Abstract
Little is known about resistance of Plasmodium falciparum to antimalarials in Sahelian countries. Here we investigated the drug susceptibilities of fresh isolates collected in Niger post-deployment of artemisinin-based combination therapies (ACTs). We found that the parasites remained highly susceptible to new (dihydroartemisinin, lumefantrine, pyronaridine, and piperaquine) and conventional (amodiaquine and chloroquine) antimalarial drugs. The introduction of ACTs in 2005 and their further deployment nationwide have therefore not resulted in a decrease in P. falciparum susceptibilities to these antimalarials.
TEXT
The WHO estimates that 50% of the world's population is exposed to malaria. In 2010, 216 million cases and more than 650,000 deaths from malaria were reported. Despite a decrease in the number of confirmed cases in some parts of the world, the situation remains heterogeneous and worrying in Africa (1). Together with respiratory infections and diarrheal diseases, malaria is one of the leading causes of death in Niger. Malaria transmission rates are high, with a mean incidence of 80 cases per 1,000 inhabitants. Despite the complementary nature of interventions in the field, which have strengthened in recent years, the number of cases has steadily risen over the last 20 years, reaching three million in 2010 (2, 3). There are several reasons for the deterioration of the public health situation with regard to malaria, and the increase in resistance to antimalarial drugs is considered a key factor. In Niger, infections are currently treated with combinations of drugs including an artemisinin (ART) derivative (artemisinin-based combination treatment, or ACT) (4). Since 2005, ACT has been proposed as the first-line treatment for the management of uncomplicated malaria. The use of such treatments throughout Niger was greatly expanded in 2010 by the implementation of a Global Fund Affordable Malaria Facility mechanism (5). Thereby, the drug pressure exerted on Plasmodium falciparum increases the risk of selection of parasites with altered susceptibility to antimalarials. This seems to be inevitable, as demonstrated by recent observations in Asia, which have revealed the presence of parasites less sensitive to artemisinins (6). The risk of emerging resistance to ACT makes it necessary to monitor the susceptibilities of parasites to antimalarial drugs, particularly those used in combination with artemisinin derivatives, on a regular basis and to search for new molecules with antimalarial activity.
In this context, a study was carried out in Niger in 2011, at Gaya in the Dosso region, 250 km south of Niamey (3.44°N, 11.9°E), to evaluate the response of P. falciparum isolates to lumefantrine (LUM) and amodiaquine diphosphate (AQ), both of which are widely used in the ACTs distributed in Niger. In addition, responses to alternative molecules, such as pyronaridine (PYD) and piperaquine (PIP), both currently not available in Niger, as well as dihydroartemisinin (DHA) and chloroquine (CQ), were investigated. P. falciparum isolates were obtained from 89 symptomatic children (<5 years old) with uncomplicated P. falciparum malaria (50.8% girls and 49.2% boys). The study protocol was approved by the National Ethics Committee for Health Research of the Nigerien Ministry of Health. The susceptibilities of the Nigerien isolates to drugs were assessed in a classical isotopic (48-h) test (7). Each isolate (5% hematocrit and 0.1 to 1% parasitemia) was tested in duplicate in microplates that were precoated with serial dilutions of drugs. Parasite growth was assessed by measuring the incorporation of [3H]hypoxanthine (0.5 μCi/well), as previously described (8). The results of the in vitro assay are expressed as the 50% inhibitory concentration (IC50) or geometric mean IC50 (GMIC50). The predosed plates were prepared monthly and stored at −20°C until use. Their suitability for in vitro testing was regularly monitored using the reference strains 3D7 Africa and W2 Indochina, maintained in continuous culture and presenting known responses to the various drugs tested. Mean IC50s were 1.8 to 2.4 nmol · liter−1 for DHA, 5.6 to 20 nmol · liter−1 for LUM, 17.4 to 43.1 nmol · liter−1 for AQ, 6.3 to 76.1 nmol · liter−1 for PYD, 10.5 to 33.0 nmol · liter−1 for PIP, and 11.4 to 230 nmol · liter−1 for CQ, respectively, for the 3D7 and W2 parasites. The cutoff IC50s for in vitro resistance to CQ, DHA, LUM, and AQ were 100, 10.5, 150, and 60 nmol · liter−1, respectively (9, 10). These thresholds allow classification of isolates as resistant or sensitive, and variations in antimalarial susceptibilities over time and between areas of endemicity can be followed. They were established using reference strains and clinical isolates. Since the cutoff IC50s for PYR and PIP have not been determined, the threshold for reduced in vitro susceptibility to these drugs was defined statistically (>2 standard deviations [SD] above the mean).
