Abstract
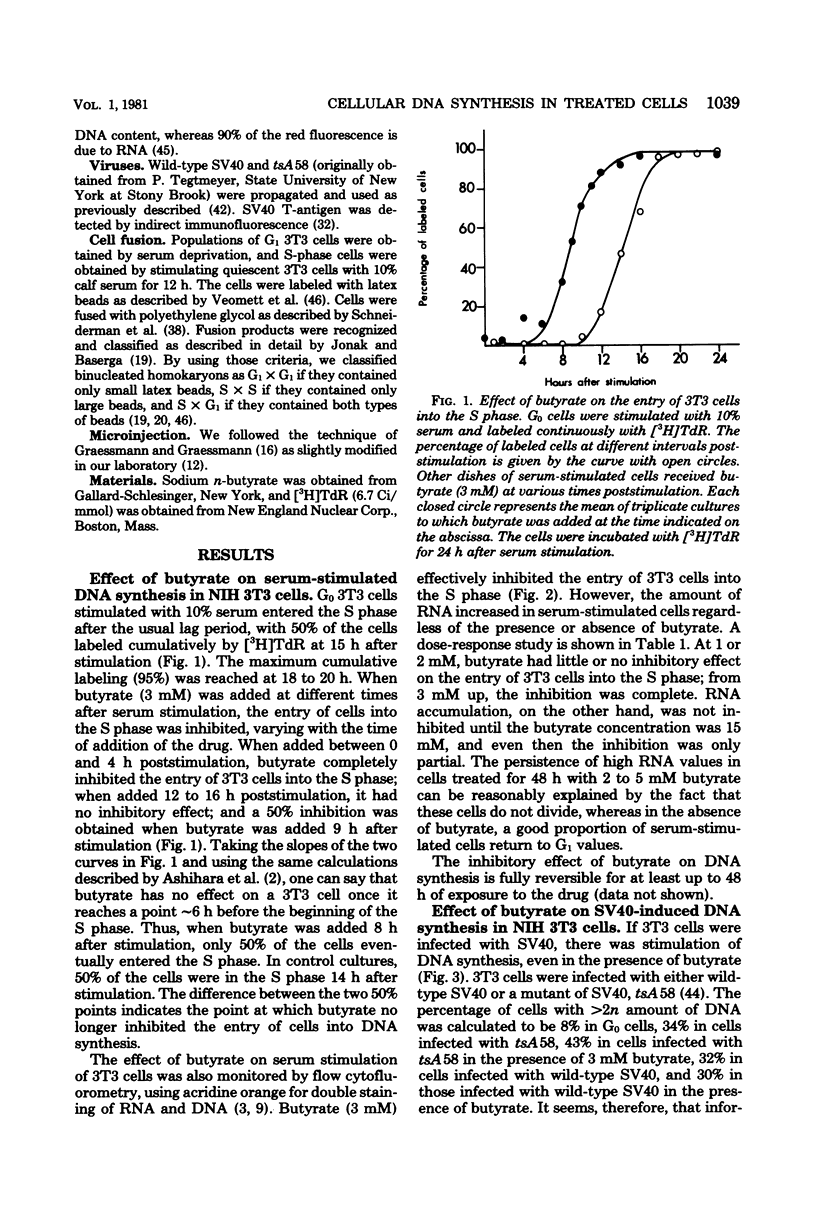
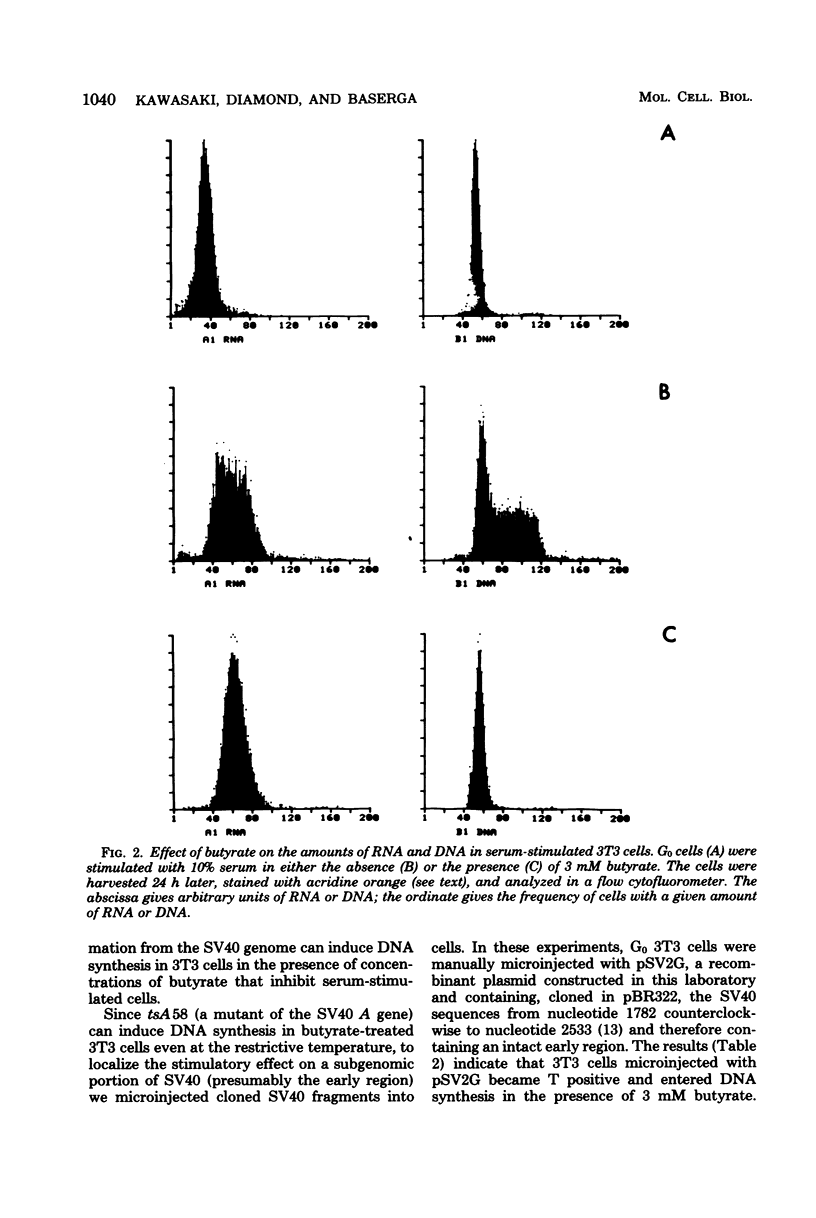
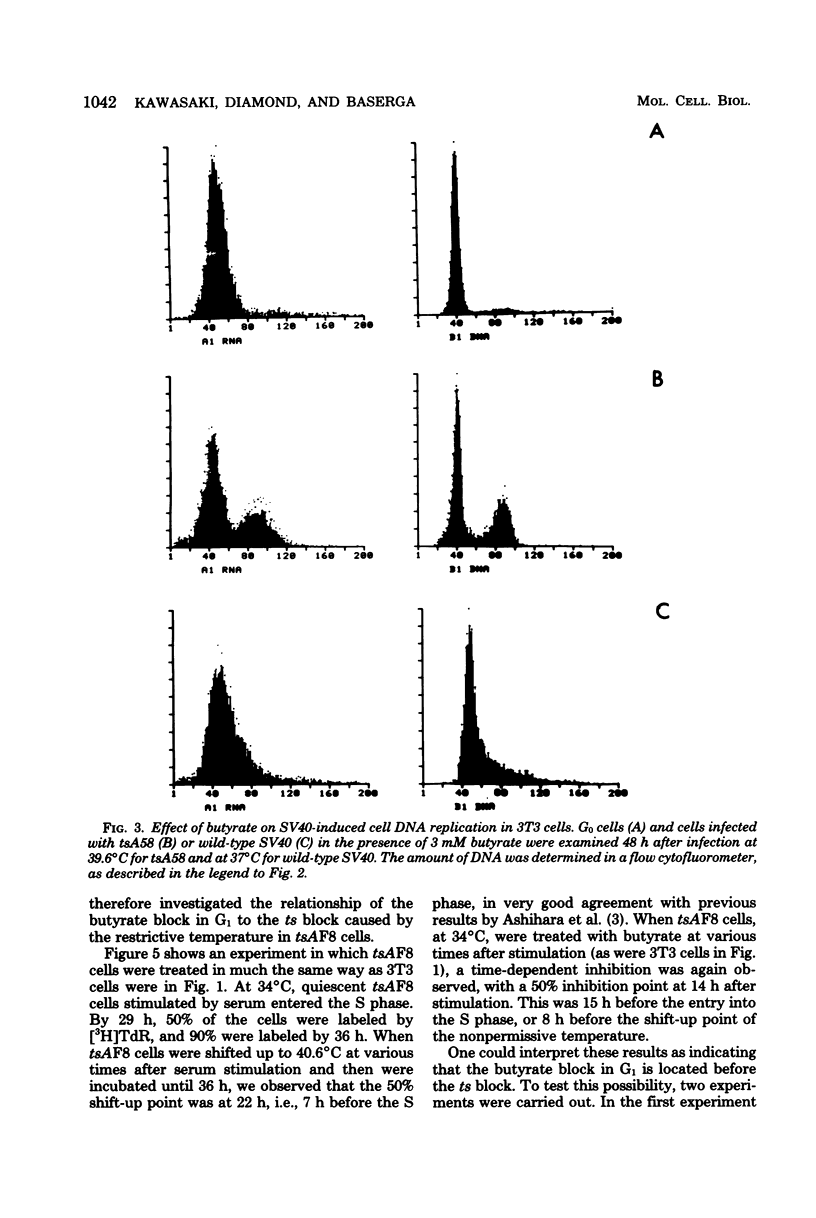
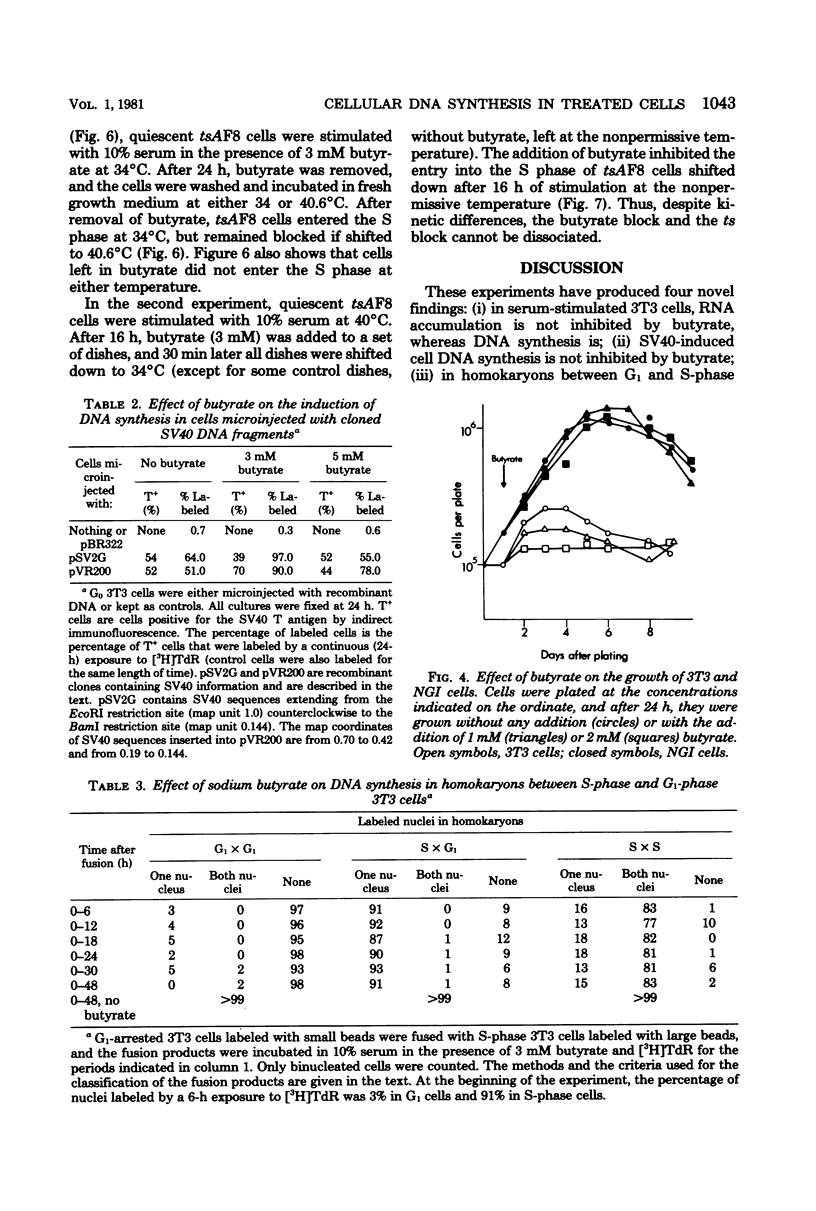
Sodium butyrate (3 mM) inhibited the entry into the S phase of quiescent 3T3 cells stimulated by serum, but had no effect on the accumulation of cellular ribonucleic acid. Simian virus 40 infection or manual microinjection of cloned fragments from the simian virus 40 A gene caused quiescent 3T3 cells to enter the S phase even in the presence of butyrate. NGI cells, a line of 3T3 cells transformed by simian virus 40, grew vigorously in 3 mM butyrate. Homokaryons were formed between G1 and S-phase 3T3 cells, Butyrate inhibited the induction of deoxyribonucleic acid synthesis that usually occurs in B1 nuclei when G1 cells are fused with S-phase cells. However, when G1 3T3 cells were fused with exponentially growing NGI cells, the 3T3 nuclei were induced to enter deoxyribonucleic acid synthesis. In tsAF8 cells, a ribonucleic acid polymerase II mutant that stops in the G1 phase of the cell cycle, no temporal sequence was demonstrated between the butyrate block and the temperature-sensitive block. These results confirm previous reports that certain virally coded proteins can induce cell deoxyribonucleic acid synthesis in the absence of cellular functions that are required by serum-stimulated cells. Our interpretation of these data is that butyrate inhibited cell growth by inhibiting the expression of genes required for the G0 leads to G1 leads to S transition and that the product of the simian virus 40 A gene overrode this inhibition by providing all of the necessary functions for the entry into the S phase.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altenburg B. C., Via D. P., Steiner S. H. Modification of the phenotype of murine sarcoma virus-transformed cells by sodium butyrate. Effects on morphology and cytoskeletal elements. Exp Cell Res. 1976 Oct 15;102(2):223–231. doi: 10.1016/0014-4827(76)90036-7. [DOI] [PubMed] [Google Scholar]
