Abstract
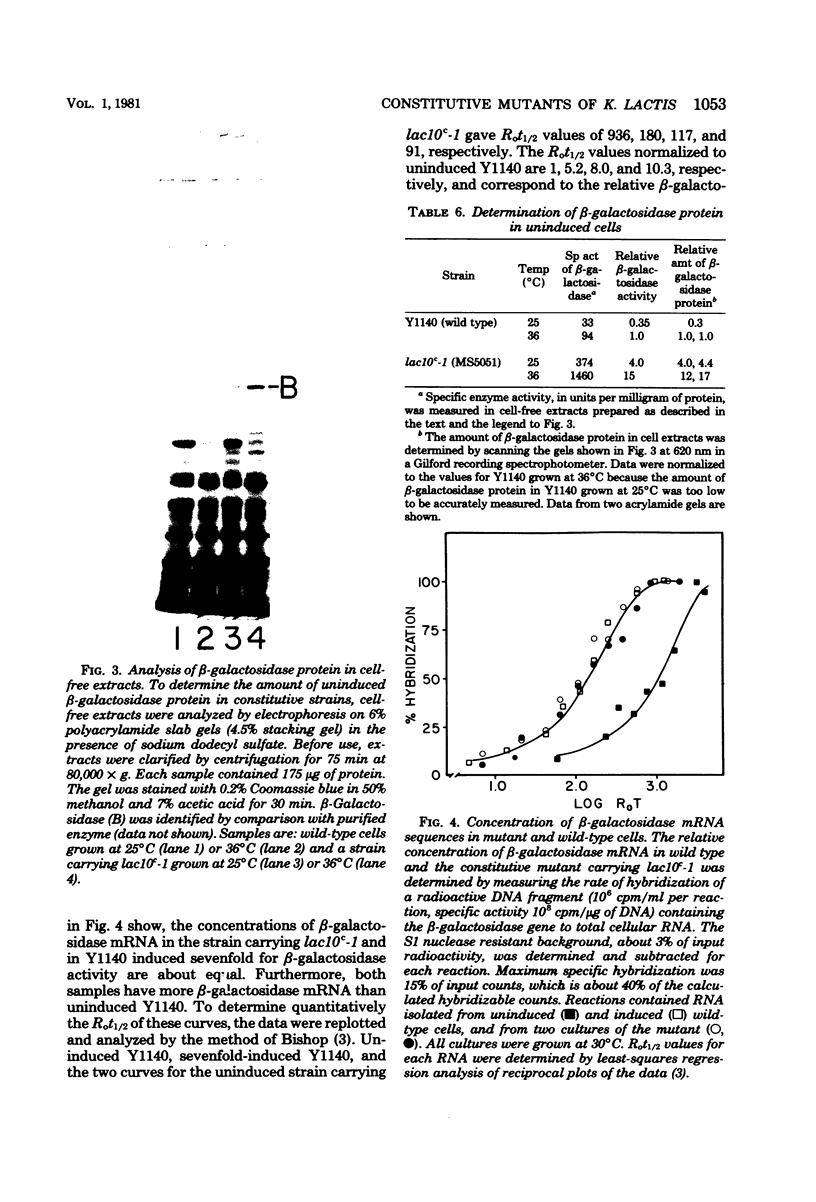
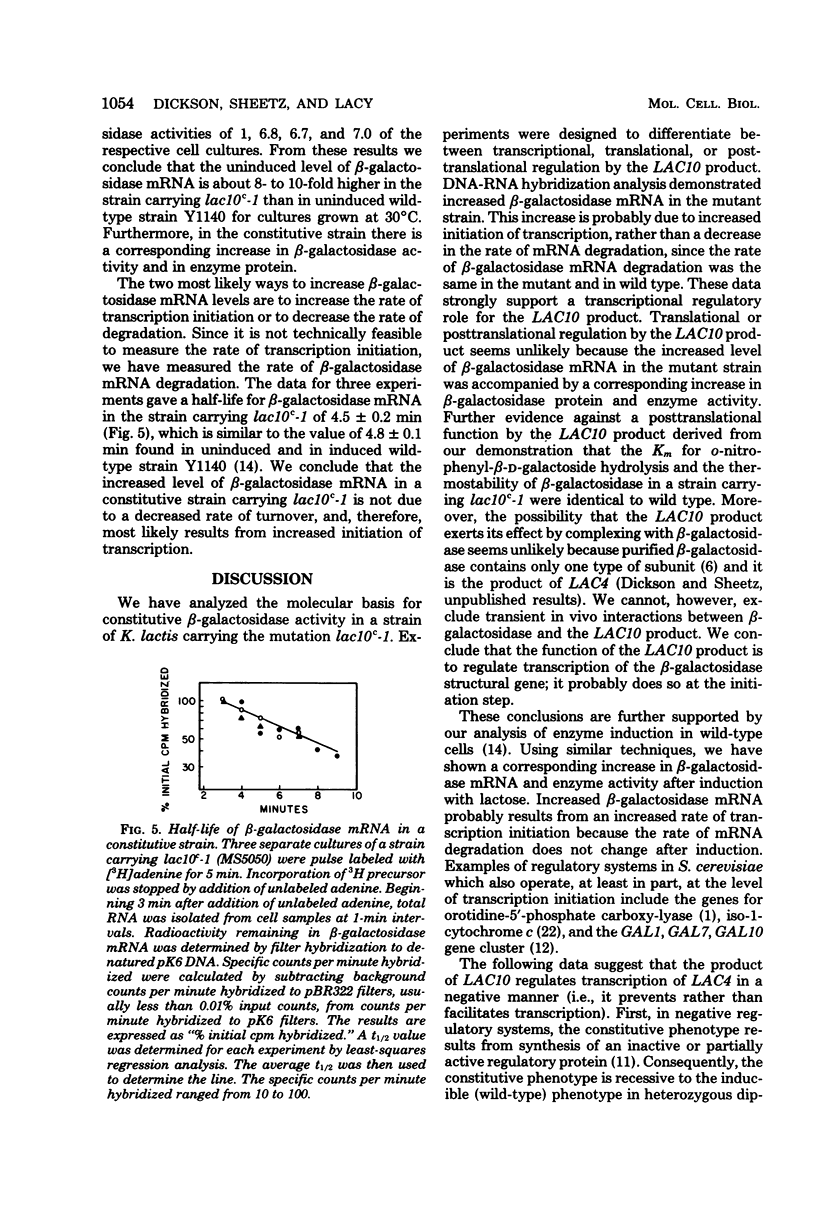
Mutants of Kluyveromyces lactis with elevated uninduced levels of beta-galactosidase (EC 32.1.2.3) activity, constitutive mutants (lac10c), were isolated and characterized to determine the basis for their constitutiveness. These lesions are not operator-type regulatory mutants because they are not closely linked to the beta-galactosidase structural gene. In a constitutive strain having a 7-fold increase in beta-galactosidase activity, the concentration of beta-galactosidase messenger ribonucleic acid (mRNA) was 8- to 10-fold higher than uninduced wild type. The half-life of beta-galactosidase mRNA was the same in the mutant strain (t1/2 = 4.5 +/- 0.2 min) as in uninduced wild-type cells (t1/2 = 4.8 +/- 0.1 min), indicating that the elevated mRNA level in the mutant was not due to a decreased rate of mRNA degradation. Consequently, we hypothesize that the LAC10 product regulates transcription of the beta-galactosidase gene; it probably affects the rate of transcription initiation. Parallel increases in enzyme protein, in constitutive levels of beta-galactosidase activity, and in mRNA further support this position, making translational or posttranslational control by LAC10 unlikely. Several types of data suggest that the LAC10 product functions as a negative regulatory element to prevent transcription. Other data demonstrate that lac10c mutations have pleiotrophic effects, there being constitutive levels not only of beta-galactosidase activity, but also the other lactose-inducible activities of galactokinase (EC 2.7.5.1), galactose-1-phosphate uridyl transferase (EC 2.7.7.10), and lactose transport. It would appear that LAC10 regulates lactose-inducible proteins.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bach M. L., Lacroute F., Botstein D. Evidence for transcriptional regulation of orotidine-5'-phosphate decarboxylase in yeast by hybridization of mRNA to the yeast structural gene cloned in Escherichia coli. Proc Natl Acad Sci U S A. 1979 Jan;76(1):386–390. doi: 10.1073/pnas.76.1.386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckwith J., Rossow P. Analysis of genetic regulatory mechanisms. Annu Rev Genet. 1974;8:1–13. doi: 10.1146/annurev.ge.08.120174.000245. [DOI] [PubMed] [Google Scholar]
