 from the Deputy for Research, Islamic Azad University, Najaf Abad branch, Isfahan, Iran.
Footnotes
Source of Support: This study was funded by grant no. 51501881216012 from the Deputy for Research, Islamic Azad University, Najaf Abad branch, Isfahan, Iran
Conflict of Interest: None declared.
REFERENCES
- 1.Ringold S, Lynm C, Glass RM. JAMA patient page. Multiple sclerosis. JAMA. 2005;293:514. doi: 10.1001/jama.293.4.514. [DOI] [PubMed] [Google Scholar]
- 2.Etemadifar M, Maghzi AH. Sharp increase in the incidence and prevalence of multiple sclerosis in Isfahan, Iran. Mult Scler. 2011;17:1022–7. doi: 10.1177/1352458511401460. [DOI] [PubMed] [Google Scholar]
- 3.Taylor BV, Lucas RM, Dear K, Kilpatrick TJ, Pender MP, van der Mei IA, et al. Latitudinal variation in incidence and type of first central nervous system demyelinating events. Mult Scler. 2010;16:398–405. doi: 10.1177/1352458509359724. [DOI] [PubMed] [Google Scholar]
- 4.Smith R, Studd JW. A pilot study of the effect upon multiple sclerosis of the menopause, hormone replacement therapy and the menstrual cycle. J R Soc Med. 1992;85:612–3. doi: 10.1177/014107689208501008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Argyriou AA, Makris N. Multiple sclerosis and reproductive risks in women. Reprod Sci. 2008;15:755–64. doi: 10.1177/1933719108324138. [DOI] [PubMed] [Google Scholar]
- 6.El-Etr M, Ghoumari A, Sitruk-Ware R, Schumacher M. Hormonal influences in multiple sclerosis: New therapeutic benefits for steroids. Maturitas. 2011;68:47–51. doi: 10.1016/j.maturitas.2010.09.014. [DOI] [PubMed] [Google Scholar]
- 7.Gold SM, Voskuhl RR. Estrogen treatment in multiple sclerosis. J Neurol Sci. 2009;286:99–103. doi: 10.1016/j.jns.2009.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Poser CM, Paty DW, Scheinberg L, McDonald WI, Davis FA, Ebers GC, et al. New diagnostic criteria for multiple sclerosis: Guidelines for research protocols. Ann Neurol. 1983;13:227–31. doi: 10.1002/ana.410130302. [DOI] [PubMed] [Google Scholar]
- 9.Kurtzke JF. Rating neurologic impairment in multiple sclerosis: An expanded disability status scale (EDSS) Neurology. 1983;33:1444–52. doi: 10.1212/wnl.33.11.1444. [DOI] [PubMed] [Google Scholar]
- 10.Tomassini V, Onesti E, Mainero C, Giugni E, Paolillo A, Salvetti M, et al. Sex hormones modulate brain damage in multiple sclerosis: MRI evidence. J Neurol Neurosurg Psychiatry. 2005;76:272–5. doi: 10.1136/jnnp.2003.033324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Duquette P, Girard M. Hormonal factors in susceptibility to multiple sclerosis. Curr Opin Neurol Neurosurg. 1993;6:195–201. [PubMed] [Google Scholar]
- 12.Dalal M, Kim S, Voskuhl RR. Testosterone therapy ameliorates experimental autoimmune encephalomyelitis and induces a T helper 2 bias in the autoantigen-specific T lymphocyte response. J Immunol. 1997;159:3–6. [PubMed] [Google Scholar]
- 13.Wei T, Lightman SL. The neuroendocrine axis in patients with multiple sclerosis. Brain. 1997;120(Pt 6):1067–76. doi: 10.1093/brain/120.6.1067. [DOI] [PubMed] [Google Scholar]
- 14.Foster SC, Daniels C, Bourdette DN, Bebo BF., Jr Dysregulation of the hypothalamic-pituitary-gonadal axis in experimental autoimmune encephalomyelitis and multiple sclerosis. J Neuroimmunol. 2003;140:78–87. doi: 10.1016/s0165-5728(03)00177-2. [DOI] [PubMed] [Google Scholar]
- 15.Cherrier MM, Matsumoto AM, Amory JK, Ahmed S, Bremner W, Peskind ER, et al. The role of aromatization in testosterone supplementation: Effects on cognition in older men. Neurology. 2005;64:290–6. doi: 10.1212/01.WNL.0000149639.25136.CA. [DOI] [PubMed] [Google Scholar]