- Ashihara T., Chang S. D., Baserga R. Constancy of the shift-up point in two temperature-sensitive mammalian cell lines that arrest in G1. J Cell Physiol. 1978 Jul;96(1):15–22. doi: 10.1002/jcp.1040960103. [DOI] [PubMed] [Google Scholar]
- Ashihara T., Traganos F., Baserga R., Darzynkiewicz Z. A comparison of cell cycle-related changes in postmitotic and quiescent AF8 cells as measured by cytofluorometry after acridine orange staining. Cancer Res. 1978 Aug;38(8):2514–2518. [PubMed] [Google Scholar]
- Baserga R. The cell cycle. N Engl J Med. 1981 Feb 19;304(8):453–459. doi: 10.1056/NEJM198102193040803. [DOI] [PubMed] [Google Scholar]
- Boffa L. C., Vidali G., Mann R. S., Allfrey V. G. Suppression of histone deacetylation in vivo and in vitro by sodium butyrate. J Biol Chem. 1978 May 25;253(10):3364–3366. [PubMed] [Google Scholar]
- Burstin S. J., Meiss H. K., Basilico C. A temperature-sensitive cell cycle mutant of the BHK cell line. J Cell Physiol. 1974 Dec;84(3):397–408. doi: 10.1002/jcp.1040840308. [DOI] [PubMed] [Google Scholar]
- Candido E. P., Reeves R., Davie J. R. Sodium butyrate inhibits histone deacetylation in cultured cells. Cell. 1978 May;14(1):105–113. doi: 10.1016/0092-8674(78)90305-7. [DOI] [PubMed] [Google Scholar]
- D'Anna J. A., Tobey R. A., Gurley L. R. Concentration-dependent effects of sodium butyrate in Chinese hamster cells: cell-cycle progression, inner-histone acetylation, histone H1 dephosphorylation, and induction of an H1-like protein. Biochemistry. 1980 Jun 10;19(12):2656–2671. doi: 10.1021/bi00553a019. [DOI] [PubMed] [Google Scholar]
