Abstract
We evaluated treatment with linezolid, dosed at 800 mg once daily for 1 to 4 months as guided by sputum culture status and tolerance and then at 1,200 mg thrice weekly until ≥1 year after culture conversion, in addition to individually optimized regimens among 10 consecutive patients with extensively drug-resistant tuberculosis or fluoroquinolone-resistant multidrug-resistant tuberculosis. All achieved stable cure, with anemia corrected and neuropathy stabilized, ameliorated, or avoided after switching to intermittent dosing. Serum linezolid profiles appeared better optimized.
TEXT
Multidrug-resistant tuberculosis (MDR-TB) and extensively drug-resistant tuberculosis (XDR-TB) have become a global epidemic (1). Fluoroquinolone-resistant MDR-TB and XDR-TB are particularly difficult to treat. Linezolid has been in off-label use for difficult MDR-TB (2).
It is unlikely that short-term treatment with linezolid can achieve a stable cure of difficult MDR-TB, but use of 600 mg linezolid twice daily beyond 2 to 4 weeks is associated with reversible hemopoietic suppression (3) and substantial peripheral neuropathy (4). Although dosage reduction to 600 mg once daily and 300 mg once daily can reduce the risk of adverse events (2, 5, 6), there may still be genuine concerns about neuropathy, treatment inadequacy, and acquired drug resistance.
In a refractory XDR-TB patient with previously acquired rifampin resistance (probably related to poor drug penetration into a large, thick-walled cavity) and subsequent intolerance to 600 mg linezolid twice daily, we attempted linezolid initially at 800 mg once daily and then at 1,200 mg thrice weekly to balance adequate drug penetration with side effects. This approach was based on the usual dose ceiling (7, 8), with possibly better drug penetration due to higher peak concentration and potentially improved tolerance as a result of an exponential decline of serum linezolid level (9). Thrice-weekly use of a higher dose instead of daily use of a lower dose may also be supported by a murine model that suggests similar linezolid activities between twice-daily dosing and once-daily dosing of the same total dose (10). The initially encouraging outcome in this difficult case prompted us to hypothesize that intermittent dosing might safely enable prolonged use of linezolid for effective treatment of difficult MDR-TB.
It has also been shown ex vivo that linezolid induces side effects by inhibiting mammalian mitochondrial protein synthesis (MPS) in a dose-dependent manner, with 50% inhibitory concentrations (IC50) being 3.37 to 5.26 mg/liter (11). Thus, the risk of side effects, which will probably decrease with time of exposure to serum linezolid levels below 3.37 mg/liter, can be evaluated by estimating the corresponding proportion of interdosing time. Additionally, if linezolid activity against Mycobacterium tuberculosis is time dependent (12, 13), treatment efficacy can be evaluated by estimating the proportion of interdosing time above the MIC (14) and that above four times the mutant prevention concentration (15), which may reduce the risk of acquired resistance.
We conducted a prospective cohort study, approved by the Ethics Committee of the Department of Health, to evaluate the above-described hypothesis. Adult patients with pulmonary XDR-TB or fluoroquinolone-resistant MDR-TB were consecutively enrolled after written informed consent was obtained. In addition to individually optimized levofloxacin-containing regimens, linezolid was dosed at 800 mg once daily for 1 to 4 months and then at 1,200 mg thrice weekly after culture conversion. The switch from daily to intermittent dosing was determined by sputum culture conversion, defined as consecutive negative cultures collected at least 30 days apart (16), and hastened by side effects, especially substantial hemopoietic suppression or neuropathy. If side effects developed, deteriorated, or persisted during intermittent linezolid dosing, consideration would be given to further reduce linezolid dosage to 600 mg thrice weekly or stopping linezolid after weighing treatment adequacy and drug toxicity. Treatment outcomes were followed with an estimation of serum linezolid profiles of patients dosed at 1,200 mg thrice weekly, 600 mg thrice weekly (including a few patients with brief exposure), and 600 mg once daily (all after brief exposure). We estimated proportions of interdosing time with levels of <3.37 mg/liter (lower limit of MPS IC50), >1 mg/liter (resistance breakpoint), and >4 mg/liter (four times the mutant prevention concentration) in a monoexponential model (9), which is represented by the following formula: elimination rate constant = [Loge (concentration 1) − Loge (concentration 2)]/time interval. For intermittent dosing, serum linezolid levels were measured 1, 2, 24, and 48 h after dosing, with proportions of interdosing time estimated by the 24- and 48-h levels or the highest attainable level at 1 or 2 h when the 48-h level was below the detectable limit of 0.2 mg/liter. For daily dosing, serum linezolid levels were measured at 2, 4, and 24 h after dosing, with proportions of interdosing time estimated by the 4- and 24-h levels.
To examine whether serum linezolid levels reflect drug levels at lung lesions, we also assessed linezolid penetrability into sputum by measuring 2-h linezolid levels in paired sputum and serum samples using high-performance liquid chromatography (17), which has been robustly used to assay linezolid levels in bone, fat, muscle, hematoma, and peritoneal dialysis fluid (18, 19). To homogenize sputum before assaying sputum linezolid levels, we added a known amount of Sputolysin that was approximately equal to the amount of the collected sputum. We found no chromatographic interference with the linezolid peak from Sputolysin.
From 16 February 2009 to 4 January 2011, we consecutively enrolled 7 patients with XDR-TB and 3 patients with fluoroquinolone-resistant MDR-TB and followed their progress through 15 February 2013. Of these 10 patients, seven (70%) had previous treatment failure or relapse of fluoroquinolone-resistant MDR-TB or XDR-TB, including four patients in whom linezolid was prematurely stopped, owing to side effects. All harbored linezolid-susceptible isolates with MIC values of ≤1 mg/liter as determined by the Bactec MGIT 960 system. Nine patients with low-level bacillary resistance to isoniazid also received high-dose isoniazid (16), which was 10 to 15 mg thrice weekly for one patient and 15 to 20 mg thrice weekly for the others. All achieved cure according to the WHO definition (16). Table 1 summarizes their clinical profiles, treatment regimens, and major side effects.
Table 1.
Clinical profiles, treatment regimens, and major side effectsa
| Patient identifier: sex/age (yr)b/initial body wt (kg)/resistance pattern | LZD dosage in mg in chronological order (duration in mo) plus other drugsc | Initial sputum smear | Initial findings on chest X-ray | Onset of sputum culture conversiond (no. of mo) | Total treatment duration [follow-upe] (no. of mo) | Major side effect(s)f |
|---|---|---|---|---|---|---|
| A: male/61/61.5/XDR | 800 OD (3.75) plus INH*/Pto*/Cy*/Cm/Z/Lv; 1,200 TIW (2.5) plus INH*/Pto*/Cy*/Cm/Z/Lv; 1,200 TIW (5.25) plus INH*/Pto*/Cy*/Z/Lv; 1,200 TIW (6.5) plus INH*/Pto*/Cy*/Lv | Pos | Cavitary, >RUL, <right lung | 2.5 | 18.0 [24.0] | Hb < 10 g/dl during daily LZD dosing |
| B: male/22/69.5/FQ-resistant MDR | 800 OD (3) plus INH*/E*/Km*/Cy*/PAS*/Lv; 1,200 TIW (3) plus INH*/E*/Km*/Cy*/PAS*/Lv; 1,200 TIW (1) plus INH*/E*/Km*/Cy*/Lv; 1,200 TIW (11) plus INH*/E*/Cy*/Lv | Neg | Noncavitary, <RUL | 0.5 | 18.0 [24.5] | No |
| C: male/43/52/XDR | 800 OD (4) plus INH*/Cy*/PAS/Pto/Lv; 1,200 TIW (12) plus INH*/Cy*/PAS/Pto/Lv; 600 TIW (4) plus INH*/Cy*/PAS/Pto/Lv | Pos | Cavitary, >right lung | 1.5 | 20.0 [19.5] | Mild PN |
| D: male/49/58.5/XDR | 800 OD (1.75) plus INH*/Cy*/Lv; 800 OD (2.5) plus INH*/Cy*/PAS/Lv; 1,200 TIW (13.75) plus INH*/Cy*/Lv | Pos | Cavitary, <RUL | 3.0 | 18.0 [18.5] | No |
