Abstract
Macrolide antibiotics are largely used in pregnancy for different bacterial infections. Their fetal safety has been studied by several groups, yielding opposing results. In particular, there have been studies claiming an association between macrolides and cardiovascular malformations. Exposure in early infancy has been associated with pyloric stenosis and intussusception. This has led to an avoidance in prescribing macrolides to pregnant women in several Scandinavian countries. The Objectives of the present study was to investigate the fetal safety of this class of drug by linking a large administrative database of drug dispensing and pregnancy outcome in Southern Israel. A computerized database of medications dispensed from 1999 to 2009 to all women registered in the Clalit health maintenance organization in southern Israel was linked with two computerized databases containing maternal and infant hospitalization records. Also, medical pregnancy termination data were analyzed. The following confounders were controlled for: maternal age, ethnicity, maternal pregestational diabetes, parity, and the year the mother gave birth or went through medical pregnancy termination. First- and third-trimester exposures to macrolide antibiotics as a group and to individual drugs were analyzed. During the study period there were 105,492 pregnancies among Clalit women that met the inclusion criteria. Of these, 104,380 ended in live births or dead fetuses and 1,112 in abortion due to medical reasons. In the first trimester of pregnancy, 1,033 women were exposed to macrolides. There was no association between macrolides and either major malformations [odds ratio (OR), 1.08; 95% confidence interval (CI), 0.84 to 1.38)] or specific malformations, after accounting for maternal age, parity, ethnicity, prepregnancy diabetes, and year of exposure. During the third trimester of pregnancy, 959 women were exposed to macrolides. There was no association between such exposure and perinatal mortality, low birth weight, low Apgar score, or preterm delivery. Similarly, no associations were demonstrated with pyloric stenosis or intussusception. Use of macrolides in the first trimester of pregnancy is not associated with an increased risk of major malformations. Exposure in the third trimester is not likely to increase neonatal risks for pyloric stenosis or intussusception in a clinically meaningful manner.
INTRODUCTION
Macrolide antibiotics are widely used for a variety of bacterial infections in pregnancy, including chlamydia, gonorrhea, Gram-positive upper respiratory infections, and premature rupture of membranes, and in cases of beta-lactam allergies (1–8). The macrolides cross the placenta, and their clearance rate in pregnancy is faster in late pregnancy (9, 10). Several studies conducted over the last 50 years failed to show an association between the use of erythromycin in the first trimester of pregnancy and the risk of major malformations for the most part (11–13). In contrast, a widely publicized Swedish study reported increased risk of cardiovascular malformations, leading several Scandinavian countries to avoid prescribing the drug to pregnant women (14). Data concerning the fetal safety of clarithromycin, azithromycin, and roxithromycin have been limited but failed to show increased fetal risks (15–20).
Recently, exposure to macrolides in very early infancy has been associated with increased risk of pyloric stenosis (21, 22) and intussusceptions (23).
Most studies to date have had small samples sizes and failed to include data on induced abortion, the exclusion of which may lead to bias toward the null (24).
The objectives of the present study were to investigate the fetal safety of this class of drugs in large administrative databases of drug dispensing and pregnancy outcome in southern Israel.
MATERIALS AND METHODS
A population-based retrospective cohort study was conducted, including all women 15 to 49 years of age who were registered in Clalit Health Services and had a delivery or a pregnancy termination due to medical reasons at Soroka Medical Center (SMC) between 1 January 1999 and 30 December 2009. Clalit Health Services is the largest health maintenance organization in the country, in which 70% of the women in the southern district of Israel 15 to 49 years of age are insured. SMC is the regional hospital, where 98% of the deliveries take place (25, 26).
Almost 70% of the population in southern Israel are Jewish and 25.4% are Bedouin Muslim. The Bedouin population composes only 3.5% of the total population of the state of Israel; however, they account approximately half of the births in southern Israel due to a high birth rate (total fertility rate of 7.5 for the Bedouin population versus 2.7 for the Jewish population) (25).
Compared to central Israel, which is the most populated and well-developed area of the state, most settlements in the southern district are ranked at low to average socioeconomic levels (27). The Israeli Central Bureau of Statistics (CBS) uses a socioeconomic scale of clusters ranked from 1 to 10, in which clusters 1 to 3, clusters 4 to 7, and cluster 8 to 10 represent low, average, and high socioeconomic ranks, respectively. Data published by the CBS show, for example, that 86% of the Jewish settlements in the southern district are ranked in clusters 4 and 5, while only 13% of the Jewish settlements in central Israel are ranked within those clusters. Similarly, 42% of the Jewish settlements in central Israel are ranked in clusters 6 and 7, whereas only 2% of the Jewish settlements in the south are ranked at that level. Furthermore, 42% of the Arabic settlements in central Israel are ranked in the low socioeconomic clusters (clusters 1 to 3), while 100% of the Bedouin settlements in the south are ranked in the low socioeconomic clusters (27).