- Darzynkiewicz Z., Traganos F., Sharpless T., Melamed M. R. Lymphocyte stimulation: a rapid multiparameter analysis. Proc Natl Acad Sci U S A. 1976 Aug;73(8):2881–2884. doi: 10.1073/pnas.73.8.2881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fallon R. J., Cox R. P. Cell cycle analysis of sodium butyrate and hydroxyurea, inducers of ectopic hormone production in HeLa cells. J Cell Physiol. 1979 Aug;100(2):251–262. doi: 10.1002/jcp.1041000206. [DOI] [PubMed] [Google Scholar]
- Floros J., Baserga R. Reactivation of G0 nuclei by S-phase cells. Cell Biol Int Rep. 1980 Jan;4(1):75–82. doi: 10.1016/0309-1651(80)90012-0. [DOI] [PubMed] [Google Scholar]
- Floros J., Jonak G., Galanti N., Baserga R. Induction of cell DNA replication in G1-specific ts mutants by microinjection of SV40 DNA. Exp Cell Res. 1981 Mar;132(1):215–223. doi: 10.1016/0014-4827(81)90097-5. [DOI] [PubMed] [Google Scholar]
- Galanti N., Jonak G. J., Soprano K. J., Floros J., Kaczmarek L., Weissman S., Reddy V. B., Tilghman S. M., Baserga R. Characterization and biological activity of cloned simian virus 40 DNA fragments. J Biol Chem. 1981 Jun 25;256(12):6469–6474. [PubMed] [Google Scholar]
- Ghosh N. K., Deutsch S. I., Griffin M. J., Cox R. P. Regulation of growth and morphological modulation of HeLa65 cells in monolayer culture by dibutyryl cyclic AMP, butyrate and their analogs. J Cell Physiol. 1975 Dec;86 (Suppl 2)(3 Pt 2):663–672. doi: 10.1002/jcp.1040860511. [DOI] [PubMed] [Google Scholar]
- Ginsburg E., Salomon D., Sreevalsan T., Freese E. Growth inhibition and morphological changes caused by lipophilic acids in mammalian cells. Proc Natl Acad Sci U S A. 1973 Aug;70(8):2457–2461. doi: 10.1073/pnas.70.8.2457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graessmann M., Graessman A. "Early" simian-virus-40-specific RNA contains information for tumor antigen formation and chromatin replication. Proc Natl Acad Sci U S A. 1976 Feb;73(2):366–370. doi: 10.1073/pnas.73.2.366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillouzo A., Tichonicky L., Boisnard-Rissel M., Kruh J. Early reversible nuclear alterations induced by sodium butyrate in cultured hepatoma cells. Cell Biol Int Rep. 1980 Oct;4(10):961–968. doi: 10.1016/0309-1651(80)90199-x. [DOI] [PubMed] [Google Scholar]
