Abstract
Artemisinin combination therapies eliminate immature Plasmodium falciparum gametocytes but not mature gametocytes, which may persist for up to 1 month posttreatment. A single dose of primaquine, which is inexpensive and effective against mature gametocytes, could be added to further reduce the potential for posttreatment parasite transmission. Currently, we have few data regarding the effectiveness or safety of doing so. We collected data from 21 therapeutic efficacy trials of the National Antimalarial Drug Resistance Monitoring System of India conducted during 2009 to 2010, wherein 9 sites used single-dose primaquine (0.75 mg/kg of body weight) administered on day 2 along with artesunate plus sulfadoxine-pyrimethamine (AS+SP) while 12 did not. We estimated the effect of primaquine on posttreatment gametocyte clearance and the total number of gametocyte-weeks as determined by microscopy. We compared the median area under the curve for gametocyte density and reported adverse events. One thousand three hundred thirty-five patients completed the antimalarial drug treatment. Adjusting for region, primaquine increased the rate of gametocyte clearance (hazard ratio, 1.9; 95% confidence interval [CI], 1.1 to 3.3), prevented 45% (95% CI, 19 to 62) of posttreatment gametocyte-weeks, and decreased the area under the gametocyte density curve over the 28-day follow-up compared to AS+SP alone (P value = 0.01). The results were robust to other adjustment sets, and the estimated effect of primaquine increased during sensitivity analysis on the measurement of exposure time. No serious adverse events were detected. In conclusion, the addition of primaquine to AS+SP was effective in reducing the posttreatment presence of P. falciparum gametocytes. Primaquine was well tolerated and could be administered along with an artemisinin combination therapy as the first-line therapy.
INTRODUCTION
The primary goal of antimalarial drug therapy is to reduce morbidity and mortality by achieving clinical and parasitological cure. A crucial secondary aim, however, is to reduce the infectious reservoir and decrease malaria transmission. Gametocytes are the sexual stage of the Plasmodium life cycle and render malaria patients infectious to mosquitoes and propagate transmission. Treatment considerations for clearing gametocytes are distinct from those for asexual stages because of the stage-specific actions of antimalarial drugs, particularly for Plasmodium falciparum (1). The chemotherapeutic control of gametocytes is largely achieved through resolving the asexual parasitemia, which prevents the generation of new gametocytes and limits the period of infectivity to the circulation time of existing gametocytes. However, gametocytemia following treatment may be prevalent in a high proportion of patients and persist for approximately up to one month (2). Eliminating the posttreatment infectious period could provide substantial impact, particularly in countries of low to moderate endemicity, where the size of the infectious reservoir is a critical determinant of transmission (3). The potential marginal direct and indirect costs of adjunct gametocyte-specific treatment during the case management process make such a strategy attractive.
Artemisinin is active against all gametocyte stages barring the fully mature stage, and this characteristic contributed to the impressive declines in transmission following its widespread implementation in several countries (4). Still, in trials with artemisinin combination therapy (ACT), mature gametocytes persisted in 10 to 30% of patients and remained infectious to mosquitoes (5–7). Primaquine, which has no activity against asexual P. falciparum parasites, is effective against mature gametocytes (8, 9). A single dose of primaquine has been used as an adjunct treatment, for its gametocytocidal effect, alongside first-line therapies in many countries since the 1960s (10, 11). The key question for P. falciparum treatment is whether a single dose of primaquine should be added to ACTs to further reduce the transmissibility of the treated infection (12). The Malaria Policy Advisory Committee of the World Health Organization (WHO) noted the gametocytocidal use of primaquine in the treatment for P. falciparum as a priority question for operational research (13).
The goal of this study was to estimate the gametocytocidal effect of adding primaquine to ACT in India. We conducted a secondary analysis of data from a national network of sentinel sites for monitoring antimalarial drug resistance. The study protocols did not contain instructions on primaquine use, as there were no policy guidelines at the time of the study. The use of the drug by some sites and not others created a natural experiment.
MATERIALS AND METHODS
Study sites and population.
We utilized data from 22 trials, which had been conducted through the National Antimalarial Drug Resistance Monitoring Network of India to assess the therapeutic efficacy of artesunate plus sulfadoxine-pyrimethamine (AS+SP) for P. falciparum malaria in 2009 and 2010 (14). Among the sites, 9 used primaquine along with AS+SP while 13 used AS+SP alone. We could not obtain data from 1 site (Gadchiroli, no primaquine use) because gametocytemia was not recorded in case record forms and slides could not be reexamined. We included all patients eligible for the WHO therapeutic efficacy trial protocol according to the following criteria: patients with P. falciparum monoinfection, febrile or with a history of fever, asexual parasite density greater than 500/μl and less than 100,000/μl, and willingness to consent to follow-up (15). We excluded pregnant patients, patients with signs of severe malaria, and patients less than 1 year of age.
Data collection.
The data collection methods have been previously described (14). Briefly, clinical and demographic information was recorded from each patient at enrollment. Patients were monitored during the first 3 days of treatment and then at weekly intervals from enrollment until day 28. Patients received AS+SP (4 mg/kg artesunate for 3 days plus 25 mg sulfadoxine and 1.25 mg pyrimethamine/kg of body weight) as per national guidelines with or without primaquine on the third day of treatment (0.75 mg/kg, for a usual adult dose of 45 mg). At each follow-up visit, a physical exam was conducted and thick and thin blood smears were prepared. Hemoglobin measurements were not conducted for any patient. Using routine oil immersion reading at a 100× lens objective on Giemsa-stained thick smears, slides were examined for white blood cell (WBC) counts of up to 200 if sexual stages and/or gametocytes stages were present or for counts of up to 1,000 WBC to declare a slide negative for both. A count of 8,000 WBC per μl of blood was assumed to obtain a final parasite density. Thus, the estimated detection limit for gametocytes would be 8/μl, although this is likely an overestimate. The data were double-entered into the WHO therapeutic efficacy database, and blood slides were cross-checked by expert microscopists.
