Abstract
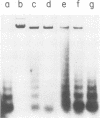
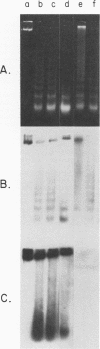
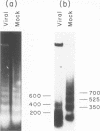
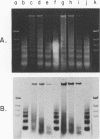
We investigated the structure of adenovirus deoxyribonucleic acid (DNA)-protein complexes in nuclei of infected cells by using micrococcal nuclease. Parental (infecting) DNA was digested into multimers which had a unit fragment size that was indistinguishable from the size of the nucleosomal repeat of cellular chromatin. This pattern was maintained in parenteral DNA throughout infection. Similar repeating units were detected in hamster cells that were nonpermissive for human adenovirus and in cells pretreated with n-butyrate. Late in infection, the pattern of digestion of viral DNA was determined by two different experimental approaches. Nuclear DNA was electrophoresed, blotted, and hybridized with labeled viral sequences; in this procedure all virus-specific DNA was detected. This technique revealed a diffuse protected band of viral DNA that was smaller than 160 base pairs, but no discrete multimers. All regions of the genome were represented in the protected DNA. To examine the nuclease protection of newly replicated viral DNA, infected cells were labeled with [3H]thymidine after blocking of cellular DNA synthesis but not viral DNA synthesis. With this procedure we identified a repeating unit which was distinctly different from the cellular nucleosomal repeat. We found broad bands with midpoints at 200, 400, and 600 