- Hagopian H. K., Riggs M. G., Swartz L. A., Ingram V. M. Effect of n-butyrate on DNA synthesis in chick fibroblasts and HeLa cells. Cell. 1977 Nov;12(3):855–860. doi: 10.1016/0092-8674(77)90284-7. [DOI] [PubMed] [Google Scholar]
- Jonak G. J., Baserga R. Cytoplasmic regulation of two G1-specific temperature-sensitive functions. Cell. 1979 Sep;18(1):117–123. doi: 10.1016/0092-8674(79)90360-x. [DOI] [PubMed] [Google Scholar]
- Jonak G. J., Baserga R. The cytoplasmic appearance of three functions expressed during the G0 leds to G1 leads to S transition is nucleus-dependent. J Cell Physiol. 1980 Nov;105(2):347–354. doi: 10.1002/jcp.1041050217. [DOI] [PubMed] [Google Scholar]
- Laughlin C., Strohl W. A. Factors regulating cellular DNA synthesis induced by adenovirus infection. II. The effects of actinomycin D on productive virus-cell systems. Virology. 1976 Oct 1;74(1):44–56. doi: 10.1016/0042-6822(76)90126-4. [DOI] [PubMed] [Google Scholar]
- Leder A., Leder P. Butyric acid, a potent inducer of erythroid differentiation in cultured erythroleukemic cells. Cell. 1975 Jul;5(3):319–322. doi: 10.1016/0092-8674(75)90107-5. [DOI] [PubMed] [Google Scholar]
- McKeehan W. L., McKeehan K. A., Calkins D. Extracellular regulation of fibroblast multiplication. Quantitative differences in nutrient and serum factor requirements for multiplication of normal and SV40 virus-transformed human lung cells. J Biol Chem. 1981 Mar 25;256(6):2973–2981. [PubMed] [Google Scholar]
- Meiss H. K., Basilico C. Temperature sensitive mutants of BHK 21 cells. Nat New Biol. 1972 Sep 20;239(90):66–68. doi: 10.1038/newbio239066a0. [DOI] [PubMed] [Google Scholar]
- Mercer W. E., Schlegel R. A. Cell cycle re-entry of quiescent mammalian nuclei following heterokaryon formation. Exp Cell Res. 1980 Aug;128(2):431–437. doi: 10.1016/0014-4827(80)90078-6. [DOI] [PubMed] [Google Scholar]