We linked 3 computerized databases which draw information directly from original sources—two from Soroka Medical Center (SMC) and one from Clalit Health Services. The SMC's Department of Obstetrics and Gynecology deliveries database includes maternal demographic information, including mother's age and ethnic group (Jewish or Bedouin Muslim), parity, health status during pregnancy and delivery, self-reported smoking status during pregnancy, gestational age at delivery, and delivery results (perinatal death, infant's birth weight, and Apgar score at 1 and 5 min). The diagnoses are reviewed routinely by a trained medical secretary before entry into the database.
Information regarding major malformations diagnosed in newborns or infants until the age of 12 months was collected from the Demog-ICD9 database, which includes demographic and medical information for patients admitted to SMC. Information on drugs dispensed to mothers during the first trimester of pregnancy was collected from the Clalit Health Services medication database, which includes information on the date the drug was dispensed, the anatomical therapeutic chemical (ATC) classification codes of the drugs (including the commercial and generic names), the dose schedule, and the dose dispensed, in terms of the defined daily dose (DDD) (28) (i.e., the assumed average maintenance dose per day). A fourth database, which included data on medical pregnancy terminations, was assembled manually from the registry of the Committee for Termination of Pregnancies at Soroka Medical Center.
The four databases were encoded and linked by personal identification numbers (numbers that are given at birth by the Interior Ministry and used throughout life) to create a registry of medications dispensed during the first trimester of pregnancy and of pregnancy outcomes. We used the unique identification number of the hospitalization given at SMC to the mother and to the newborn to link the mother and the infant's identification number.
The study was approved by the local institutional ethics committee in accordance with the principles of the Declaration of Helsinki. In accordance with Ministry of Health regulations, the institutional ethics committee did not require written informed consent because the data were obtained anonymously from medical files, with no participation of patients.
Study design.
The exposure groups were defined as exposure to any macrolide (erythromycin, azithromycin, clarithromycin, or roxithromycin) during the first trimester of pregnancy, or during the third trimester of pregnancy. The unexposed groups were infants or fetuses that were not exposed to any macrolide during the first or third trimester, respectively. The analysis also investigated specific macrolide drugs. An infant or fetus was defined as “exposed” if a macrolide was dispensed to the mother during the first 13 weeks of pregnancy (first trimester group) or during the last 12 weeks of pregnancy (third trimester group). The first day of the last menstrual period was defined as the first day of gestation.
First-trimester exposure to macrolide was also characterized by the total number of defined daily doses (DDD) dispensed. The DDD for macrolides is as follows (28): erythromycin, 1 g; erythromycin ethyl succinate, 2 g; azithromycin, 0.5 g; clarithromycin, 1 g; roxithromycin, 0.3 g. The total defined daily doses dispensed during the first trimester were stratified into three categories: 1 to 5, 6 to 10, and 11 and more.
We investigated the risk of major malformations after exposure to macrolides during the first trimester of pregnancy for live births and stillbirths and for pregnancy terminations due to medical reasons. We used the definitions of major and minor congenital malformations developed by the Metropolitan Atlanta Congenital Defects Program of the Centers for Disease Control and Prevention (CDC) (29–31). Chromosomal diseases were excluded. In subclass analyses of major malformations, the following specific defects were examined (ICD9 codes are in parentheses): anencephaly (740); spina bifida (741); other anomaly of the nervous system (742); anomalies of the eye (743; anomalies of the ear, face, and neck (744); bulbus cordis anomalies and anomalies of cardiac septal closure (745); other anomalies of the heart (746); other anomalies of the circulatory system (747); anomalies of the respiratory system (748); cleft palate and lip (749); other anomalies of the upper alimentary tract (750); other anomalies of the digestive system (751); genital anomalies (752); anomalies of the urinary system (753); musculoskeletal deformities (754); other anomalies of the limbs (755); other musculoskeletal anomalies (756); and anomalies of the integument (757).
In a separate analysis we investigated the risk of pyloric stenosis (ICD9 code 7505) or intussusception (ICD9 code 5600) in infants exposed to macrolides during the last 12 weeks of pregnancy.