- Bishop J. O. DNA-RNA hybridization. Acta Endocrinol Suppl (Copenh) 1972;168:247–276. doi: 10.1530/acta.0.071s247. [DOI] [PubMed] [Google Scholar]
- Broach J. R. Galactose regulation in Saccharomyces cerevisiae. The enzymes encoded by the GAL7, 10, 1 cluster are co-ordinately controlled and separately translated. J Mol Biol. 1979 Jun 15;131(1):41–53. doi: 10.1016/0022-2836(79)90300-0. [DOI] [PubMed] [Google Scholar]
- Dickson R. C., Dickson L. R., Markin J. S. Purification and properties of an inducible beta-galactosidase isolated from the yeast Kluyveromyces lactis. J Bacteriol. 1979 Jan;137(1):51–61. doi: 10.1128/jb.137.1.51-61.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickson R. C. Expression of a foreign eukaryotic gene in Saccharomyces cerevisiae: beta-galactosidase from Kluyveromyces lactis. Gene. 1980 Sep;10(4):347–356. doi: 10.1016/0378-1119(80)90155-9. [DOI] [PubMed] [Google Scholar]
- Dickson R. C., Markin J. S. Molecular cloning and expression in E. coli of a yeast gene coding for beta-galactosidase. Cell. 1978 Sep;15(1):123–130. doi: 10.1016/0092-8674(78)90088-0. [DOI] [PubMed] [Google Scholar]
- Douglas H. C., Hawthorne D. C. Regulation of genes controlling synthesis of the galactose pathway enzymes in yeast. Genetics. 1966 Sep;54(3):911–916. doi: 10.1093/genetics/54.3.911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Englesberg E., Irr J., Power J., Lee N. Positive control of enzyme synthesis by gene C in the L-arabinose system. J Bacteriol. 1965 Oct;90(4):946–957. doi: 10.1128/jb.90.4.946-957.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Englesberg E., Wilcox G. Regulation: positive control. Annu Rev Genet. 1974;8:219–242. doi: 10.1146/annurev.ge.08.120174.001251. [DOI] [PubMed] [Google Scholar]