- Mori Y., Akedo H., Tanigaki Y., Tanaka K. M., Okada M., Nakamura N. Effect of sodium butyrate on the production of serotonin, histamine and glycosaminoglycans by cultured murine mastocytoma cells. Exp Cell Res. 1980 Jun;127(2):465–470. doi: 10.1016/0014-4827(80)90455-3. [DOI] [PubMed] [Google Scholar]
- Norwood T. H., Pendergrass W. R., Martin G. M. Reinitiation of DNA synthesis in senescent human fibroblasts upon fusion with cells of unlimited growth potential. J Cell Biol. 1975 Mar;64(3):551–556. doi: 10.1083/jcb.64.3.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norwood T. H., Pendergrass W. R., Sprague C. A., Martin G. M. Dominance of the senescent phenotype in heterokaryons between replicative and post-replicative human fibroblast-like cells. Proc Natl Acad Sci U S A. 1974 Jun;71(6):2231–2235. doi: 10.1073/pnas.71.6.2231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- POPE J. H., ROWE W. P. DETECTION OF SPECIFIC ANTIGEN IN SV40-TRANSFORMED CELLS BY IMMUNOFLUORESCENCE. J Exp Med. 1964 Aug 1;120:121–128. doi: 10.1084/jem.120.2.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardee A. B. A restriction point for control of normal animal cell proliferation. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1286–1290. doi: 10.1073/pnas.71.4.1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peden K. W., Pipas J. M., Pearson-White S., Nathans D. Isolation of mutants of an animal virus in bacteria. Science. 1980 Sep 19;209(4463):1392–1396. doi: 10.1126/science.6251547. [DOI] [PubMed] [Google Scholar]
- Pochron S., Rossini M., Darzynkiewicz Z., Traganos F., Baserga R. Failure of accumulation of cellular RNA in hamster cells stimulated to synthesize DNA by infection with adenovirus 2. J Biol Chem. 1980 May 25;255(10):4411–4413. [PubMed] [Google Scholar]