Statistical analysis.
We used the SPSS program, version 17 (IBM SPSS; Somers, NY), for statistical analysis. Characteristics of mothers from the exposed and unexposed groups were compared by the chi square or Fisher exact test for categorical variables and the Student t test for continuous variables. We used a multivariate logistic-regression model to determine whether exposure to macrolides was independently associated with an increased risk of major congenital malformations adjusting for maternal age, parity, ethnic group (i.e., Jewish versus Bedouin Muslim), pregestational diabetes mellitus, and year of birth or medical pregnancy termination. A categorical multivariate logistic-regression model was constructed to determine whether greater exposure in terms of defined daily dose (DDD) was associated with an increased risk for major congenital malformations. Odd ratios and 95% confidence intervals were computed. Similar methods were used to estimate the risks of pyloric stenosis or intussusceptions after third-trimester exposure to macrolides.
RESULTS
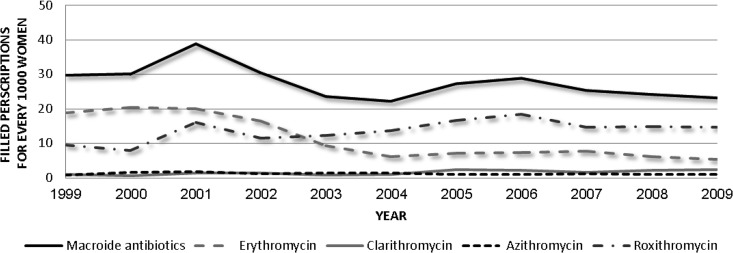
There were 105,492 births and 1,112 pregnancy terminations at SMC between 1999 and 2009. Overall, 1,033 fetuses were exposed to macrolides during the first trimester of pregnancy, and 959 during the third trimester. Table 1 presents the distribution of the different macrolide antibiotics in these two groups. The use of macrolides by women during pregnancy remained stable during the study period (Fig. 1).
Table 1.
Distribution of macrolides dispensed during the first and third trimesters of pregnancy
| No. of prescriptions dispensed in: |
||
|---|---|---|
| First trimester | Third trimester | |
| Macrolide | 1,033 | 959 |
| Erythromycin | 325 | 446 |
| Roxithromycin | 535 | 476 |
| Clarithromycin | 141 | 9 |
| Azithromycin | 46 | 34 |
Fig 1.
Annual trend of macrolides dispensed to pregnant women in the study.
Characteristics of mothers exposed and unexposed to macrolides are presented in Table 2.
Table 2.
Comparison of the characteristics of women exposed and unexposed to macrolides during the first trimester of pregnancy
| Characteristic | No. (%) of women |
P value | |
|---|---|---|---|
| With exposure to macrolides (n = 1,033) | Without exposure to macrolides (n = 104,459) | ||
| Ethnicity | |||
| Jewish | 339 (32.8) | 37,461 (35.9) | 0.044 |
| Bedouin | 693 (67.2) | 66,988 (64.1) | |
| Pregestational diabetes | 23 (2.2) | 1,054 (1.0) | <0.001 |
| Maternal smoking during pregnancy | 26 (2.5) | 2,074 (2.0) | 0.224 |
| Maternal age (yr) (mean ± SD) | 29.77 ± 5.92 | 28.68 ± 5.85 | <0.001 |
| Parity (mean ± SD) | 2.21 ± 0.72 | 2.08 ± 0.72 | <0.001 |
The adjusted risk for major congenital malformations following exposure to macrolides during the first trimester of pregnancy was 1.074 (0.839 to 1.376) (Table 3). Macrolide exposure was not associated with increased risk for cardiovascular malformations (Table 4) or central nervous system, musculoskeletal, gastrointestinal, or urogenital malformations. Similar results were obtained for each macrolide separately In addition, there was no significant dose response in the association between macrolides and major malformations in univariate analyses or after adjustment using a categorical multiple logistic regression (Table 5).
Table 3.
Multiple logistic regression analysis for the risk of major malformations among infants born to women exposed during the first trimester to macrolides vs the unexposed
| Variable | Major malformation OR (95% CI)a | P value |
|---|---|---|
| Macrolide exposure | 1.074 (0.839–1.376) | 0.57 |
| Maternal age | 1.019 (1.013–1.025) | <0.001 |
| Pregestational diabetes | 1.678 (1.370–2.055) | <0.001 |
| Parity | 0.959 (0.946–0.972) | <0.001 |
| Ethnicity (Bedouin/Jewish) | 1.453 (1.362–1.550) | <0.001 |
| Year of birth/pregnancy termination | 1.025 (1.017–1.033) | <0.001 |
OR, odds ratio; CI, confidence interval.
Table 4.
Multiple logistic regression analysis for the risk of cardiovascular malformations among women exposed to macrolides during the first trimester of pregnancy
| Variable | Cardiovascular malformation OR (95% CI) | P value |
|---|---|---|
| Macrolide exposure | 0.953 (0.649–1.400) | 0.81 |
| Maternal age | 1.019 (1.010–1.028) | <0.001 |
| Pregestational diabetes | 1.945 (1.487–2.544) | <0.001 |
| Parity | 0.991 (0.971–1.011) | 0.39 |
| Ethnicity (Bedouin/Jewish) | 1.246 (1.133–1.372) | <0.001 |
OR, odds ratio; CI, confidence interval.
Table 5.
Categorical multiple logistic regression analysis for the effect of macrolide dose on the risk for major malformations
| Variable | Major malformation OR (95% CI)a | P value |
|---|---|---|
| DDDb | ||
| Unexposed | Reference | |
| 1–5 | 0.928 (0.287–2.999) | 0.9 |
| 6–10 | 1.095 (0.282–4.246) | 0.89 |
| >10 | 1.322 (0.365–4.790) | 0.78 |
| Maternal age (yr) | 1.019 (1.013–1.025) | <0.001 |
| Pregestational diabetes | 1.712 (1.398–2.097) | <0.001 |
| Parity | 0.959 (0.945–0.972) | <0.001 |
| Ethnicity (Bedouin/Jewish) | 1.451 (1.360–1.549) | <0.001 |
| Year of birth or pregnancy termination | 1.025 (1.017–1.033) | <0.001 |
OR, odds ratio; CI, confidence interval.
DDD, defined daily dose. For a description, see the text.
Exposure to macrolides in the third trimester was not associated with increased risk of either pyloric stenosis or intussusception (Table 6). However, due to the very small numbers of observations in the exposed group, no attempt was made to perform multivariate logistic regression.
Table 6.
Crude odds ratio for pyloric stenosis and intussusception after third trimester exposure to macrolides
| Diagnosis | OR (95% CI)a | No. (%) of subjects |
P value | |
|---|---|---|---|---|
| With exposure to macrolides (n = 952) | Without exposure to macrolides (n = 101,879) | |||
| Pyloric stenosis | —b | 0 | 50 (0.0) | —b |
| Intussusception | 1.049 (0.146–7.528) | 1 (0.1) | 102 (0.1) | 0.61 |
OR, odds ratio; CI, confidence interval.
Due to zero cases in the exposed, OR could not be calculated.
DISCUSSION
Macrolides are widely used in pregnancy, and therefore establishing their fetal safety is critical in ensuring evidence-based safe use during fetal development. In the study period, the dispensing of macrolides to pregnant women stayed stable between 20 and 40 prescriptions for 1,000 women in the study region. Starting in 2003, we saw an increase in use of the newer macrolides, led by roxithromycin (Fig. 1).
Our large population-based study failed to show an association between first-trimester macrolide exposure and either overall major malformations or specific malformations. In particular, our study could not confirm the Swedish study claiming an increase in cardiovascular malformations in connection with macrolides (14). Similarly, we did not detect risks for any other adverse fetal outcome. Moreover, by calculating the dose of exposure, we could rule out a dose response relationship between macrolides and any of these risks.
Our study is unique in using one of very few administrative databases that also capture malformations among the subgroup undergoing pregnancy termination. Our recent study showed that not including data on induced abortion creates bias toward the null, which may be critical when the results do not suggest an increased risk in the exposed group (24).
Our attempts to verify fetal risks for pyloric stenosis were limited by an absence of pyloric stenosis cases among the 952 exposed infants/fetuses, compared to 50 cases out of the 101,879 unexposed infants/fetuses. There was one case of intussusception among the exposed infants/fetuses, compared to 102 cases among the unexposed infants/fetuses, suggesting no increased risk. In both cases, our observed rates of these adverse outcomes were lower in the exposed than among the unexposed infants/fetuses, suggesting that a clinically meaningful increased risk is highly unlikely.