| E: female/41/54.6/XDR | 800 OD (3.75) plus INH*/Cy/Z/Lv; 1,200 TIW (4.75) plus INH*/Cy/Z/Lv; 1,200 TIW (0.5) plus INH*/Z/Lv; 1,200 TIW (2.75) plus INH*/Lv; 600 TIW (4.25) plus INH*/Lv | Pos | Cavitary, <RUL | 2.5 | 16.0 [21.5] | Hb < 10 g/dl during daily LZD dosing; mild PN† |
| F: male/31/54.5/XDR | 600 BD (0.5) plus PAS*/Cy/Am/Mox; 800 OD (3.0) plus PAS*/Cy/Pto/Am/Lv; 800 OD (0.75) plus INH*/PAS*/Cy/Pto/Lv; 1,200 OD (1)g plus INH*/PAS*/Cy/Pto/Lv; 1,200 TIW (3.5) plus INH*/PAS*/Lv; 600 TIW (6.0) plus INH*/PAS*/Lv; 600 TIW (3.25) plus INH*/Lv | Pos | Cavitary, >right lung | 4.0 | 18.0 [12.0] | Hb < 10 g/dl during daily LZD dosing; mild PN† |
| G: male/53/54/XDR | 800 OD (0.75) plus PAS*/Cfz/Cm/Cy/Lv; 800 OD (1.5) plus Pto*/PAS*/Cfz/Cm/Lv; 800 OD (0.75) plus Pto*/PAS*/Cfz/Lv; 1,200 TIW (2.0) plus Pto*/PAS*/Lv; 1,200 TIW (7.5) plus INH*/PAS*/Lv; 1,200 TIW (5.5) plus INH*/Lv | Neg | Cavitary, <RUL | 1.0 | 18.0 [9.0] | No |
| H: female/49/39.7/XDR | 800 OD (2.5) plus Pto*/PAS*/Cy/Lv; 1,200 TIW (8.5) plus INH*/Pto*/PAS*/Lv; 1,200 TIW (2.25) plus INH*/Pto*/Lv; 1,200 TIW (3.0) plus INH*/Lv | Pos | Cavitary, <RUL | 5.0 | 16.25 [10.5] | No |
| I: female/32/53/FQ-resistant MDR | 800 OD (1.25) plus Km*/Pto*/Cy*/PAS*/Lv; 1,200 TIW (0.75) plus Km*/Pto*/Cy*/PAS*/Lv; 1,200 TIW (2.25) plus Km*/Pto*/Lv; 1,200 TIW (2.5) plus Km*/PAS*/Lv; 1,200 TIW (5.25) plus PAS*/Lv | Neg | Noncavitary, <RUL | <0.5 (within 3 days) | 12.0 [6.0] | No |
| Jh: male/31/51.5/FQ-resistant MDR | 600 BD (0.5) plus Z*/Am*/Cy*/Mox; 800 OD (2.75) plus Z*/Am*/Pto*/Cy*/Lv; 1,200 TIW (6.0) plus Z*/Pto*/Cy*/Lv; Nil (8.0) plus INH*/Z*/Pto*/Cy*/Lv; Nil (6.0) plus INH*/Z* | Neg | Noncavitary, <RUL | 2.0 | 23.25 [16.0] | Hb < 10 g/dl throughout daily LZD dosing; severe PN |
Seven patients (A to G) had previous treatment failure of XDR-TB or relapse of FQ-resistant MDR-TB/XDR-TB. Linezolid was previously given for 2 to 4 months in four patients (A, B, C, E) and stopped because of side effects. Abbreviations: Am, amikacin; BD, twice daily; Cfz, clofazimine; Cm, capreomycin; Cy, cycloserine; DST, drug susceptibility testing; E, ethambutol; FQ, fluoroquinolone; Hb, hemoglobin; INH, isoniazid; Km, kanamycin; Lv, levofloxacin; LZD, linezolid; MDR, multidrug resistant; Mox, moxifloxacin; Neg, negative; OD, once daily; PAS, para-aminosalicylic acid; Pos, positive; Pto, prothionamide; PN, peripheral neuropathy; RUL, right upper lobe; TIW, thrice weekly; XDR, extensively drug resistant; Z, pyrazinamide.
Age when linezolid-containing regimen was started.
An asterisk denotes the presence of in vitro activity according to drug susceptibility testing. Isoniazid is considered as having in vitro activity if its MIC is >0.2 mg/liter and ≤1 mg/liter.
Counted from start of treatment. Sputum culture conversion was defined as having two sets of consecutive negative cultures, from samples collected at least 30 days apart (16).
Counted from treatment completion to the last date of clinical assessment involving sputum and chest radiographic examination.
A dagger denotes partial improvement in peripheral neuropathy.
Instead of giving linezolid at 1,200 mg thrice weekly immediately after linezolid at 800 mg once daily, linezolid at 1,200 mg 5 times per week was given because sputum acid-fast bacillus smear was positive, with subsequent culture found to be negative.
Patient J also had graft-versus-host disease after receiving a bone marrow transplant for leukemia. He also received thalidomide when peripheral neuropathy occurred. Linezolid was stopped in view of good clinical progress and severe peripheral neuropathy.
Adverse events were more common during daily than intermittent linezolid dosing. During daily dosing, hemoglobin dropped below 10 g/dl in 4 (40%) patients, with normalization during intermittent dosing, and peripheral neuropathy developed in three (30%). Of the seven patients with no peripheral neuropathy during daily dosing, one (14%) experienced peripheral neuropathy soon after switching to intermittent dosing. Thus, the risk of peripheral neuropathy compared favorably with that of 300 mg linezolid once daily, which ranged from 25% (20) to 50% (6). Two (50%) of the four patients with peripheral neuropathy gradually improved during intermittent dosing and further improved after receiving 600 mg linezolid thrice weekly. Neuropathy remained unchanged in the other two patients.
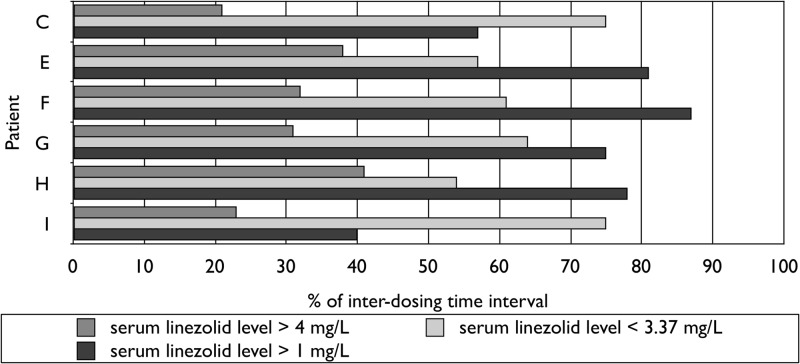
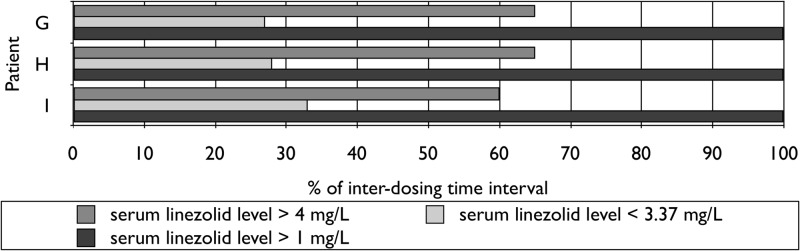
For eight patients dosed at 1,200 mg thrice weekly, the serum linezolid profiles estimated were <3.37 mg/liter in 34 to 62% of the interdosing time interval, >1 mg/liter in 60 to 100%, and >4 mg/liter in 34 to 61% (Fig. 1). For six of the eight patients dosed at 600 mg thrice weekly (including three after brief exposure), corresponding proportions were 54 to 75%, 40 to 87%, and 21 to 41%, respectively (Fig. 2). For three of the eight patients dosed at 600 mg once daily (all after brief exposure), corresponding proportions were 27 to 33%, 100%, and 60 to 65%, respectively (Fig. 3). Longer exposure to serum linezolid levels below 3.37 mg/liter could explain why neuropathy was stabilized, ameliorated, or avoided after switching from daily to prolonged intermittent dosing and why neuropathy further ameliorated in two patients given 600 mg linezolid thrice weekly. Substantial proportions of interdosing time above the resistance breakpoint and four times the mutant prevention concentration were consistent with stable cure. Although it has been shown ex vivo that linezolid activity is concentration independent at concentrations above twice the MIC (12, 13), thereby favoring more frequent dosing of smaller doses, our findings suggest that less frequent dosing of higher doses is also efficacious by a time-dependent mechanism, with an additional potential of reducing acquired resistance that remains to be further evaluated.
Fig 1.
Serum pharmacokinetics of oral linezolid at 1,200 mg thrice weekly.
Fig 2.
Serum pharmacokinetics of oral linezolid at 600 mg thrice weekly.
Fig 3.
Serum pharmacokinetics of oral linezolid at 600 mg once daily.
In line with previous findings in healthy volunteers (9) and patients with non-TB diseases (21), we found that linezolid penetrated well into sputum, with a median sputum-to-serum linezolid ratio of 1.0 (range, 0.7 to 1.5).
Major limitations of our study included a small sample size, incomplete sampling of all enrolled cases for estimating serum linezolid profiles, possible selection bias, and the lack of a concurrent control group. However, seven patients with previous treatment failure or relapse might serve as their own internal controls. It was difficult to delineate the role of other second-line drugs, notably newer-generation fluoroquinolones and high-dose isoniazid. We admit the need for caution in extrapolating ex vivo findings of MPS IC50, and in vitro observations regarding the mutant prevention concentration, to in vivo manifestations.
In summary, this case series suggests that intermittent dosing after initial daily dosing may better optimize the serum linezolid profile, thereby enabling safe, efficacious, and prolonged use of linezolid to achieve better outcomes of fluoroquinolone-resistant MDR-TB and XDR-TB. Further exploration of intermittent dosing is warranted to validate our findings.
ACKNOWLEDGMENT
We are indebted to all nurses working in the Tuberculosis and Chest Service, Department of Health, Hong Kong SAR, China, for their support.
Footnotes
Published ahead of print 6 May 2013
REFERENCES
- 1. World Health Organization 2010. Multidrug and extensively drug-resistant TB (M/XDR-TB): 2010 global report on surveillance and response. WHO/HTM/TB/2010.3. World Health Organization, Geneva, Switzerland: http://whqlibdoc.who.int/publications/2010/9789241599191_eng.pdf [Google Scholar]
- 2. Sotgiu G, Centis R, D'Ambrosio L, Alffenaar JW, Anger HA, Caminero JA, Castiglia P, De Lorenzo S, Ferrara G, Koh WJ, Schecter GF, Shim TS, Singla R, Skrahina A, Spanevello A, Udwadia ZF, Villar M, Zampogna E, Zellweger JP, Zumla A, Migliori GB. 2012. Efficacy, safety and tolerability of linezolid containing regimens in treating MDR-TB and XDR-TB: systematic review and meta-analysis. Eur. Respir. J. 40:1430–1442 [DOI] [PubMed] [Google Scholar]
- 3. Gerson SL, Kaplan SL, Bruss JB, Le V, Arellano FM, Hafkin B, Kuter DJ. 2002. Hematologic effects of linezolid: summary of clinical experience. Antimicrob. Agents Chemother. 46:2723–2726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bressler AM, Zimmer SM, Gilmore JL, Somani J. 2004. Peripheral neuropathy associated with prolonged use of linezolid. Lancet Infect. Dis. 4:528–531 [DOI] [PubMed] [Google Scholar]
- 5. Koh W-J, Kwon OJ, Gwak H, Chung JW, Cho S-N, Kim WS, Shim TS. 2009. Daily 300 mg dose of linezolid for the treatment of intractable multidrug-resistant and extensively drug-resistant tuberculosis. J. Antimicrob. Chemother. 64:388–391 [DOI] [PubMed] [Google Scholar]
- 6. Lee M, Lee J, Carroll MW, Choi H, Min S, Song T, Via LE, Goldfeder LC, Kang E, Jin B, Park H, Kwak H, Kim H, Jeon HS, Jeong I, Joh JS, Chen RY, Olivier KN, Shaw PA, Follmann D, Song SD, Lee JK, Lee D, Kim CT, Dartois V, Park S-K, Cho S-N, Barry CE., III 2012. Linezolid for treatment of chronic extensively drug-resistant tuberculosis. N. Engl. J. Med. 367:1508–1518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bailey EM, Faber MD, Nafziger DA. 2001. Linezolid for treatment of vancomycin-resistant enterococcal peritonitis. Am. J. Kidney Dis. 38:E20. [DOI] [PubMed] [Google Scholar]
- 8. Pfizer 2008. Zyvox US physician prescribing information. Pfizer, New York, NY [Google Scholar]
- 9. Conte JE, Jr, Golden JA, Kipps J, Zurlinden E. 2002. Intrapulmonary pharmacokinetics of linezolid. Antimicrob. Agents Chemother. 46:1475–1480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Williams KN, Stover CK, Zhu T, Tasneen R, Tyagi S, Grosset JH, Nuermberger E. 2009. Promising antituberculosis activity of the oxazolidinone PNU-100480 relative to that of linezolid in a murine model. Antimicrob. Agents Chemother. 53:1314–1319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. McKee EE, Ferguson M, Bentley AT, Marks TA. 2006. Inhibition of mammalian mitochondrial protein synthesis by oxazolidinones. Antimicrob. Agents Chemother. 50:2042–2049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wallis RS, Jakubiec WM, Kumar V, Silvia AM, Paige D, Dimitrova D, Li X, Ladutko L, Campbell S, Friedland G, Mitton-Fry M, Miller PF. 2010. Pharmacokinetics and whole-blood bactericidal activity against Mycobacterium tuberculosis of single doses of PNU-100480 in healthy volunteers. J. Infect. Dis. 202:745–751 [DOI] [PubMed] [Google Scholar]
- 13. Wallis RS, Jakubiec W, Kumar V, Bedarida G, Silvia A, Paige D, Zhu T, Mitton-Fry M, Ladutko L, Campbell S, Miller PF. 2011. Biomarker-assisted dose selection for safety and efficacy in early development of PNU-100480 for tuberculosis. Antimicrob. Agents Chemother. 55:567–574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Nuermberger E, Grosset J. 2004. Pharmacokinetic and pharmacodynamic issues in the treatment of mycobacterial infections. Eur. J. Clin. Microbiol. Infect. Dis. 23:243–255 [DOI] [PubMed] [Google Scholar]
- 15. Rodríguez JC, Cebrián L, López M, Ruiz M, Jiménez I, Royo G. 2004. Mutant prevention concentration: comparison of fluoroquinolones and linezolid with Mycobacterium tuberculosis. J. Antimicrob. Chemother. 53:441–444 [DOI] [PubMed] [Google Scholar]
- 16. World Health Organization 2008. Guidelines for the programmatic management of drug-resistant tuberculosis. WHO/HTM/TB/2008.402. World Health Organization, Geneva, Switzerland [Google Scholar]
- 17. Tobin CM, Sunderland J, White LO, MacGowan AP. 2001. A simple, isocratic high-performance liquid chromatography assay for linezolid in human serum. J. Antimicrob. Chemother. 48:605–608 [DOI] [PubMed] [Google Scholar]
- 18. Lovering AM, Zhang J, Bannister GC, Lankester BJA, Brown JHM, Narendra G, MacGowan AP. 2002. Penetration of linezolid into bone, fat, muscle and haematoma of patients undergoing routine hip replacement. J. Antimicrob. Chemother. 50:73–77 [DOI] [PubMed] [Google Scholar]
- 19. Tobin CM, Sunderland J, Lovering AM, MacGowan AP. 2003. A high performance liquid chromatography (HPLC) assay for linezolid in continuous ambulatory peritoneal dialysis fluid (CAPDF). J. Antimicrob. Chemother. 51:1041–1042 [DOI] [PubMed] [Google Scholar]
- 20. Koh W-J, Kang YR, Jeon K, Kwon OJ, Lyu J, Kim WS, Shim TS. 2012. Daily 300 mg dose of linezolid for multidrug-resistant and extensively drug-resistant tuberculosis: updated analysis of 51 patients. J. Antimicrob. Chemother. 67:1503–1507 [DOI] [PubMed] [Google Scholar]
- 21. Saralaya D, Peckham DG, Hulme B, Tobin CM, Denton M, Conway S, Etherington C. 2004. Serum and sputum concentrations following the oral administration of linezolid in adult patients with cystic fibrosis. J. Antimicrob. Chemother. 53:325–328 [DOI] [PubMed] [Google Scholar]