- Rao P. N., Johnson R. T. Mammalian cell fusion: studies on the regulation of DNA synthesis and mitosis. Nature. 1970 Jan 10;225(5228):159–164. doi: 10.1038/225159a0. [DOI] [PubMed] [Google Scholar]
- Ringertz N. R., Bolund L. Reactivation of chick erythrocyte nuclei by somatic cell hybridization. Int Rev Exp Pathol. 1974;13(0):83–116. [PubMed] [Google Scholar]
- Rossini M., Baserga R. RNA synthesis in a cell cycle-specific temperature sensitive mutant from a hamster cell line. Biochemistry. 1978 Mar 7;17(5):858–863. doi: 10.1021/bi00598a017. [DOI] [PubMed] [Google Scholar]
- Rossini M., Baserga S., Huang C. H., Ingles C. J., Baserga R. Changes in RNA polymerase II in a cell cycle-specific temperature-sensitive mutant of hamster cells. J Cell Physiol. 1980 Apr;103(1):97–103. doi: 10.1002/jcp.1041030114. [DOI] [PubMed] [Google Scholar]
- Rossini M., Weinmann R., Baserga R. DNA synthesis in temperature-sensitive mutants of the cell cycle infected by polyoma virus and adenovirus. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4441–4445. doi: 10.1073/pnas.76.9.4441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneiderman S., Farber J. L., Baserga R. A simple method for decreasing the toxicity of polyethylene glycol in mammalian cell hybridization. Somatic Cell Genet. 1979 Mar;5(2):263–269. doi: 10.1007/BF01539165. [DOI] [PubMed] [Google Scholar]
- Scott W. A., Brockman W. W., Nathans D. Biological activities of deletion mutants of simian virus 40. Virology. 1976 Dec;75(2):319–334. doi: 10.1016/0042-6822(76)90031-3. [DOI] [PubMed] [Google Scholar]
- Shales M., Bergsagel J., Ingles C. J. Defective RNA polymerase II in the G1 specific temperature sensitive hamster cell mutant TsAF8. J Cell Physiol. 1980 Dec;105(3):527–532. doi: 10.1002/jcp.1041050317. [DOI] [PubMed] [Google Scholar]