The mean (95% confidence interval [CI]) age (in months) of the patients included in the in vitro study was 58.8(50.6 to 67). The geometric mean (95% CI) parasitemia (percent infected red blood cells) at admission was 2.9 (0.5 to 5.3). Results were interpretable for at least one of the drugs tested for 37 (41.6%) of the 89 fresh isolates tested. The low yield for the in vitro assay observed for 52 isolates may have been due to suboptimal storage and transportation conditions of parasitized blood or to the presence of traces of the drug in the samples, rather than limitations of the technical approach.
The in vitro responses obtained with samples from Niger are shown in Table 1. All isolates reacted in a satisfactory manner to DHA (GMIC50 of 2.9; 95% CI, 2.2 to 3.5), with an IC50 of <10.5. Our data can be compared with those found earlier for clinical isolates collected in Cameroon and in other African countries in 1992 and 2002, before ACTs were deployed: the GMIC50 values reported are 1.29 nM · liter−1 for DHA and 3.46 to 5.66 nM · liter−1 for various artemisinin derivatives (arteether, artemether, and artelinate) (11, 12). Thus, after several years of extensive use of artesunate plus amodiaquine and of artemether plus lumefantrine as first-line treatments for P. falciparum malaria in Niger, there is no evidence of a decline in sensitivity to artemisinin. Our data are similar to those reported in Senegal and Congo and are consistent with findings of studies indicating a lack of decrease in sensitivity to artesunate in Gabon, Senegal, and Djibouti despite the extensive and growing use of ACT in these regions (12–18). A decrease in parasite susceptibility to artemisinin derivatives has been reported to date only in Southeast Asia along the border between Thailand and Cambodia. In this region, resistance is characterized by a decrease in susceptibility to both artemisinins and ACTs and by delayed parasite clearance times (6, 19). However, since decreases in sensitivity to ACT in vivo are difficult to measure in vitro, caution is necessary when trying to determine the clinical significance of the in vitro responses of parasites to artemisinin derivatives (20). The P. falciparum ATPase6 (PfATPase6) S769N mutation, which has been described as a potential molecular marker for P. falciparum resistance to artemisinin in South America, was not observed in 92 samples collected in Niger in 2003 and 2006 (21, 22).
Table 1.
In vitro susceptibilities of fresh Nigerien isolates of Plasmodium falciparum to new and conventional antimalarial drugsa
| Antimalarial | No. of isolates tested | GMIC50 (nM) | 95% CI | IC50 |
Q1 25% | Q3 75% | Cut-off (nM) | R/S | % R | |
|---|---|---|---|---|---|---|---|---|---|---|
| Min | Max | |||||||||
| DHA | 37 | 2.9 | 2.2–3.5 | 0.7 | 7.3 | 1.8 | 4.6 | 10.5 | 0/37 | 0 |
| LUM | 33 | 7.1 | 3.9–10.3 | 0.2 | 44.3 | 3.9 | 14.7 | 150 | 2/33 | 0 |
| PIP | 34 | 24.2 | 0.3–48.0 | 2.5 | 311.5 | 12 | 59.6 | 150 | 2/34 | 6 |
| CQ | 29 | 12.7 | 1.9–23.4 | 2.1 | 144.7 | 8.6 | 18.9 | 100 | 2/29 | 7 |
| AQ | 26 | 17.3 | 11.9–22.6 | 6.9 | 64.2 | 11.3 | 24.6 | 60 | 1/26 | 4 |
| PYD | 34 | 9.8 | 7.7–11.9 | 4.7 | 32.9 | 6.5 | 14.3 | 20 | 1/34 | 3 |
Dihydroartemisinin (DHA) (D7439), pyronaridine (PYD) (P0049), chloroquine (CQ) (C6628), and ammodiaquine diphosphate (AQ) (A2799) were obtained from Sigma Chemicals. Piperaquine (PIP) was obtained from Sigma Tau, and lumefantrine (LUM) was obtained from Novartis. The final concentrations ranged from 0.02 to 200 nM for DHA, from 1.95 to 2,000 nM for AQ and PIP, from 2.44 to 2,500 nM for CQ, and from 4.88 to 5,000 nM for PYD. GMIC50, geometric mean IC50; Min, minimum; Max, maximum; Q1 25%, 1st quartile; Q2 75%, 3rd quartile; R/S, no. resistant/no. susceptible (according to the in vitro cutoff); % R, percent resistant.
The ACT concept is based on the use of a combination of an artemisinin derivative and another antimalarial drug with a different mode of action and a slower clearance rate. Despite the danger signs concerning the emergence of resistance along the Thai-Cambodian border, mutual drug protection seems to have been successfully achieved in areas of higher endemicity, particularly in Africa, where in vivo and in vitro monitoring over the last decade has revealed no evidence of a decrease in the susceptibility of parasites to ACT (4, 23). The success of ACT depends almost entirely on the efficacy of the partner molecule. Our data show that isolates from Niger have remained highly susceptible to current partner drugs, with IC50s ranging from 0.2 to 44.3 nmol · liter−1 (GMIC50, 7.1 nmol · liter−1; 95% CI, 3.9 to 10.3 nmol · liter−1) and from 6.9 to 64.2 nmol · liter−1 (GMIC50, 17.3 nmol · liter−1; 95% CI, 11.9 to 22.6 nmol · liter−1) for LUM and AQ, respectively. These values are lower than those reported in Cameroon a decade ago, where the GMIC50 for LUM was 11.9 nm · liter−1 and that for AQ was 29.0 nm · liter−1 (12). The IC50 of LUM was <150 nmol · liter−1 for all isolates. The IC50 of AQ was <60 nmol · liter−1in all but one isolate (64.2 nmol · liter−1). This finding is of particular concern, because the use of AQ-containing combinations has been shown to select for infections that are less responsive to AQ in Uganda (24). However, no clear relationship was observed between the treatment response and the in vitro IC50 of AQ in Gabon, and selection of this type does not seem to be occurring in Niger, while resistances to AQ have already been reported in some other African countries (25, 26). Similarly, no sign of a decrease in susceptibility to LUM has been detected. This observation is consistent with earlier molecular data showing the prevalence of mutations of P. falciparum mdr1 (Pfmdr1) sequences to be low in isolates from Niger, particularly for the Y86N mutation, which is thought to confer a selective advantage on parasites, rendering them tolerant to LUM (22, 27). PYD and PIP have been developed as alternative antimalarial drugs for inclusion in ART-containing combinations for the treatment of multidrug-resistant malaria. PIP is a 4-aminoquinoline that has been in clinical use in China for decades, but few data have been published addressing its activity against African isolates in vitro. PYD, which was first synthesized in China in the 1970s, is a Mannich base structurally related to AQ. It has potent in vitro activity against erythrocyte stages of P. falciparum and is effective against CQ-resistant isolates (28). The IC50s for PYD ranged from 4.7 to 32.9 nmol · liter−1 (GMIC50, 9.8 nmol · liter−1; 95% CI, 7.7 to 11.9 nmol · liter−1). The range of IC50s for PIP was broader, extending from 2.5 to 311.5 nmol · liter−1 (GMIC50, 24.2 nmol · liter−1; 95% CI, 0.3 to 48.0 nmol · liter−1). The IC50s of PIP were <150 nmol · liter−1 for all but two isolates (279.9 and 311.5 nmol · liter−1), whereas those of PYD were <20 nmol · liter−1 in all but one isolate (32.9 nmol · liter−1). These data demonstrate that the ACT components PIP and PYD have excellent antimalarial activity against Nigerien isolates. These activities are broadly similar to those reported in Africa (12–14, 18). In Niger, the first cases of CQ resistance in vitro were reported at the end of the 1980s. These data are ancient and concern only a small number of isolates. The few clinical trials carried out since then have confirmed the circulation of parasites resistant to this antimalarial drug but with an exceptionally low treatment failure rate, i.e., below 16% (29, 30). Consistent with these data, most of the Nigerien samples examined in the present study were found to respond adequately to CQ in vitro (CQ susceptible [CQS], 93%), with IC50s ranging from 2.1 to 144.7 nmol · liter−1 (GMIC50, 12.7 nmol · liter−1; 95% CI, 1.9 to 23.4 nmol · liter−1). Only two samples had IC50s of >100 (120 and 144.7 nmol · liter−1, respectively). There is a positive correlation between polymorphism at position 76 in the P. falciparum crt (Pfcrt) gene and the CQ-resistant (CQR) in vitro response. We therefore assessed the prevalence of the mutant 76T Pfcrt allele by PCR-restriction fragment length polymorphism (RFLP) (2 DNA samples were not available for typing) (31). Briefly, crt sequences (280-bp fragment) were amplified by conventional PCR with sense (5′CGACCTTAACAGATGGCTCA3′) and antisense (5′TTTGAATTTCCCTTTTTA TTTCCA3′) primers and digested with ApoI. Overall (Fig. 1), 23 samples harboring a wild-type K76 Pfcrt allele had IC50s between 2.1 and 38.2 nmol · liter−1 (GMIC50, 10.9 nmol · liter−1; 95% CI, 7.5 to 14.3 nmol · liter−1), whereas three others with a mutant 76T Pfcrt allele had significantly higher IC50s (Mann-Whitney rank sum test, P = 0.005), ranging from 83.4 to 144.7 nmol · liter−1 (GMIC50, 113.1 nmol · liter−1; 95% CI, 78.3 to 147.9 nmol · liter−1). One isolate (no. 147) with a mixed restriction pattern had an IC50 of 24.4 nmol · liter−1. All CQS isolates other than no. 59 had wild-type Pfcrt K76; isolate no. 59 had an IC50 of 83 nmol · liter−1. The two CQR isolates had a mutated Pfcrt 76T. Taken together, the in vitro and molecular data indicate a high level of susceptibility of Nigerien isolates to CQ. This observation must be viewed in light of the results of in vivo tests conducted in Niger a decade ago, for which a treatment success rate of about 85% was reported for CQ. The trend in 2011 (11% of isolates presenting a mutated codon 76) is therefore distinctly different from those reported for molecular studies carried out between 2003 and 2007 in Niger, in which the prevalence of Pfcrt 76T was reported to be between 32.4% and 50.8% (22, 32). However, given the limited number of isolates examined and the short time interval between these studies, it is too soon to draw any firm conclusions about a shift toward an improved susceptibility of parasites to CQ in Niger.
Fig 1.
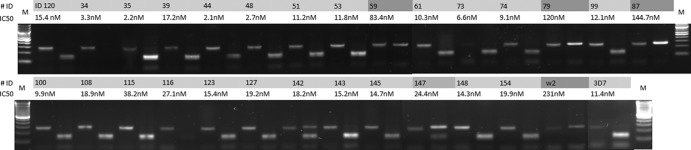
ApoI restriction digestion of Pfcrt fragments and genotype distribution among Nigerien isolates. Sample identification numbers (#ID) and the IC50 of CQ are indicated. For each sample, the first and second lanes correspond to undigested and ApoI-digested PCR products, respectively. M, SmartLadder SF (Eurogentec).
The degree of in vitro cross-reactivity in drug responses was assessed by pairwise correlation analyses of IC50s. A significant, positive association between CQ IC50s in vitro and those of PYD and DHA and between the responses to AQ and PYD was detected. No significant correlation was found between the IC50s of the other antimalarial drugs (Table 2). However, the positive correlations observed are very weak (r2 value between 0.36 and 0.5) and may suggest a shared mode of “resistance” to antimalarials rather than a common mechanism of action of the drugs. Consistent with this interpretation, studies with mammalian cells have shown that changes in mdr gene expression in response to toxic stress may have pleiotropic consequences, affecting the sensitivity to several drugs simultaneously.
Table 2.