Case and predictor definitions.
Primaquine therapy was the main exposure. We obtained primaquine use information from the site physician. We defined pretreatment gametocytemia as the presence of gametocytes in peripheral blood on any day between day 0 and day 2 in a patient eligible for a therapeutic efficacy study in 2009 or 2010. We defined posttreatment gametocyte clearance as the first day without gametocytemia after day 2 in a patient with pretreatment gametocytemia. We defined an event of posttreatment gametocytemia as the presence of gametocytes on any follow-up day after day 2 in a patient eligible for the therapeutic efficacy study in 2009 or 2010. We excluded gametocytemia at the time of failure for patients with recrudescence or reinfection (16). For measuring exposure time in weeks, we censored at the end of the interval. We reported the data in person-weeks, instead of person-days, to enable comparisons with previous literature. For the measurement of posttreatment gametocyte circulation, patients who completed the follow-up accrued an additional week of exposure in order to incorporate the contribution of individuals who remained positive at the end of day 28. Thus, the total posttreatment exposure time of 32 days is congruent with biological estimates of the maximum circulation time of gametocytes (2).
We compared covariates associated with gametocytemia in prior literature: age, sex, season, region, previous antimalarial drug intake, current fever, history of fever, and asexual parasite density (17). We designated “region” using geographic clusters associated with different malaria ecotypes, which also correspond to different transmission intensities: western India as Gujarat, Mumbai, and Rajasthan, central India as Andhra Pradesh, Chhattisgarh, Gadchiroli, Jharkhand, Madhya Pradesh, and Orissa, and northeast India as the Assam, Meghalaya, and West Bengal sites (18, 19). We classified “season” by month of enrollment: monsoon (June to August), postmonsoon (September to November), and winter (December and January).
Data analysis.
We included in our analysis all patients who completed treatment. Missing gametocytemia data due to withdrawal and loss to follow-up during the treatment phase constituted less than 3% of the overall sample. None of the covariates had missing data. We tabulated the clinical and demographic characteristics, as well as the primary and secondary study outcomes, of patients who received and did not receive primaquine. We calculated the mean and median gametocyte clearance times by treatment group and compared them using the t test and rank sum tests. We calculated the incidence, incidence rate ratios, and incidence rate differences over follow-up time among the primaquine and no-primaquine groups.
We modeled the effect of primaquine by estimating the posttreatment clearance rates of gametocytes among patients with gametocytemia at enrollment and by comparing the circulation of posttreatment gametocyte-weeks among the entire population. We used the Cox regression with the Efron method for ties (20) with clustered (on site) robust standard error and the Poisson regression using general estimating equations with unstructured correlation matrixes and robust clustered (on patient) standard error, respectively. We constructed a causal diagram of the effect of primaquine on gametocytemia to identify adjustment sets using the DAG program v2.1 (http://epi.dife.de/dag). Based on our diagram, adjustment on district was sufficient to control confounding bias. However, we controlled for region instead since the exposure, primaquine, did not vary by district. To assess the robustness of the measure of effect to uncontrolled confounding, we also produced estimates adjusted for age and parasite density, variables strongly associated with gametocytemia, as well as estimates adjusted for all covariates whose distribution differed between the two arms (P value < 0.10). We included an interaction term between region and primaquine and assessed effect measure modification using the Wald test (P value < 0.2). We used a backwards elimination strategy to retain confounders that changed the estimate by at least 10%. In the Cox model, we compared the model with and without discrete (marginal) time due to the large number of tied events and assessed the proportional hazards assumption by examining the −log[log(survival)] over time for parallel trends between the groups. We compared the Poisson model to a negative binomial model using a likelihood ratio test (P value < 0.05) to ensure appropriate fit with outcome dispersion.
The time at risk was measured in intervals and not continuously, i.e., measured on day 7, day 14, etc. A patient gametocytemic at day 7 and not gametocytemic on day 14 may have cleared gametocytes at any point during the interval, which potentially misclassifies the exposure time. Based on previous studies, we conducted a sensitivity analysis assuming a 50% reduction in time at risk in a given interval among primaquine-exposed patients. We plotted the mean area under the curve (AUC) for gametocyte density for each group by study visit. We excluded outliers of high pretreatment gametocyte density (maximum, >2,000/μl, n = 5) that disproportionally influenced the AUC. We calculated the AUC per day using the formula of Méndez et al. without log transformation and with the addition of the area for gametocytemia remaining at the end of follow-up (21). We compared the median AUC per day for each group using the rank sum test. We imported the final data set into STATA (v10) and used it for all analyses.
Study power.
For gametocyte clearance, our sample size of approximately 230 patients with pretreatment gametocytemia and α of 0.05 provides more than 90% power to detect a hazard ratio of 0.5, assuming 10% withdrawal and 80% of patients experiencing the outcome during the follow-up period. With a no-primaquine to primaquine ratio of approximately 2:1, α of 0.05, and assuming the prevalence of gametocytemia among the exposed as 5%, we calculated more than 90% power to detect a gametocyte-week incidence rate ratio greater than 2.
Ethical clearance.
The Scientific Advisory Committee of the National Institute of Malaria Research approved the original trials, and the Institutional Review Board of the University of North Carolina approved the secondary analysis study.
RESULTS
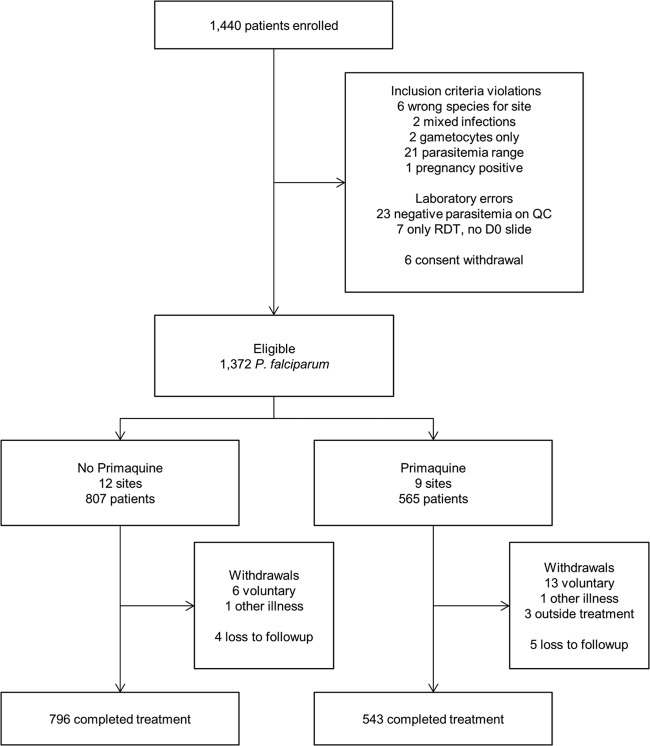
During 2009 to 2010, 1,372 eligible patients were recruited among 21 sites (Fig. 1). Nine sites, where 543 patients completed treatment, used primaquine, while 11 sites, where 796 patients completed treatment, did not use primaquine. Study withdrawal was higher in the primaquine group (P value = 0.02), but 11 of the 16 withdrawals were from two sites in western India where most patients were migrant workers. In each group, 2 patients missing gametocytemia data were excluded; the final complete case population was 1,335 patients (541 receiving primaquine and 794 not receiving primaquine). Among treatment failures, 4 of 12 recrudescent patients, 0 of 2 P. falciparum reinfection patients, and 0 of 6 Plasmodium vivax reinfection patients were gametocytemic at the time of failure. The clinical, demographic, and treatment efficacy as well as parasite clearance outcomes were similar between study groups, with the exceptions of a higher proportion of males, adults, and patients from northeast India in the primaquine-unexposed group (Table 1). Critically, the pretreatment gametocytemia prevalence in the no-primaquine group (18%, n = 141/794) was similar to the primaquine group (20%, n = 107/541). The prevalence of gametocytemia between the arms was similar on day 0, day 1, and day 2 and subsequently declined among the primaquine-exposed arm starting on day 3 (Fig. 2).
Fig 1.
Flowchart of patients eligible for treatment with primaquine from the National Antimalarial Drug Resistance Monitoring System, India, 2009 to 2010. QC, quality control; RDT, rapid diagnostic test; D0, day zero.
Table 1.
Demographics, clinical characteristics, and primary outcomes by primaquine receipt status of patients from the National Antimalarial Drug Resistance Monitoring System, India, 2009 to 2010a
| Characteristic | Value | No primaquine |
Primaquine |
||
|---|---|---|---|---|---|
| % | No.(n = 794) | % | No.(n = 541) | ||
| Area | Central | 39.3 | 312 | 77.4 | 419 |
| Western | 31.4 | 249 | 22.6 | 122 | |
| Northeastern | 29.3 | 233 | 0.0 | 0 | |
| Sex | Male | 63.7 | 506 | 49.4 | 267 |
| Female | 36.3 | 288 | 50.6 | 274 | |
| Age category (yr) | 0–4 | 5.5 | 44 | 11.3 | 61 |
| 5–9 | 14.1 | 112 | 28.3 | 153 | |
| 10–14 | 16.5 | 131 | 19.8 | 107 | |
| 15–49 | 56.0 | 445 | 34.4 | 186 | |
| ≥50 | 7.8 | 62 | 6.3 | 34 | |
| Asexual parasite density (no./μl) | <5,000 | 36.5 | 290 | 35.1 | 190 |
| 5,000–10,000 | 18.1 | 144 | 15.9 | 86 | |
| 10,000–50,000 | 38.8 | 308 | 38.4 | 208 | |
| ≥50,000 | 6.5 | 52 | 10.5 | 57 | |
| Febrile (≥37.5°C) | No | 31.4 | 249 | 29.0 | 157 |
| Yes | 68.6 | 545 | 71.0 | 384 | |
| History of fever | No | 2.3 | 18 | 0.0 | 0 |
| Yes | 97.7 | 776 | 100.0 | 541 | |
| Previous drug intake | No | 98.4 | 781 | 97.8 | 529 |
| Yes | 1.0 | 8 | 1.7 | 9 | |
| Unknown | 0.6 | 5 | 0.6 | 3 | |
| Primary classification | ACPR | 96.3 | 765 | 97.4 | 527 |
| ETF | 0.3 | 2 | 0.2 | 1 | |
| LTF | 0.6 | 5 | 0.2 | 1 | |
| LPF | 1.1 | 9 | 0.6 | 3 | |
| LFU | 1.6 | 13 | 1.3 | 7 | |
| WTH | 0.0 | 0 | 0.4 | 2 | |
| Parasite clearance time (h) | ≤24 | 58.9 | 468 | 62.5 | 338 |
| 24–48 | 27.1 | 215 | 25.3 | 137 | |
| 48–72 | 11.7 | 93 | 11.3 | 61 | |
| ≥72 | 2.0 | 16 | 0.9 | 5 | |
| Pretreatment gametocytemia | No | 82.2 | 653 | 80.2 | 434 |
| Yes | 17.8 | 141 | 19.9 | 107 | |
Abbreviations: ACPR, adequate clinical and parasitological response; ETF, early treatment failure; LTF, late treatment failure; LPF, late parasitological failure; LFU, loss to follow-up; WTH, withdrawal.
Fig 2.
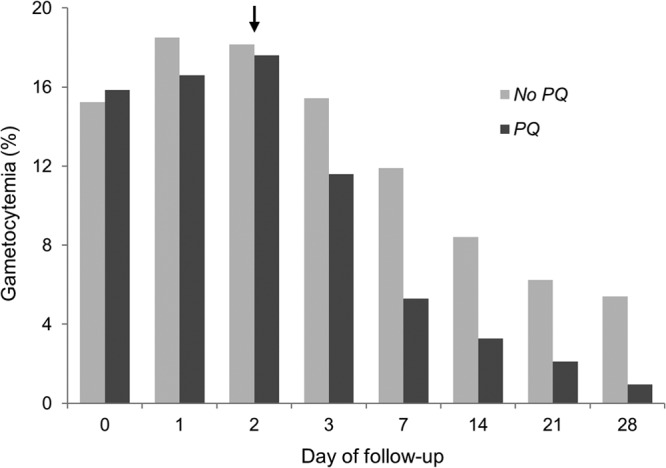
Prevalence of gametocytemia by treatment arm at each study visit among patients from the National Antimalarial Drug Resistance Monitoring System, India, 2009 to 2010. PQ, primaquine; arrow, administration of primaquine.
Among 248 patients with pretreatment gametocytemia, the median time to gametocyte clearance was 7 days (interquartile range, 3 to 14) in patients who received primaquine and 14 days (interquartile range, 3 to 28) in patients who did not (P value < 0.001). Primaquine with AS+SP cleared gametocytes faster than AS+SP alone with only 3% survival of gametocytemia compared to 23% at the end of follow-up (Fig. 3). The crude hazard ratio for the effect of primaquine on the rate of gametocyte clearance was 2.2 (95% confidence interval [CI], 1.2 to 4.2) over 28 days. Adjusted for region, the addition of primaquine resulted in an increased rate of gametocyte clearance 1.9 times (95% CI, 1.1 to 3.3) that of AS+SP alone over 28 days. The effect measure for primaquine was robust to adjusting for age and parasite density or all covariates selected by univariate associations (data not shown). We did not detect any modification of the effect with time or region.
Fig 3.
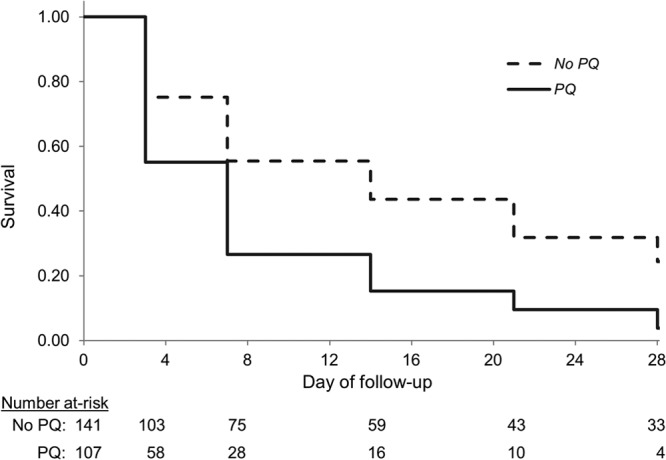
Clearance of gametocytes by treatment arm among patients with pretreatment gametocytemia from the National Antimalarial Drug Resistance Monitoring System, India, 2009 to 2010. PQ, primaquine.
The development of posttreatment gametocytemia among patients who were pretreatment negative for gametocytemia was lower (0.7%, 3/434) in the primaquine group than in the no-primaquine group (2.8%, 18/653) (P value = 0.02). Primaquine reduced the absolute and relative rates of posttreatment gametocyte circulation among the population (Table 2). The incidence rate ratio for the effect of primaquine on the number of posttreatment gametocyte-weeks was 0.3 (95% CI, 0.2 to 0.6). Adjusted for region, primaquine decreased the number of posttreatment gametocyte weeks by 45% (95% CI, 19 to 62). The effect measure for primaquine was robust to adjusting for age and parasite density or all covariates selected by univariate associations (data not shown). We did not detect any modification of the effect with region or overdispersion of the outcome.
Table 2.
Posttreatment gametocyte circulation by primaquine group among patients from the National Antimalarial Drug Resistance Monitoring System, India, 2009 to 2010a
| Time unit | Arm | No. of gametocytes | Total | IR | IRR | 95% CI | IRD | 95% CI |
|---|---|---|---|---|---|---|---|---|
| Days | PQ | 473 | 17,052 | 2.77 | 0.43 | 0.39, 0.47 | −0.037 | −0.033 to −0.041 |
| No PQ | 1,620 | 25,019 | 6.48 | |||||
| Weeks | PQ | 92 | 2,436 | 3.78 | 0.47 | 0.37, 0.60 | −0.042 | −0.030 to −0.054 |
| No PQ | 285 | 3,574 | 7.97 |
Abbreviations: PQ, primaquine; IR, incidence rate per 100-person-time; IRR, incidence rate ratio; IRD, incidence rate difference; CI, confidence interval.
In our sensitivity analysis, to examine the potential bias of our conservative assumption of midinterval gametocyte clearance in both arms, the estimated effect of primaquine increased. The crude and region-adjusted hazard ratios for primaquine on the rate of gametocyte clearance were 2.8 (95% CI, 1.5 to 5.5) and 2.5 (95% CI, 1.4 to 4.3) over 28 days. The crude and region-adjusted incidence rate ratios for primaquine on the number of gametocyte-weeks were 0.2 (95% CI, 0.1 to 0.3) and 0.3 (95% CI, 0.2 to 0.4) over 28 days. The mean AUC of each group at a study visit decreased over time compared to no primaquine (Fig. 4). The 90th to 99th percentile AUC per day was 2.2 to 79 gametocytes/μl for primaquine and 9.1 to 198 gametocytes/μl for AS+SP alone (P value = 0.004).
Fig 4.
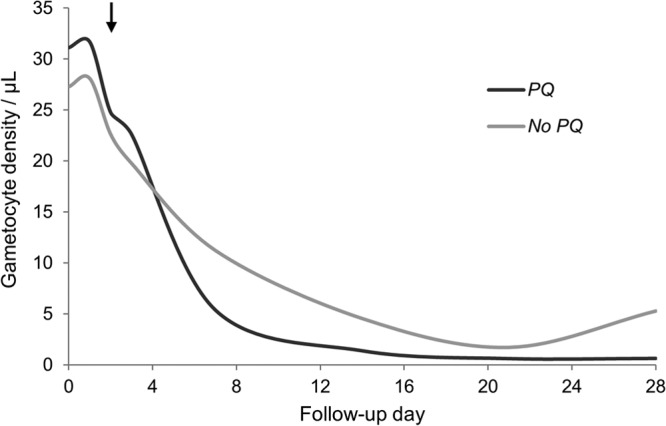
Area under the curve for the mean gametocyte density by treatment arm at each study visit among patients from the National Antimalarial Drug Resistance Monitoring System, India, 2009 to 2010. PQ, primaquine; arrow, administration of primaquine.
Adverse events reported during follow-up included vomiting (8) and fever (2) in the AS+SP-alone arm and vomiting (14) and jaundice (1) in the primaquine arm. Only 5 of the 14 patients who experienced vomiting did so on the day of primaquine administration. The case of jaundice occurred in a 5-year-old, male child on day 14 and self-resolved.
DISCUSSION
The addition of primaquine to AS+SP reduced posttreatment carriage of P. falciparum. The single dose of primaquine accelerated gametocyte clearance by 1 week in patients who were positive pretreatment, decreased the incidence of gametocytemia in patients who were negative pretreatment, and was well tolerated.
In those receiving only AS+SP alone, the prevalence of gametocytemia at the end of follow-up was 5% among all patients and 24% among those initially positive for gametocytes. While the choice of partner drug may also affect posttreatment gametocytemia prevalence, as SP monotherapy is associated with increased posttreatment gametocytemia (17, 22), our observations were similar to those observed for other combination therapies elsewhere (17, 23). We measured the effect of primaquine through gametocyte clearance, the number of gametocyte-weeks of circulation, and the area under the curve using gametocyte density over time. By any approach, the addition of primaquine provided substantial benefit even in the presence of an artemisinin-based treatment. Our sensitivity analysis suggests that our estimates were conservative and the effect of primaquine may be larger. Other estimates of the effect of primaquine with an ACT compared to ACT alone include a 92% reduction in gametocyte-weeks in Burma (24), 83% reduction in the prevalence on any day of follow-up in Tanzania (25), the acceleration of gametocyte clearance by a week in Colombia (26), and 2.4 times the rate of gametocyte clearance in Indonesia (27). While our estimate of clearance time and clearance rate was similar to the estimates from the low-transmission setting of the Colombian trial and Indonesia, the reduction of posttreatment gametocytemia was lower than the other two studies in high-transmission areas. The difference in primaquine effectiveness could be due to the different host immunity or pharmacokinetics of the study population, but there is also the possibility of reduced primaquine efficacy in India. Since the extensive malaria eradication program in the 1960s, India has a long history of primaquine use. Several studies previously reported the frequent persistence of gametocytemia following primaquine administration (28, 29) and the slower clearance of gametocytes after treatment with primaquine than with a novel 8-aminoquinoline in Western India (30).
While primaquine certainly works, several operational questions remain regarding its safety, optimal dose, and the day of administration. The primary concern with primaquine use is safety, given the risk of hemolysis in individuals with G6PD deficiency (8). In previous single-dose primaquine trials, hemoglobin concentrations among exposed patients were 5% lower at day 7 (recovered by the end of the month) or the mean increase over the study was 0.3g/dl less than in patients who received only ACT (24, 25). Recent research also suggests that hemolysis may occur in non-G6PD individuals as well, although severe anemia was rare (31). However, hemolysis and side effects will vary by population, and in the context of already vulnerable populations (32), more assessments, particularly postmarketing pharmacovigilance, are needed. We could not assess the effect of single-dose primaquine on hemoglobin levels, as these data were unavailable. The current dose of primaquine was determined through limited experiments in the 1960s (10, 11). Lower, or higher, doses of primaquine may be equally, or more, effective as well as safe—Thailand, for example, uses a 30-mg adult dose of primaquine for gametocytocidal therapy (33). A 4-arm, randomized study of different doses is under way in Uganda and should help determine the trade-offs, if any, between primaquine efficacy and safety (34). Recently, the World Health Organization issued new guidelines for the use of 0.25 mg/kg single-dose primaquine in areas currently not implementing a gametocytocide treatment (35). The recommendation considered the risk of hemolysis with the current dose and gray literature evidence of similar transmission-blocking efficacy using lower doses of primaquine (36). The day of primaquine administration varies from day 0 to day 2 with little evidence available to guide policy. Modeling studies have suggested that later administration, a week after ACT treatment, may have the most transmission-reducing effect (37). However, such a regimen could be operationally difficult to implement.
Our study had several limitations. We used microscopy for the measurement of gametocytemia, which is less sensitive than molecular techniques. However, in studies comparing the two methods, the latter increased the magnitude of gametocytemia but did not alter its age structure, circulation time estimates, or other trends (2, 38). While submicroscopic-density infections can infect mosquitoes, the probability of infection, the proportion of mosquitoes infected, and the density of infection in mosquitoes are positively correlated with gametocyte density (39, 40). Second, primaquine use was not randomized, and the gametocyte data were analyzed retrospectively. While we controlled for several sets of covariates and assessed effect measure modification, confounding due to unmeasured causes may bias our estimates, and the possibility of heterogeneity of the effect cannot be excluded with the available power (41). Third, initial gametocytemia among patients may have varied depending on the form of their recruitment. The direction of bias is unclear, as active case detection could detect infections earlier in the disease course, prior to self-referral, or later in the disease course, after symptom attenuation, compared to passive case detection. Finally, we used the presence of gametocytes as a proxy for infectiousness. Infectivity is modified by a number of factors, and its assessment through membrane-feeding experiments, which are labor-intensive and time-consuming, could preclude large, multisite trials needed for generalizable results (42). However, recently reviewed evidence suggests that primaquine may be effective in sterilizing gametocytes prior to their clearance and underlines the importance of measuring actual transmission (43).
We conclude that primaquine reduced the prevalence and duration of posttreatment P. falciparum gametocytemia compared to AS+SP alone. Single-dose primaquine could be provided along with ACT in India to improve malaria control. We outlined several avenues of operational research needed for optimizing the adjunctive use of primaquine. Future studies should use more-sensitive detection techniques to improve estimates of gametocytemia and, preferably, direct measures of transmission.
ACKNOWLEDGMENTS
N.K.S. was supported by a fellowship from the Paul and Daisy Soros Foundation and National Institutes of Health Medical Scientist Training Program grant GM008719. This paper was cleared by the NIMR publication screening committee (approval no. 017/2012).
N.K.S., S.R.M., N.V., and N.M. designed the study, and B.S., A.A., N.V., and N.M. collected the original data. N.K.S., C.P., J.J.J., A.S., S.R.M., P.D.M.M., and N.M. analyzed the data, N.K.S. wrote the first draft of the manuscript, and all authors wrote the final report.
We declare that we have no conflicts of interest.
Footnotes
Published ahead of print 15 April 2013
REFERENCES
- 1. Bruce-Chwatt LJ. 1962. Classification of antimalarial drugs in relation to different stages in the life-cycle of the parasite: commentary on a diagram. Bull. World Health Organ. 27:287–290 [PMC free article] [PubMed] [Google Scholar]
- 2. Bousema T, Okell L, Shekalaghe S, Griffin JT, Omar S, Sawa P, Sutherland C, Sauerwein R, Ghani AC, Drakeley C. 2010. Revisiting the circulation time of Plasmodium falciparum gametocytes: molecular detection methods to estimate the duration of gametocyte carriage and the effect of gametocytocidal drugs. Malar. J. 9:136. 10.1186/1475-2875-9-136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Okell LC, Drakeley CJ, Bousema T, Whitty CJM, Ghani AC. 2008. Modelling the impact of artemisinin combination therapy and long-acting treatments on malaria transmission intensity. PLoS Med. 5:e226. 10.1371/journal.pmed.0050226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bousema JT, Schneider P, Gouagna LC, Drakeley CJ, Tostmann A, Houben R, Githure JI, Ord R, Sutherland CJ, Omar SA, Sauerwein RW. 2006. Moderate effect of artemisinin-based combination therapy on transmission of Plasmodium falciparum. J. Infect. Dis. 193:1151–1159 [DOI] [PubMed] [Google Scholar]
- 5. Chen PQ, Li GQ, Guo XB, He KR, Fu YX, Fu LC, Song YZ. 1994. The infectivity of gametocytes of Plasmodium falciparum from patients treated with artemisinin. Chin. Med. J. 107:709–711 [PubMed] [Google Scholar]
- 6. Drakeley CJ, Jawara M, Targett GAT, Walraven G, Obisike U, Coleman R, Pinder M, Sutherland CJ. 2004. Addition of artesunate to chloroquine for treatment of Plasmodium falciparum malaria in Gambian children causes a significant but short-lived reduction in infectiousness for mosquitoes. Trop. Med. Int. Health 9:53–61 [DOI] [PubMed] [Google Scholar]
- 7. Targett G, Drakeley C, Jawara M, Von Seidlein L, Coleman R, Deen J, Pinder M, Doherty T, Sutherland C, Walraven G, Milligan P. 2001. Artesunate reduces but does not prevent posttreatment transmission of Plasmodium falciparum to Anopheles gambiae. J. Infect. Dis. 183:1254–1259 [DOI] [PubMed] [Google Scholar]
- 8. Vale N, Moreira R, Gomes P. 2009. Primaquine revisited six decades after its discovery. Eur. J. Med. Chem. 44:937–953 [DOI] [PubMed] [Google Scholar]
- 9. Grewal RS. 1981. Pharmacology of 8-aminoquinolines. Bull. World Health Organ. 59:397–406 [PMC free article] [PubMed] [Google Scholar]
- 10. Burgess RW, Bray RS. 1961. The effect of a single dose of primaquine on the gametocytes, gametogony and sporogony of Laverania falciparum. Bull. World Health Organ. 24:451–456 [PMC free article] [PubMed] [Google Scholar]
- 11. Gunders AE. 1961. The effect of a single dose of pyrimethamine and primaquine in combination upon gametocytes and sporogony of Laverania falcipara (Plasmodium falciparum) in Liberia. Bull. World Health Organ. 24:650–653 [PMC free article] [PubMed] [Google Scholar]
- 12. White N. 2008. The role of anti-malarial drugs in eliminating malaria. Malar. J. 7(Suppl 1):S8. 10.1186/1475-2875-7-S1-S8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Secretariat MPAC. 2012. Inaugural meeting of the malaria policy advisory committee to the WHO: conclusions and recommendations. Malar. J. 11:137. 10.1186/1475-2875-11-137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mishra N, Singh J, Srivastava B, Arora U, Shah NK, Ghosh S, Bhatt R, Sharma S, Das M, Kumar A, Anvikar A, Kaitholia K, Gupta R, Sonal G, Dhariwal A, Valecha N. 2012. Monitoring antimalarial resistance in India via sentinel sites: outcomes and risk factors for treatment failure, 2009-2010. Bull. World Health Organ. 91:1–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. World Health Organization 2009. Methods for surveillance of antimalarial drug efficacy. World Health Organization, Geneva, Switzerland [Google Scholar]
- 16. Tjitra E, Suprianto S, Anstey NM. 2002. Higher gametocyte prevalence following failure of treatment of Plasmodium falciparum malaria with sulfadoxine-pyrimethamine and the combination of chloroquine plus sulfadoxine-pyrimethamine: implications for progression of anti-folate resistance. Trans. R. Soc. Trop. Med. Hyg. 96:434–437 [DOI] [PubMed] [Google Scholar]
- 17. Bousema T, Drakeley C. 2011. Epidemiology and infectivity of Plasmodium falciparum and Plasmodium vivax gametocytes in relation to malaria control and elimination. Clin. Microbiol. Rev. 24:377–410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sharma VP. 1999. Current scenario of malaria in India. Parassitologia 41:349–353 [PubMed] [Google Scholar]
- 19. Sonal GS, Thakor HG, Joshi C, Arora P, Gupta RKD, Dhariwal AC. 2010. Epidemiological status of malaria and scaling up of interventions in India. J. Indian Med. Assoc. 108:840–843 [PubMed] [Google Scholar]
- 20. Hertz-Picciotto I, Rockhill B. 1997. Validity and efficiency of approximation methods for tied survival times in Cox regression. Biometrics 53:1151–1156 [PubMed] [Google Scholar]
- 21. Méndez F, Muñoz Á, Plowe CV. 2006. Use of area under the curve to characterize transmission potential after antimalarial treatment. Am. J. Trop. Med. Hyg. 75:640–644 [PubMed] [Google Scholar]
- 22. Sowunmi A, Fateye BA. 2003. Plasmodium falciparum gametocytaemia in Nigerian children: before, during and after treatment with antimalarial drugs. Trop. Med. Int. Health 8:783–792 [DOI] [PubMed] [Google Scholar]
- 23. Sinclair D, Zani B, Donegan S, Olliaro P, Garner P. 2009. Artemisinin-based combination therapy for treating uncomplicated malaria. Cochrane Database Syst. Rev. 6:CD007483. 10.1002/14651858.CD008492.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Smithuis F, Kyaw MK, Phe O, Win T, Aung PP, Oo APP, Naing AL, Nyo MY, Myint NZH, Imwong M, Ashley E, Lee SJ, White NJ. 2010. Effectiveness of five artemisinin combination regimens with or without primaquine in uncomplicated falciparum malaria: an open-label randomised trial. Lancet Infect. Dis. 10:673–681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Shekalaghe S, Drakeley C, Gosling R, Ndaro A, Van Meegeren M, Enevold A, Alifrangis M, Mosha F, Sauerwein R, Bousema T. 2007. Primaquine clears submicroscopic Plasmodium falciparum gametocytes that persist after treatment with sulphadoxine-pyrimethamine and artesunate. PLoS One 2:e1023. 10.1371/journal.pone.0001023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Vásquez AM, Sanín F, Alvarez LG, Tobón A, Ríos A, Blair S. 2009. Therapeutic efficacy of a regimen of artesunate-mefloquine-primaquine treatment for Plasmodium falciparum malaria and treatment effects on gametocytic development. Biomedica 29:307–319 (In Spanish.) [PubMed] [Google Scholar]