base pairs, as well as the limit digest material revealed by blotting. High-resolution acrylamide gel electrophoresis revealed that the viral species comprised a series of closely spaced bands ranging in size from less than 30 to 250 base pairs.
Full text
PDF


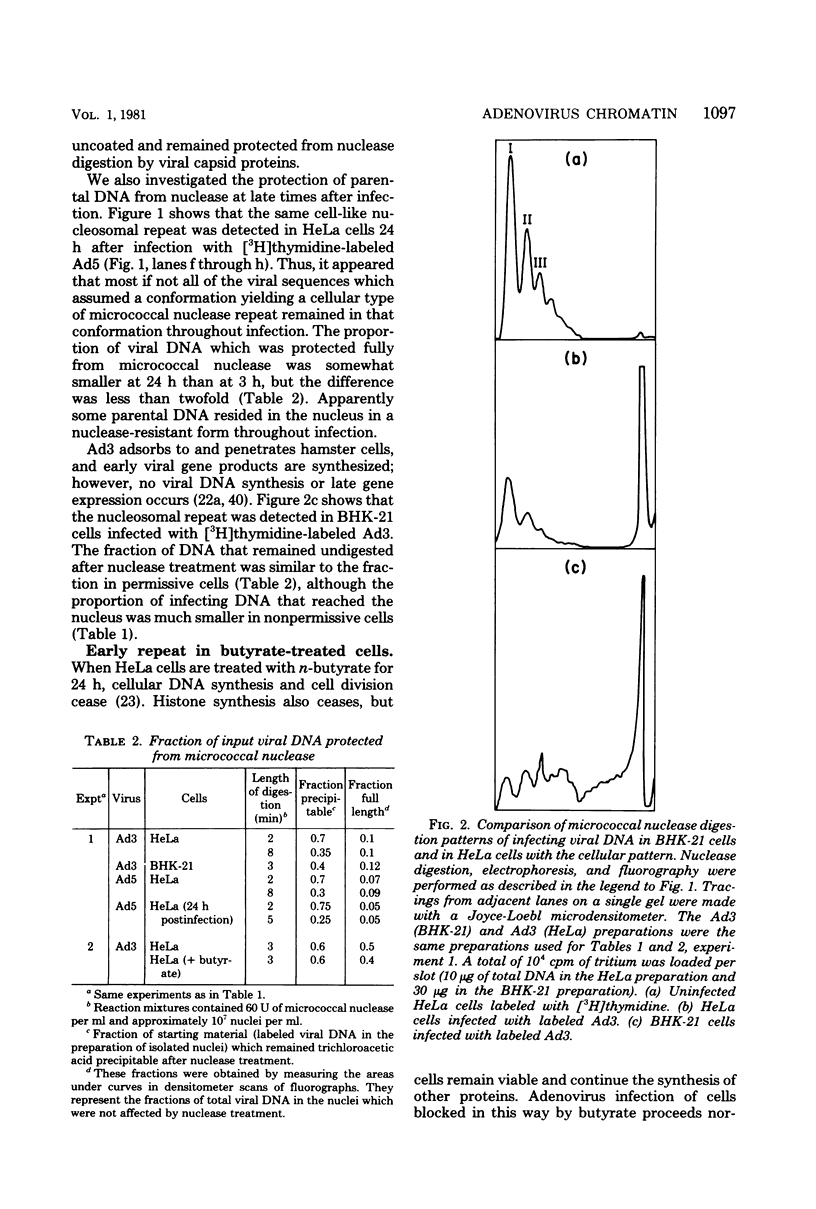


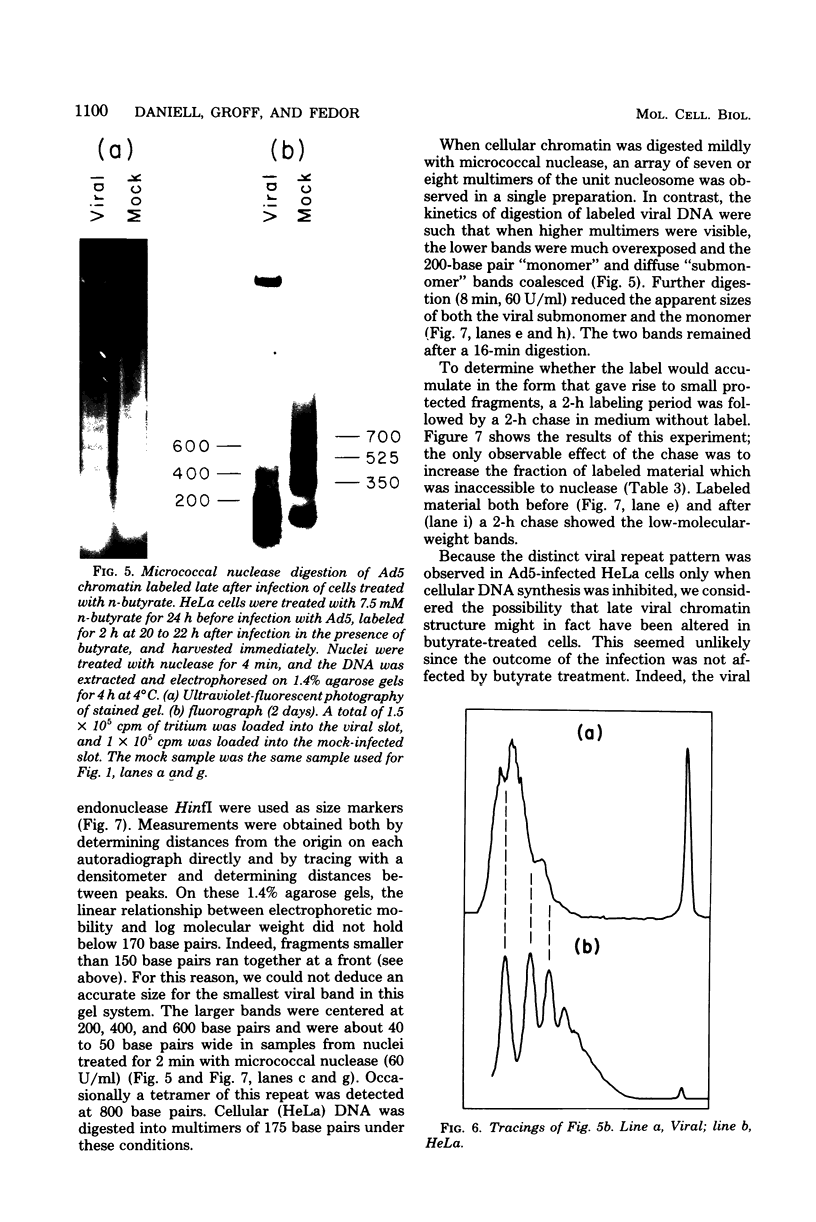
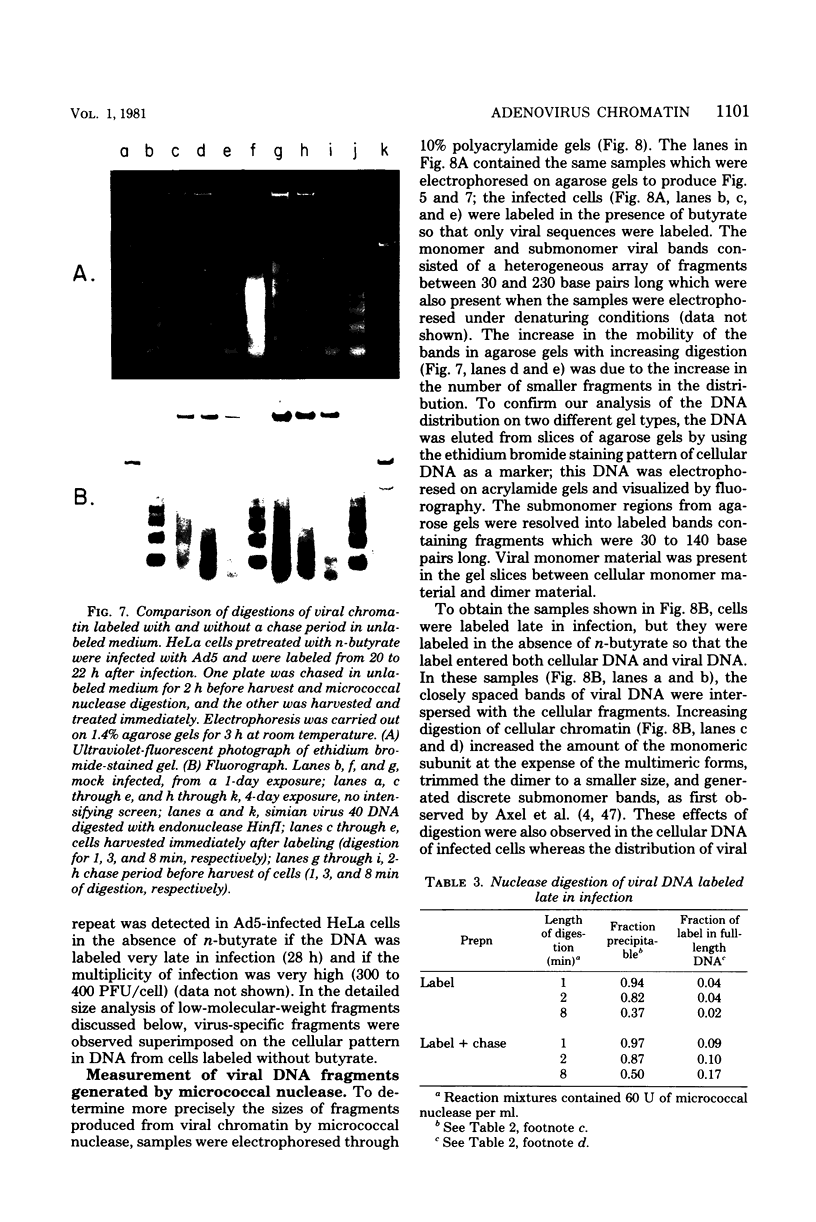



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abboud M. M., Horwitz M. S. The DNA polymerases associated with the adenovirus type 2 replication complex: effect of 2'-3'-dideoxythymidine-5'-triphosphate on viral DNA synthesis. Nucleic Acids Res. 1979 Mar;6(3):1025–1039. doi: 10.1093/nar/6.3.1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alwine J. C., