Abstract
We report the findings of a study examining the relationship between in vitro daptomycin-rifampin synergy and the therapeutic outcome of 12 patients with complex deep methicillin-resistant Staphylococcus aureus (MRSA) infections treated for prolonged periods with this combination. Checkerboard synergy was found in nine cases and was 100% predictive of therapeutic success; absence of synergy was found in three cases, two of which were therapeutic failures (P = 0.045). No relationship was observed between synergy and outcome by time-kill assessment. Checkerboard synergy may predict clinical response to daptomycin plus rifampin for complex invasive MRSA infections requiring prolonged treatment.
TEXT
Daptomycin is being increasingly used for the treatment of complicated Gram-positive infections, such as osteomyelitis and infections of prosthetic devices caused by methicillin-resistant Staphylococcus aureus (MRSA) (1, 2), but the evidence for the effectiveness of combination regimens with daptomycin has been limited. In cases of primary treatment failure with S. aureus infections, rifampin has been recommended as an adjunctive therapeutic agent (3). Studies with in vitro and in vivo pharmacodynamic models of biofilm-associated infection support the use of daptomycin in combination with rifampin, where synergistic activity has been associated primarily with preventing the emergence of daptomycin-nonsusceptible strains (4–6). We have recently reported a high rate of success with daptomycin in combination with rifampin for treatment of complicated bone and joint infections (7), and in this study, we report the relationship between treatment outcome and in vitro synergy with complex deep MRSA infections.
Patients with invasive MRSA infections treated with daptomycin plus rifampin between 2005 and 2008 at the University of Wisconsin Hospital and Clinics in Madison, WI, were retrospectively reviewed. Clinical and microbiologic treatment outcomes were determined using standard definitions for osteomyelitis and nonosteomyelitis infections at clinical endpoints of 1 year and 4 weeks after discontinuation of therapy, respectively (1, 8), and compared to the results of in vitro synergy testing of the causative strain.
The MRSA isolates from 12 patients treated with the study combination were retrieved from the hospital clinical microbiology laboratory and analyzed. Daptomycin (Cubist, Lexington, MA) and rifampin (Sigma-Aldrich, St. Louis, MO) susceptibility testing was performed by broth microdilution on all isolates (9). Antibiotic susceptibility in biofilm was also determined since most patients had infection sources that commonly involve biofilm formation. The MIC and minimum biofilm eradication concentration (MBEC) were determined using a previously described transferable solid-phase pin-lid method (10). Synergy testing was evaluated by two methods: (i) checkerboard analysis with standard definitions of fractional inhibitory concentrations (FIC) (synergy, FIC ≤ 0.5; indifference, FIC of >0.5 but ≤4; antagonism, FIC > 4) (11); (ii) 24-hour time-kill studies, with concentrations of daptomycin that are 0.5 to 4 times the organism MIC alone and concentrations of rifampin that are 0.5 times the MIC (synergy, ≥2 logs kill; additivity, 1 to 2 logs kill; indifference, 1 log kill to 1 log growth; antagonism, ≥1 log growth) (12). In this fashion, at least one of the antibiotics (rifampin) was present in a concentration with a minimal effect on growth when used alone in order to assess its interaction with daptomycin. All synergy studies were performed in duplicate. The relationship between in vitro synergy and clinical success was analyzed by Fisher's exact test using GraphPad Prism software (GraphPad, La Jolla, CA).
As expected, the MRSA infections in these patients were diverse and included five cases of refractory osteomyelitis, two infected postoperative surgical wounds, and one case each of prosthetic valve endocarditis, septic arthritis, a deep body cavity abscess, an infected prosthesis, and an infected hemodialysis catheter with bacteremia. Clinical cure (resolution of all signs and symptoms and no additional antibiotic therapy needed) or improvement (partial resolution of signs and symptoms) was documented in 10 of the 12 patients (83%). Only two patients failed with daptomycin-plus-rifampin therapy (Table 1).
Table 1.
Patient and isolate characteristics of cases treated with daptomycin plus rifampin for invasive methicillin-resistant Staphylococcus aureus infectionsa
| Subject no. | Age and sex | Comorbidity(ies) | Infection type | MICs (μg/ml) | DAP (mg/kg of body wt) + RIF (mg) therapy | Clinical outcome | Synergy analysis |
|
|---|---|---|---|---|---|---|---|---|
| Checkerboardb | Time-killc | |||||||
| 1 | 66, F | DM, HTN | Osteomyelitis | DAP, 0.13; RIF, 0.03 | 4 mg/kg DAP q24h for 135 days; 600 mg RIF p.o. QD for 175 days | Cure | S | S |
| 2 | 59, M | CKD, HTN, obesity | Osteomyelitis | DAP, 0.13; RIF, 0.03 | 6 mg/kg DAP q48h for 84 days; 600 mg RIF p.o. QD for 23 days | Cure | S | S |
| 3 | 47, M | HTN, SLE, obesity, MRSA colonization | Postoperative wound | DAP, 0.13; RIF, 0.06 | 6 mg/kg DAP q48h for 90 days; 300 mg RIF p.o. q24h for 14 days | Cure | S | Add |
| 4 | 31, M | ICU stay, ESRD HD, MRSA colonization | Endovascular/HD line | DAP, 0.25; RIF, 0.06 | 8 mg/kg DAP q48h for 90 days; 600 mg RIF p.o. q24h for 85 days | Cure | S | S |
| 5 | 30, M | DM | Osteomyelitis | DAP, 0.25; RIF, 0.03 | 4 mg/kg DAP q24h for 46 days; 300 mg RIF p.o. q12h for 43 days | Improvement | I | I |
| 6 | 22, M | Obesity | Septic arthritis | DAP, 0.13; RIF, 0.03 | 4 mg/kg DAP q24h for 68 days; 600 mg RIF p.o. q24h for 15 days | Cure | S | S |
| 7 | 42, M | DM, CKD with transplant | Osteomyelitis | DAP, 0.13; RIF, 0.06 | 4 mg/kg DAP q24h for 42 days; 600 mg RIF p.o. q24h for 6 days | Cure | S | S |
| 8 | 53, F | HTN | Joint prosthesis | DAP, 0.25; RIF, 0.03 | 4 mg/kg DAP q24h for 68 days; 600 mg RIF p.o. q24h for 39 days | Failure | I | Ant |
| 9 | 53, F | ESRD HD, DM, HTN, obesity | Osteomyelitis | DAP, 0.25; RIF, 0.06 | 6 mg/kg DAP q48h for 43 days; 600 mg RIF p.o. q24h for 39 days | Cure | S | S |
| 10 | 76, M | ESRD HD, DM, COPD, HTN | Postoperative wound | DAP, 1; RIF, 8 | 6 mg/kg DAP q48h for 43 days; 300 mg RIF p.o. q24h for 43 days | Cure | S | I |
| 11 | 50, M | ICU stay, obesity | Prosthetic valve endocarditis | DAP, 0.25; RIF, 0.06 | 6 mg/kg DAP q24h for 100 days; 600 mg RIF p.o. q24h for 115 days | Cure | S | I |
| 12 | 47, F | ICU stay | Deep abscess | DAP, 0.25; RIF, 0.03 | 6 mg/kg DAP q24h for 13 days; 600 mg RIF p.o. q12h for 83 days | Failure | I | I |
F, female; M, male; DM, diabetes mellitus; HTN, hypertension; CKD, chronic kidney disease; SLE, systemic lupus erythematosus; ICU, intensive care unit; ESRD, end-stage renal disease; HD, hemodialysis; COPD, chronic obstructive pulmonary disease; p.o., orally; q24h, every 24 h; QD, once daily; S, synergy; I, indifference; Add, additivity; Ant, antagonism.
Classification based on fractional inhibitory concentration results.
Classification based on time-kill curves with DAP at 0.5 times the MIC and RIF at 0.5 times the MIC.
The susceptibilities of the collected pathogens against daptomycin and vancomycin are listed in Table 1: all were susceptible to daptomycin (range, 0.13 to 1.0 μg/ml), and all but one were susceptible to rifampin (range, 0.03 to 8.0 μg/ml). In separate susceptibility experiments with biofilm cultures, up to an 8-fold increase in MIC was seen with daptomycin (the median MIC was 2.0 μg/ml in biofilm versus 0.25 μg/ml in planktonic cultures) while there was no difference with rifampin (0.03 μg/ml in both planktonic and biofilm cultures). Similar fold changes occurred between cidal concentrations in planktonic (minimum bactericidal concentration [MBC]) and biofilm (MBEC) cultures (data not shown). The isolates from the two patients with microbiologic failure were susceptible to daptomycin.
By checkerboard assay, daptomycin and rifampin were synergistic against the infecting strain in nine (75%) of the 12 cases; indifference was found in the remaining three MRSA cases, and no antagonism was detected. In a correlation of clinical outcome to the results of synergy testing by checkerboard with the infecting strains, checkerboard synergy was found in the nine cases and was 100% predictive of therapeutic success; absence of synergy was found in three cases, two of which were therapeutic failures (P = 0.046; Fisher's exact test).
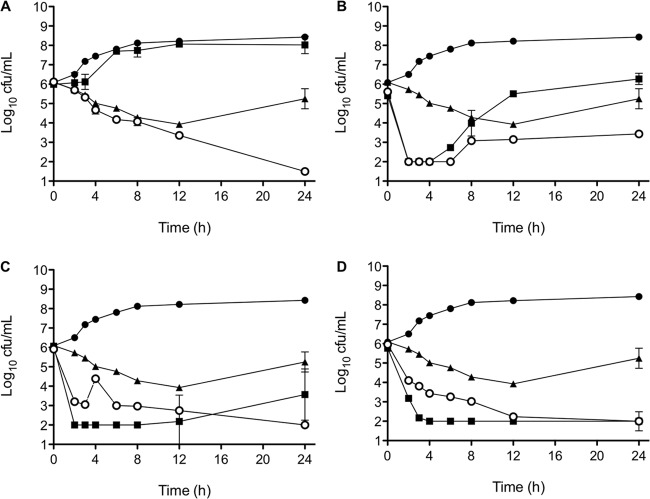
Synergy was also examined by time-kill curves. The representative results from a single MRSA strain are displayed in Fig. 1. At exposures of 0.5 times the MIC of daptomycin and rifampin, six of the 12 strains tested exhibited synergy while the remaining strains had indifference (4), additivity (1), or antagonism (1). However, when daptomycin concentrations were increased to 4 times the MIC, with rifampin held at 0.5 times the MIC, no synergy was found. At daptomycin concentrations of 4 times the MIC, 11 of the 12 isolates showed indifference while one isolate exhibited antagonism. In the first 4 h of exposure, antagonism was often present with high daptomycin concentrations when combined with rifampin, a finding which is consistent with other reports with similar concentration ratios (5, 13). There was no correlation between patient outcome and in vitro synergy by time-kill assay.
Fig 1.
Results of the killing curve study with daptomycin (DAP) and rifampin (RIF) activity alone and in combination against a single MRSA strain from a subject. (A) DAP at 0.5 times the MIC and RIF at 0.5 times the MIC; (B) DAP at 1 times the MIC and RIF at 0.5 times the MIC; (C) DAP at 2 times the MIC and RIF at 0.5 times the MIC; (D) DAP at 4 times the MIC and RIF at 0.5 times the MIC. Black circles, growth control; squares, DAP; triangles, RIF; white circles, DAP plus RIF. The analysis shows synergy with low concentrations of both DAP and RIF but early antagonism as DAP concentrations increased.
Daptomycin in combination with adjunctive antibiotics, such as rifampin, aminoglycosides, or co-trimoxazole, may improve antimicrobial killing or inhibit the emergence of resistance compared to daptomycin alone (5, 14). New Infectious Diseases Society of America (IDSA) guidelines for the treatment of prosthetic joint infections recommend combining rifampin with primary therapy (15), but published clinical experiences with daptomycin combined with rifampin have been very limited. We have recently reported an 86% rate of success at 3 to 5 years in a cohort of 29 patients with osteomyelitis or infected total joint prostheses, most of whom had failed conventional therapy, usually with vancomycin (7).
Previous in vitro synergy studies with daptomycin and rifampin have demonstrated an additive effect in laboratory MRSA strains (4, 7). This combination in our study was synergistic or indifferent in all of our strains in the checkerboard assay, while no synergy was found in the majority of the strains with the time-kill technique. Our study is unique in that we correlate the findings of in vitro synergy testing with clinical treatment outcome in patients with complex deep MRSA infections. Daptomycin plus rifampin showed in vitro synergy by checkerboard testing in 75% of the isolates, which correlated 100% with clinical cure in patients with complex MRSA infections treated with this combination. We hypothesize that the checkerboard method may better assess the range of antibiotic concentration ratios that occurs in sequestered infection sites in which antibiotic penetration may be delayed and highly variable. This method may not be ideal to predict outcomes of acute bloodstream infections, in which antibiotic pharmacokinetics can be more directly estimated with static or dynamic pharmacokinetic (PK)/pharmacodynamic (PD) models. Our study is limited by a relatively small number of observations and requires further validation, but the checkerboard method for demonstrating in vitro synergy between daptomycin and rifampin may be a reliable predictor of therapeutic success in complex MRSA infections treated with this novel combination.
ACKNOWLEDGMENTS
This work was supported by a grant from the Cubist Investigator-Initiated Study program (Cubist Pharmaceuticals) and a gift for research in infectious disease from the Oscar Rennebohm Foundation of Madison, WI.
Footnotes
Published ahead of print 6 May 2013
REFERENCES
- 1. Lamp KC, Friedrich LV, Mendez-Vigo L, Russo R. 2007. Clinical experience with daptomycin for the treatment of patients with osteomyelitis. Am. J. Med. 120(Suppl 1):S13–S20 [DOI] [PubMed] [Google Scholar]
- 2. Forrest GN, Donovan BJ, Lamp KC, Friedrich LV. 2008. Clinical experience with daptomycin for the treatment of patients with documented gram-positive septic arthritis. Ann. Pharmacother. 42:213–217 [DOI] [PubMed] [Google Scholar]
- 3. Liu C, Bayer A, Cosgrove SE, Daum RS, Fridkin SK, Gorwitz RJ, Kaplan SL, Karchmer AW, Levine DP, Murray BE, Rybak MJ, Talan DA, Chambers HF. 2011. Clinical practice guidelines by the Infectious Diseases Society of America for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children. Clin. Infect. Dis. 52:e18–e55 [DOI] [PubMed] [Google Scholar]
- 4. Murillo O, Garrigós C, Pachón ME, Euba G, Verdaguer R, Cabellos C, Cabo J, Gudiol F, Ariza J. 2009. Efficacy of high doses of daptomycin versus alternative therapies against experimental foreign-body infection by methicillin-resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 53:4252–4257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Rose WE, Leonard SN, Rybak MJ. 2008. Evaluation of daptomycin pharmacodynamics and resistance at various dosage regimens against Staphylococcus aureus isolates with reduced susceptibilities to daptomycin in an in vitro pharmacodynamic model with simulated endocardial vegetations. Antimicrob. Agents Chemother. 52:3061–3067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Saleh-Mghir A, Muller-Serieys C, Dinh A, Massias L, Cremieux AC. 2011. Adjunctive rifampin is crucial to optimizing daptomycin efficacy against rabbit prosthetic joint infection due to methicillin-resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 55:4589–4593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rose WE, Hatch JB, Maki DG.Abstr. 47th Annu. Meet. Infect. Dis. Soc. Am., abstr 896.2009. [Google Scholar]
- 8. Mohr JF, Friedrich LV, Yankelev S, Lamp KC. 2009. Daptomycin for the treatment of enterococcal bacteraemia: results from the Cubicin Outcomes Registry and Experience (CORE). Int. J. Antimicrob. Agents 33:543–548 [DOI] [PubMed] [Google Scholar]
- 9. Clinical and Laboratory Standards Institute 2011. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically; approved standard—eleventh edition. Clinical and Laboratory Standards Institute, Wayne, PA [Google Scholar]
- 10. Ceri H, Olson ME, Stremick C, Read RR, Morck D, Buret A. 1999. The Calgary Biofilm Device: new technology for rapid determination of antibiotic susceptibilities of bacterial biofilms. J. Clin. Microbiol. 37:1771–1776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Odds FC. 2003. Synergy, antagonism, and what the chequerboard puts between them. J. Antimicrob. Chemother. 52:1. [DOI] [PubMed] [Google Scholar]
- 12. Chin JN, Rybak MJ, Cheung CM, Savage PB. 2007. Antimicrobial activities of ceragenins against clinical isolates of resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 51:1268–1273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Credito K, Lin G, Appelbaum PC. 2007. Activity of daptomycin alone and in combination with rifampin and gentamicin against Staphylococcus aureus assessed by time-kill methodology. Antimicrob. Agents Chemother. 51:1504–1507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Steed ME, Vidaillac C, Rybak MJ. 2010. Novel daptomycin combinations against daptomycin-nonsusceptible methicillin-resistant Staphylococcus aureus in an in vitro model of simulated endocardial vegetations. Antimicrob. Agents Chemother. 54:5187–5192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Osmon DR, Berbari EF, Berendt AR, Lew D, Zimmerli W, Steckelberg JM, Rao N, Hanssen A, Wilson WR. 2013. Diagnosis and management of prosthetic joint infection: clinical practice guidelines by the Infectious Diseases Society of America. Clin. Infect. Dis. 56:e1–e25 [DOI] [PubMed] [Google Scholar]