- 16.Grinsted L, Heltberg A, Hagen C, Djursing H. Serum sex hormone and gonadotropin concentrations in premenopausal women with multiple sclerosis. J Intern Med. 1989;226:241–4. doi: 10.1111/j.1365-2796.1989.tb01387.x. [DOI] [PubMed] [Google Scholar]
- 17.Kim SB, Hill M, Kwak YT, Hampl R, Jo DH, Morfin R. Neurosteroids: Cerebrospinal fluid levels for Alzheimer’s disease and vascular dementia diagnostics. J Clin Endocrinol Metab. 2003;88:5199–206. doi: 10.1210/jc.2003-030646. [DOI] [PubMed] [Google Scholar]
- 18.Roberts E, Fauble TJ. In: Oral DHEA in multiple sclerosis: results of a phase one, open study, in The Biologic Role of DHEA. Kalimi M, Regelson W, editors. New York: Walter De Gruyter; 1990. pp. 81–93. [Google Scholar]
- 19.Nicot A. Gender and sex hormones in multiple sclerosis pathology and therapy. Front Biosci. 2009;14:4477–515. doi: 10.2741/3543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Garcia-Estrada J, Del Rio JA, Luquin S, Soriano E, Garcia-Segura LM. Gonadal hormones down-regulate reactive gliosis and astrocyte proliferation after a penetrating brain injury. Brain Res. 1993;628:271–8. doi: 10.1016/0006-8993(93)90964-o. [DOI] [PubMed] [Google Scholar]
- 21.Bovolenta P, Wandosell F, Nieto-Sampedro M. CNS glial scar tissue: A source of molecules which inhibit central neurite outgrowth. Prog Brain Res. 1992;94:367–79. doi: 10.1016/s0079-6123(08)61765-3. [DOI] [PubMed] [Google Scholar]
- 22.Pozzilli C, Falaschi P, Mainero C, Martocchia A, D’Urso R, Proietti A, et al. MRI in multiple sclerosis during the menstrual cycle: Relationship with sex hormone patterns. Neurology. 1999;53:622–4. doi: 10.1212/wnl.53.3.622. [DOI] [PubMed] [Google Scholar]
- 23.Bansil S, Lee HJ, Jindal S, Holtz CR, Cook SD. Correlation between sex hormones and magnetic resonance imaging lesions in multiple sclerosis. Acta Neurol Scand. 1999;99:91–4. doi: 10.1111/j.1600-0404.1999.tb00663.x. [DOI] [PubMed] [Google Scholar]
- 24.Riccio P, Haas H, Liuzzi GM, Rossano R. New diagnostic and therapeutic options for the treatment of multiple sclerosis. Clinical Applications of Immunomics. 2009;2:205–26. [Google Scholar]
- 25.Bodhankar S, Wang C, Vandenbark AA, Offner H. Estrogen-induced protection against experimental autoimmune encephalomyelitis is abrogated in the absence of B cells. Eur J Immunol. 2011;41:1165–75. doi: 10.1002/eji.201040992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ghoumari AM, Ibanez C, El-Etr M, Leclerc P, Eychenne B, O’Malley BW, et al. Progesterone and its metabolites increase myelin basic protein expression in organotypic slice cultures of rat cerebellum. J Neurochem. 2003;86:848–59. doi: 10.1046/j.1471-4159.2003.01881.x. [DOI] [PubMed] [Google Scholar]
- 27.Shelly S, Boaz M, Orbach H. Prolactin and autoimmunity. Autoimmun Rev. 2012;11:A465–70. doi: 10.1016/j.autrev.2011.11.009. [DOI] [PubMed] [Google Scholar]
- 28.Draca S, Levic Z. The possible role of prolactin in the immunopathogenesis of multiple sclerosis. Med Hypotheses. 1996;47:89–92. doi: 10.1016/s0306-9877(96)90444-2. [DOI] [PubMed] [Google Scholar]
- 29.Heidbrink C, Hausler SF, Buttmann M, Ossadnik M, Strik HM, Keller A, et al. Reduced cortisol levels in cerebrospinal fluid and differential distribution of 11beta-hydroxysteroid dehydrogenases in multiple sclerosis: Implications for lesion pathogenesis. Brain Behav Immun. 2010;24:975–84. doi: 10.1016/j.bbi.2010.04.003. [DOI] [PubMed] [Google Scholar]
- 30.Huitinga I, Erkut ZA, van Beurden D, Swaab DF. The hypothalamo-pituitary-adrenal axis in multiple sclerosis. Ann N Y Acad Sci. 2003;992:118–28. doi: 10.1111/j.1749-6632.2003.tb03143.x. [DOI] [PubMed] [Google Scholar]