Correlation coefficients for in vitro antimalarial drug susceptibilities of Nigerien isolates
| Drugs compared | No. of isolates | Correlation of responses |
|
|---|---|---|---|
| r value | P value | ||
| LUM and AQ | 20 | 0.006 | 0.97 |
| CQ and PIP | 27 | 0.051 | 0.8 |
| LUM and CQ | 22 | 0.08 | 0.72 |
| DHA and LUM | 24 | 0.081 | 0.7 |
| DHA and PIP | 27 | 0.133 | 0.57 |
| PIP and PYD | 25 | 0.166 | 0.43 |
| LUM and PYD | 25 | 0.202 | 0.33 |
| DHA and PYD | 27 | 0.246 | 0.22 |
| LUM and PIP | 20 | 0.254 | 0.28 |
| AQ and PIP | 24 | 0.285 | 0.18 |
| DHA and AQ | 22 | 0.304 | 0.17 |
| AQ and CQ | 23 | 0.358 | 0.09 |
| DHA and CQ | 24 | 0.415 | 0.04 |
| CQ and PYD | 25 | 0.429 | 0.03 |
| AQ and PYD | 23 | 0.496 | 0.02 |
An inverse relationship between the levels of malaria transmission and resistance was found, and the highly diverse epidemiological contexts of the various collection sites undoubtedly influence the results of resistance studies (33). The Gaya site, on the border with Benin, has one of the highest levels of malaria endemicity in Niger (34). This region belongs to the Sudanese zone, which is the most highly populated, and may not be representative of northern regions of Niger in terms of malaria endemicity. Nevertheless, our results indicate that the parasites of this part of Africa have retained a high degree of susceptibility to the new therapeutic combinations and, unexpectedly, to CQ. These observations are reassuring, because Niger has adopted ACTs as the first-line treatment for malaria, and AQ and LUM are the leading partner drugs used in ACTs in this region. These data support the continuing collaboration of the health authorities of Niger with the Global Fund and the strengthening of the AMFm (Affordable Medicines Facility—malaria) system established in this country in 2010 with the aim of making ACTs more accessible to the population (5), yet no official decision has been announced about whether and how to continue the program (35). Further investigations are required to confirm these preliminary observations, particularly in vivo using the WHO therapeutic efficacy test; indeed, it is necessary to check that the global trend observed by in vitro testing applies to in vivo conditions and to extend monitoring to northern sentinel sites. This will make it possible to take into account the possible effects of the malaria transmission level on both the nature and prevalence distribution of resistance to antimalarial drugs in Niger.
ACKNOWLEDGMENTS
This study was supported by the Global Fund (AMFm Phase 1, Niger) and by the Rotary Foundation (ACTION PALU Global Grant 25008).
We thank Sigma Tau and Novartis for supplying piperaquine and lumefantrine, respectively. We are grateful to Ibrahim Maman Laminou for his help in sample collection.
Footnotes
Published ahead of print 22 April 2013
REFERENCES
- 1. WHO 2010. World Malaria report. WHO, Geneva, Switzerland: http://www.who.int/malaria/world_malaria_report_2010/worldmalariareport2010.pdf [Google Scholar]
- 2. United Nations Development Programme 2007. Human development report/2008. Fighting climate change: human solidarity in a divided world. United Nations Development Programme, New York, NY: http://hdr.undp.org/en/media/HDR_20072008_EN_Complete.pdf [Google Scholar]
- 3. WHO 2011. World health statistics. WHO, Geneva, Switzerland: http://www.who.int/gho/publications/world_health_statistics/EN_WHS2011_Full.pdf [Google Scholar]
- 4. Eastman RT, Fidock DA. 2009. Artemisinin-based combination therapies: a vital tool in efforts to eliminate malaria. Nat. Rev. Microbiol. 7:864–874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. AMFm Task Force 2007. Affordable medicines facility—malaria (AMFm). Roll Back Malaria Partnership, Geneva, Switzerland: http://www.rollbackmalaria.org/partnership/tf/globalsubsidy/AMFmTechProposal.pdf [Google Scholar]
- 6. Dondorp AM, Nosten F, Yi P, Das D, Phyo AP, Tarning J, Lwin KM, Ariey F, Hanpithakpong W, Lee SJ, Ringwald P, Silamut K, Imwong M, Chotivanich K, Lim P, Herdman T, An SS, Yeung S, Singhasivanon P, Day NP, Lindegardh N, Socheat D, White NJ. 2009. Artemisinin resistance in Plasmodium falciparum malaria. N. Engl. J. Med. 361:455–467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Desjardins RE, Canfield CJ, Haynes JD, Chulay JD. 1979. Quantitative assessment of antimalarial activity in vitro by a semiautomated microdilution technique. Antimicrob. Agents Chemother. 16:710–718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lim P, Chim P, Sem R, Nemh S, Poravuth Y, Lim C, Seila S, Tsuyuoka R, Denis MB, Socheat D, Fandeur T. 2005. In vitro monitoring of Plasmodium falciparum susceptibility to artesunate, mefloquine, quinine and chloroquine in Cambodia: 2001–2002. Acta Trop. 93:31–40 [DOI] [PubMed] [Google Scholar]
- 9. Basco LK, Bickii J, Ringwald P. 1998. In vitro activity of lumefantrine (Benflumetol) against clinical isolates of Plasmodium falciparum in Yaounde, Cameroon. Antimicrob. Agents Chemother. 42:2347–2351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Pradines B, Hovette P, Fusai T, Atanda HL, Baret E, Cheval P, Mosnier J, Callec A, Cren J, Amalvict R, Gardair JP, Rogier C. 2006. Prevalence of in vitro resistance to eleven standard or new antimalarial drugs among plasmodium falciparum isolates from Pointe-Noire, Republic of the Congo. J. Clin. Microbiol. 44:2404–2408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Basco LK, Le Bras J. 1993. In vitro activity of artemisinin derivatives against African isolates and clones of Plasmodium falciparum. Am. J. Trop. Med. Hyg. 49:301–307 [DOI] [PubMed] [Google Scholar]
- 12. Basco LK, Ringwald P. 2003. In vitro activities of piperaquine and other 4-aminoquinolines against clinical isolates of Plasmodium falciparum in Cameroon. Antimicrob. Agents Chemother. 47:1391–1394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Pascual A, Parola P, Benoit-Vical F, Simon F, Malvy D, Picot S, Delaunay P, Basset D, Maubon D, Faugère B, Ménard G, Bourgeois N, Oeuvray C, Didillon E, Rogier C, Pradines B. 2012. Ex vivo activity of the ACT new components pyronaridine and piperaquine in comparison with conventional ACT drugs against isolates of Plasmodium falciparum. Malar. J. 11:45. 10.1186/1475-2875-11-45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Briolant S, Henry M, Oeuvray C, Amalvict R, Baret RE, Didillon E, Rogier C, Pradines B. 2010. Absence of association between piperaquine in vitro responses and polymorphisms in the pfcrt, pfmdr1, pfmrp, and pfnhe genes in Plasmodium falciparum. Antimicrob.Agents Chemother. 54:3537–3544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Nsobya SL, Kiggundu M, Nanyunja S, Joloba M, Greenhouse B, Rosenthal PJ. 2010. In vitro sensitivities of Plasmodium falciparum to different antimalarial drugs in Uganda. Antimicrob. Agents Chemother. 54:1200–1206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Fall B, Diawara S, Sow K, Baret E, Diatta B, Fall KB, Mbaye PS, Fall F, Diémé Y, Rogier C, Wade B, Bercion R, Pradines B. 2011. Ex vivo susceptibility of Plasmodium falciparum isolates from Dakar, Senegal, to seven standard anti-malarial drugs. Malar. J. 10:310. 10.1186/1475-2875-10-310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kremsner PG, Taylor T, Issifou S, Kombila M, Chimalizeni Y, Kawaza K, Bouyou Akotet MK, Duscha M, Mordmüller B, Kösters K, Humberg A, Miller RS, Weina P, Duparc S, Möhrle J, Kun JF, Planche T, Teja-Isavadharm P, Simpson JA, Köhler C, Krishna S. 2012. A simplified intravenous artesunate regimen for severe malaria. J. Infect. Dis. 205:312–319 [DOI] [PubMed] [Google Scholar]
- 18. Mwai L, Kiara SM, Abdirahman A, Pole L, Rippert A, Diriye A, Bull P, Marsh K, Borrmann S, Nzila A. 2009. In vitro activities of piperaquine, lumefantrine, and dihydroartemisinin in Kenyan Plasmodium falciparum isolates and polymorphisms in pfcrt and pfmdr1. Antimicrob. Agents Chemother. 