- Hopper J. E., Broach J. R., Rowe L. B. Regulation of the galactose pathway in Saccharomyces cerevisiae: induction of uridyl transferase mRNA and dependency on GAL4 gene function. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2878–2882. doi: 10.1073/pnas.75.6.2878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JACOB F., MONOD J. Genetic regulatory mechanisms in the synthesis of proteins. J Mol Biol. 1961 Jun;3:318–356. doi: 10.1016/s0022-2836(61)80072-7. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lacy L. R., Dickson R. C. Transcriptional regulation of the Kluyveromyces lactis beta-galactosidase gene. Mol Cell Biol. 1981 Jul;1(7):629–634. doi: 10.1128/mcb.1.7.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Metzenberg R. L., Chia W. Genetic control of phosphorus assimilation in Neurospora crassa: dose-dependent dominance and recessiveness in constitutive mutants. Genetics. 1979 Nov;93(3):625–643. doi: 10.1093/genetics/93.3.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ptashne M., Backman K., Humayun M. Z., Jeffrey A., Maurer R., Meyer B., Sauer R. T. Autoregulation and function of a repressor in bacteriophage lambda. Science. 1976 Oct 8;194(4261):156–161. doi: 10.1126/science.959843. [DOI] [PubMed] [Google Scholar]
- Segawa T., Fukasawa T. The enzymes of the galactose cluster in Saccharomyces cerevisiae. Purification and characterization of galactose-1-phosphate uridylyltransferase. J Biol Chem. 1979 Nov 10;254(21):10707–10709. [PubMed] [Google Scholar]
- Sheetz R. M., Dickson R. C. Mutations affecting synthesis of beta-galactosidase activity in the yeast Kluyveromyces lactis. Genetics. 1980 Aug;95(4):877–890. doi: 10.1093/genetics/95.4.877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zitomer R. S., Montgomery D. L., Nichols D. L., Hall B. D. Transcriptional regulation of the yeast cytochrome c gene. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3627–3631. doi: 10.1073/pnas.76.8.3627. [DOI] [PMC free article] [PubMed] [Google Scholar]