- Soprano K. J., Jonak G. J., Galanti N., Floros J., Baserga R. Identification of an SV40 DNA sequence related to the reactivation of silent rRNA genes in human greater than mouse hybrid cells. Virology. 1981 Feb;109(1):127–136. doi: 10.1016/0042-6822(81)90477-3. [DOI] [PubMed] [Google Scholar]
- Soprano K. J., Rossini M., Croce C., Baserga R. The role of large T antigen in simian virus 40-induced reactivation of silent rRNA genes in human-mouse hybrid cells. Virology. 1980 Apr 30;102(2):317–326. doi: 10.1016/0042-6822(80)90099-9. [DOI] [PubMed] [Google Scholar]
- Stein G. H., Yanishevsky R. M. Entry into S phase is inhibited in two immortal cell lines fused to senescent human diploid cells. Exp Cell Res. 1979 Apr;120(1):155–165. doi: 10.1016/0014-4827(79)90546-9. [DOI] [PubMed] [Google Scholar]
- Tegtmeyer P. Function of simian virus 40 gene A in transforming infection. J Virol. 1975 Mar;15(3):613–618. doi: 10.1128/jvi.15.3.613-618.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traganos F., Darzynkiewicz Z., Sharpless T., Melamed M. R. Simultaneous staining of ribonucleic and deoxyribonucleic acids in unfixed cells using acridine orange in a flow cytofluorometric system. J Histochem Cytochem. 1977 Jan;25(1):46–56. doi: 10.1177/25.1.64567. [DOI] [PubMed] [Google Scholar]
- Veomett G., Prescott D. M., Shay J., Porter K. R. Reconstruction of mammalian cells from nuclear and cytoplasmic components separated by treatment with cytochalasin B. Proc Natl Acad Sci U S A. 1974 May;71(5):1999–2002. doi: 10.1073/pnas.71.5.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidali G., Boffa L. C., Mann R. S., Allfrey V. G. Reversible effects of Na-butyrate on histone acetylation. Biochem Biophys Res Commun. 1978 May 15;82(1):223–227. doi: 10.1016/0006-291x(78)90599-5. [DOI] [PubMed] [Google Scholar]
- Wright J. A. Morphology and growth rate changes in Chinese hamster cells cultured in presence of sodium butyrate. Exp Cell Res. 1973 Apr;78(2):456–460. doi: 10.1016/0014-4827(73)90091-8. [DOI] [PubMed] [Google Scholar]