Our study has several limitations that need to be acknowledged:
Similar to all administrative database studies, our study assumed that a medication being dispensed is also a drug being taken. In previous studies of our databases, it has been shown that in cases of deep vein thrombosis and familial Mediterranean fever, there was close agreement between dispensing records and women's reports on adherence in pregnancy (32). It is conceivable that infection in pregnancy would be similarly associated with high adherence rates. In addition, our study population represents well the unique nature of southern Israel, but it has a limited ability to generalize the socioeconomic distribution of the Israeli population.
In conclusion, our study strengthens the rational for using macrolides when needed in pregnancy, by providing data on fetal safety based on a large cohort. There are high levels of anxiety among prescribers and pregnant women, often leading to suboptimal drug therapy even in life-threatening maternal conditions (33). By empowering the use of macrolides in pregnancy, it is hoped that the present study contributes to rational drug use in pregnancy.
Footnotes
Published ahead of print 6 May 2013
REFERENCES
- 1. Katzung BG, Masters SB, Trevor AJ. 2009. Basic and clinical pharmacology, 11th ed p 799–801 McGraw-Hill, New York, NY [Google Scholar]
- 2. Chambers HF. 2011. Protein synthesis inhibitors and miscellaneous antibacterial agents, p 1521–1547 In Brunton LL, Chabner BA, Knollmann BC. (ed). Goodman & Gilman's the pharmacological basis of therapeutics, 12th ed McGraw Hill, New York, NY [Google Scholar]
- 3. Jain R, Danziger LH. 2004. The macrolide antibiotics: a pharmacokinetic and pharmacodynamic overview current pharmaceutical design. Curr. Pharm. Des. 10:3045–3053 [DOI] [PubMed] [Google Scholar]
- 4. Bush MR, Rosa C. 1994. Azithromycin and erythromycin in the treatment of cervical chlamydial infection during pregnancy. Obstet. Gynecol. 84:61–63 [PubMed] [Google Scholar]
- 5. Adair CD, Gunter M, Stovall TG, McElroy G, Veille JC, Ernest JM. 1998. Chlamydia in pregnancy: a randomized trial of azithromycin and erythromycin. Obstet. Gynecol. 91:165–168 [DOI] [PubMed] [Google Scholar]
- 6. Ogasawara K, Goodwin M. 1999. Efficacy of Azithromycin in reducing lower genital ureaplasma urealyticum colonization in women at risk for preterm delivery. J. Matern. Fetal Med. 8:12–16 [DOI] [PubMed] [Google Scholar]
- 7. Kenyon S, Boulvain M, Neilson JP. 2009. Antibiotics for preterm rupture of membranes. The Cochrane Collaboration. John Wiley & Sons, Ltd., New York, NY [Google Scholar]
- 8. Edwards M, Rainwater K, Carter S. 1994. Comparison of azithromycin and erythromycin, for Chlamydia cervicitis in pregnancy. Am. J. Obstet. Gynecol. 170:419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Heikkinen T, Laine K, Neuvonen PJ, Ekblad U. 2000. The transplacental transfer of the macrolide antibiotics erythromycin, roxithromycin and azithromycin. Br. J. Obstet. Gynaecol. 107:770–775 [DOI] [PubMed] [Google Scholar]
- 10. Ramsey PS, Vaules MB, Vasdev GM. 2003. Maternal and transplacental pharmacokinetics of azithromycin. Am. J. Obstet. Gynecol. 188:714–718 [DOI] [PubMed] [Google Scholar]
- 11. Briggs GG, Freeman RK, Yaffe SJ. 2011. Drugs in pregnancy and lactation: a reference guide to fetal and neonate risk, 9th ed Lippincott Williams & Wilkins, Philadelphia, PA [Google Scholar]
- 12. Heinonen OP, Slone D, Shapiro S. 1977. Birth defects and drugs in pregnancy. Publishing Science Group, Littleton, MA [Google Scholar]
- 13. Romoren M, Lindbaek M. 2012. Pregnancy outcome after gestational exposure to erythromycin—a population based register study from Norway. Br. J. Clin. Pharmacol. 74:1053–1062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Källén B, Olausson P. 2003. Maternal drug use in early pregnancy and infant cardiovascular defect. Reprod. Toxicol. 17:255–261 [DOI] [PubMed] [Google Scholar]
- 15. Bar-Oz B, Diav-Citrin O, Shechtman S. 2008. Pregnancy outcome after gestational exposure to the new macrolides: a prospective multi-center observational study. Eur. J. Obstet. Gynecol. Reprod. Biol. 141:31–34 [DOI] [PubMed] [Google Scholar]
- 16. Bar-Oz B, Weber-Schoendorfer C. 2012. The outcomes of pregnancy in women exposed to the new macrolides in the first trimester. Drug Saf. 35:589–598 [DOI] [PubMed] [Google Scholar]
- 17. Sarkar M, Woodland C, Koren G. 2006. Pregnancy outcome following gestational exposure to azithromycin. BMC Pregnancy Childbirth 6:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chun JY, Han JY, Ahn HK. 2006. Fetal outcome following roxithromycin exposure in early pregnancy. J. Matern. Fetal Neonatal. Med. 19:189–192 [DOI] [PubMed] [Google Scholar]
- 19. Einarson A, Phillip E, Mawji F, D'Alimonte D, et al. 1998. A prospective controlled multicentre study of clarithromycin in pregnancy. Am. J. Perinatol. 15:523–525 [DOI] [PubMed] [Google Scholar]
- 20. Drinkard CR, Shatin D, Clouse J. 2000. Postmarketing surveillance of medications and pregnancy outcomes: clarithromycin and birth malformations. Pharmacoepidemiol. Drug Saf. 9:549–556 [DOI] [PubMed] [Google Scholar]
- 21. Cooper WO, Griffin MR, Arbogast P. 2002. Very early exposure to erythromycin and infantile hypertrophic pyloric stenosis. Arch. Pediatr. Adolesc. Med. 156:647–650 [DOI] [PubMed] [Google Scholar]
- 22. Hauben M, Amsden G. 2002. The association of erythromycin and infantile hypertrophic pyloric stenosis, causal or coincidental? Drug Safety 25:929–942 [DOI] [PubMed] [Google Scholar]
- 23. Hviid A, Svanström H. 2009. Antibiotic use and intussusception in early childhood. J. Antimicrob. Chemother. 64:642–648 [DOI] [PubMed] [Google Scholar]
- 24. Levy A, Matok I, Gorodischer R, Sherf M, Wiznitzer A, Uziel E, Koren G. 2012. Bias toward the null hypothesis in pregnancy drug studies that do not include data on medical terminations of pregnancy: the folic acid antagonists. J. Clin. Pharmacol. 52:78–83 [DOI] [PubMed] [Google Scholar]
- 25. The Israeli Central Bureau of Statistics (CBS) 2013. Statistical abstracts of Israel 2008. No. 59. Population by district, subdistrict and religion. http://www.cbs.gov.il/reader/shnaton/templ_shnaton_e.html?num_tab=st02_06x&CYear=2008 Accessed 7 April 2013
- 26. The Israeli Central Bureau of Statistics (CBS) 2013. Patterns of fertility in 2006. http://www.cbs.gov.il/hodaot2007n/01_07_215b.pdf Accessed 7 April 2013
- 27.The Israeli Central Bureau of Statistics (CBS) Local authorities by socio-economic index value, rank and cluster membership. 2013. [Accessed 7 April 2013]. http://www.cbs.gov.il/hodaot2013n/24_13_087_local_autorities_a2.xls.
- 28. WHO Collaborating Centre for Drug Statistics Methodology 2012. ATC/DDD index 2013. http://www.whocc.no/atcddd/ Accessed 15 February 2012
- 29. Centers for Disease Control and Prevention 2011. Metropolitan Atlanta Congenital Defects Program. http://www.cdc.gov/ncbddd/bd/macdp.htm Accessed 6 June 2011
- 30. Correa-Villasenor A, Cragan J, Kucik J, O'Leary L, Siffel C, Williams L. 2003. The Metropolitan Atlanta Congenital Defects Program: 35 years of birth defects surveillance at the Centers for Disease Control and Prevention. Birth Defects Res. A Clin. Mol. Teratol. 67:617–624 [DOI] [PubMed] [Google Scholar]
- 31. Rasmussen SA, Olney RS, Holmes LB, Lin AE, Keppler-Noreuil KM, Moore CA. 2003. Guidelines for case classification for the National Birth Defects Prevention Study. Birth Defects Res. A Clin. Mol. Teratol. 67:193–201 [DOI] [PubMed] [Google Scholar]
- 32. Matok I, Gorodischer R, Koren G, Eyal Sheiner E, Wiznitzer A, Levy A. 2009. The safety of metoclopramide use in the first trimester of pregnancy. N. Engl. J. Med. 360:2528–2535 [DOI] [PubMed] [Google Scholar]
- 33. Koren G, Pastuszak AP, Ito S. 1998. Drugs in pregnancy. N. Engl. J. Med. 338:1128–1137 [DOI] [PubMed] [Google Scholar]