53:5069–5073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Phyo AP, Nkhoma S, Stepniewska K, Ashley EA, Nair S, McGready R, ler Moo C, Al-Saai S, Dondorp AM, Lwin KM, Singhasivanon P, Day NP, White NJ, Anderson TJ, Nosten F. 2012. Emergence of artemisinin-resistant malaria on the western border of Thailand: a longitudinal study. Lancet 379:1960–1966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Dondorp AM, Fairhurst RM, Slutsker L, Macarthur JR, Breman JG, Guerin PJ, Wellems TE, Ringwald P, Newman RD, Plowe CV. 2011. The threat of artemisinin-resistant malaria. N. Engl. J. Med. 365:1073–1075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Jambou R, Legrand E, Niang M, Khim N, Lim P, Volney B, Ekala MT, Bouchier C, Esterre P, Fandeur T, Mercereau-Puijalon O. 2005. Resistance of Plasmodium falciparum field isolates to in-vitro artemether and point mutations of the SERCA-type PfATPase6. Lancet 366:1960–1963 [DOI] [PubMed] [Google Scholar]
- 22. Ibrahim ML, Steenkeste N, Khim N, Adam HH, Konaté L, Coppée JY, Ariey F, Duchemin JB. 2009. Field-based evidence of fast and global increase of Plasmodium falciparum drug-resistance by DNA-microarrays and PCR/RFLP in Niger. Malar. J. 8:32. 10.1186/1475-2875-8-32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. WHO 2000. Global report on antimalarial efficacy and drug resistance: 2010. WHO, Geneva, Switzerland: whqlibdoc.who.int/publications/2010/9789241500470_eng.pdf [Google Scholar]
- 24. Nawaz F, Nsobya SL, Kiggundu M, Joloba M, Rosenthal PJ. 2009. Selection of parasites with diminished drug susceptibility by amodiaquine-containing antimalarial regimens in Uganda. J. Infect. Dis. 200:1650–1657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Aubouy A, Mayombo J, Keundjian A, Bakary M, Le Bras J, Deloron P. 2004. Lack of prediction of amodiaquine efficacy in treating Plasmodium falciparum malaria by in vitro tests. Am. J. Trop. Med. Hyg. 713:294–296 [PubMed] [Google Scholar]
- 26. Folarin OA, Bustamante C, Gbotosho GO, Sowunmi A, Zalis MG, Oduola AM, Happi CT. 2011. In vitro amodiaquine resistance and its association with mutations in pfcrt and pfmdr1 genes of Plasmodium falciparum isolates from Nigeria. Acta Trop. 120:224–230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sisowath C, Strömberg J, Mårtensson A, Msellem M, Obondo C, Björkman A, Gil JP. 2005. In vivo selection of Plasmodium falciparum pfmdr1 86N coding alleles by artemether-lumefantrine (Coartem). J. Infect. Dis. 191:1014–1017 [DOI] [PubMed] [Google Scholar]
- 28. Nosten F, White NJ. 2007. Artemisinin-based combination treatment of falciparum malaria. Am. J. Trop. Med. Hyg. 77:181–192 [PubMed] [Google Scholar]
- 29. Parola P, Ali I, Djermakoye F, Crassard N, Bendavid C, Faugère B, Condomines P. 1999. Chloroquine sensitivity of Plasmodium falciparum at the Gamkalley Clinic and the Nigerian armed forces PMI (Niamey, Niger). Bull. Soc. Pathol. Exot. 92:317–319 (In French.) [PubMed] [Google Scholar]
- 30. Dugelay F, Adehossi E, Adamou S, Ousmane I, Parzy D, Delmont J, Parola P. 2003. Efficacy of chloroquine in the treatment of uncomplicated, Plasmodium falciparum malaria in Niamey, Niger, in 2001. Ann. Trop. Med. Parasitol. 97:83–86 [DOI] [PubMed] [Google Scholar]
- 31. Fidock DA, Nomura T, Talley AK, Cooper RA, Dzekunov SM, Ferdig MT, Ursos LM, Sidhu AB, Naudé B, Deitsch KW, Su XZ, Wootton JC, Roepe PD, Wellems TE. 2000. Mutations in the P. falciparum digestive vacuole transmembrane protein PfCRT and evidence for their role in chloroquine resistance. Mol. Cell 6:861–871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ibrahim ML, Gay-Andrieu F, Adehossi E, Lacroix V, Randrianarivelojosia M, Duchemin JB. 2007. Field-based evidence for the linkage of pfcrt and pfdhfr drug-resistant malaria genotypes and clinical profiles of severe malaria in Niger. Microbes Infect. 9:599–604 [DOI] [PubMed] [Google Scholar]
- 33. Ariey F, Robert V. 2003. The puzzling links between malaria transmission and drug resistance. Trends Parasitol. 19:158–160 [DOI] [PubMed] [Google Scholar]
- 34. Doudou MH, Mahamadou A, Ouba I, Lazoumar R, Boubacar B, Arzika I, Zamanka H, Ibrahim ML, Labbo R, Maiguizo S, Girond F, Guillebaud J, Maazou A, Fandeur T. 2012. A refined estimate of the malaria burden in Niger. Malar. J. 11:89. 10.1186/1475-2875-11-89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Maxmen A. 2012. Malaria plan under scrutiny. Nature 490:13–14 [DOI] [PubMed] [Google Scholar]