- 27. Sutanto I, Suprijanto S, Kosasih A, Dahlan MS, Sjafruddin D, Kusriastuti R, Hawley WA, Lobo NF, ter Kuile FO. 2013. The effect of primaquine on gametocyte development and clearance in the treatment of uncomplicated falciparum malaria with dihydroartemisinin-piperaquine in South Sumatra, Western Indonesia: an open label randomized controlled trial. Clin. Infect. Dis. 56:685–693 [DOI] [PubMed] [Google Scholar]
- 28. Gogtay NJ, Chogle AR, Sorabjee JS, Marathe SN, Kshirsagar NA. 1999. Poor gametocytocidal activity of 45 mg primaquine in chloroquine-treated patients with acute, uncomplicated, Plasmodium falciparum malaria in Mumbai (Bombay): an issue of public-health importance. Ann. Trop. Med. Parasitol. 93:813–816 [DOI] [PubMed] [Google Scholar]
- 29. Kamtekar KD, Gogtay NJ, Dalvi SS, Karnad DR, Chogle AR, Aigal U, Kshirsagar NA. 2004. A prospective study evaluating the efficacy of a single, 45-mg dose of primaquine, as a gametocytocidal agent, in patients with Plasmodium falciparum malaria in Mumbai, India. Ann. Trop. Med. Parasitol. 98:453–458 [DOI] [PubMed] [Google Scholar]
- 30. Gogtay N, Kamtekar K, Dalvi S, Mehta S, Chogle A, Aigal U, Kshirsagar N. 2006. A randomized, parallel study of the safety and efficacy of 45 mg primaquine versus 75 mg bulaquine as gametocytocidal agents in adults with blood schizonticide-responsive uncomplicated falciparum malaria [ISCRTN50134587]. BMC Infect. Dis. 6:16. 10.1186/1471-2334-6-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Shekalaghe SA, Ter Braak R, Daou M, Kavishe R, Van den Bijllaardt W, Van den Bosch S, Koenderink JB, Luty AJF, Whitty CJM, Drakeley C, Sauerwein RW, Bousema T. 2010. In Tanzania, hemolysis after a single dose of primaquine coadministered with an artemisinin is not restricted to glucose-6-phosphate dehydrogenase-deficient (G6PD A−) individuals. Antimicrob. Agents Chemother. 054:01762–1768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Baird JK, Surjadjaja C. 2011. Consideration of ethics in primaquine therapy against malaria transmission. Trends Parasitol. 27:11–16 [DOI] [PubMed] [Google Scholar]
- 33. Congpuong K, Bualombai P, Banmairuroi V, Na-Bangchang K. 2010. Compliance with a three-day course of artesunate-mefloquine combination and baseline anti-malarial treatment in an area of Thailand with highly multidrug resistant falciparum malaria. Malar. J. 9:43. 10.1186/1475-2875-9-43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Eziefula A, Staedke S. 2011. Evaluation of the gametocytocidal efficacy and safety of primaquine in uncomplicated falciparum malaria in Uganda. NIH Clinical Trials Registry; http://clinicaltrials.gov/show/NCT01365598 [Google Scholar]
- 35. Malaria Policy Advisory Committee to the WHO 2012. Malaria Policy Advisory Committee to the WHO: conclusions and recommendations of September 2012 meeting. Malar. J. 11:424. 10.1186/1475-2875-11-424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. White NJ, Qiao LG, Qi G, Luzzatto L. 2012. Rationale for recommending a lower dose of primaquine as a Plasmodium falciparum gametocytocide in populations where G6PD deficiency is common. Malar. J. 11:418. 10.1186/1475-2875-11-418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Lawpoolsri S, Klein EY, Singhasivanon P, Yimsamran S, Thanyavanich N, Maneeboonyang W, Hungerford LL, Maguire JH, Smith DL. 2009. Optimally timing primaquine treatment to reduce Plasmodium falciparum transmission in low endemicity Thai-Myanmar border populations. Malar. J. 8:159. 10.1186/1475-2875-8-159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ouédraogo AL, Bousema T, De Vlas SJ, Cuzin-Ouattara N, Verhave Drakeley J-PC, Luty AJF, Sauerwein R. 2010. The plasticity of Plasmodium falciparum gametocytaemia in relation to age in Burkina Faso. Malar. J. 9:281. 10.1186/1475-2875-9-281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Schneider P, Bousema JT, Gouagna LC, Otieno S, Van De Vegte-Bolmer M, Omar SA, Sauerwein RW. 2007. Submicroscopic Plasmodium falciparum gametocyte densities frequently result in mosquito infection. Am. J. Trop. Med. Hyg. 76:470–474 [PubMed] [Google Scholar]
- 40. Ouédraogo AL, Bousema T, Schneider P, De Vlas SJ, Ilboudo-Sanogo E, Cuzin-Ouattara N, Nébié I, Roeffen W, Verhave JP, Luty AJF, Sauerwein R. 2009. Substantial contribution of submicroscopical Plasmodium falciparum gametocyte carriage to the infectious reservoir in an area of seasonal transmission. PLoS One 4:e8410. 10.1371/journal.pone.0008410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Localio AR, Berlin JA, Ten Have TR, Kimmel SE. 2001. Adjustments for center in multicenter studies: an overview. Ann. Intern. Med. 135:112–123 [DOI] [PubMed] [Google Scholar]
- 42. Awono-Ambene HP, Diawara L, Robert V. 2001. Comparison of direct and membrane feeding methods to infect Anopheles arabiensis with Plasmodium falciparum. Am. J. Trop. Med. Hyg. 64:32–34 [DOI] [PubMed] [Google Scholar]
- 43. White NJ. 2013. Primaquine to prevent transmission of falciparum malaria. Lancet Infect. Dis. 13:175–181 [DOI] [PubMed] [Google Scholar]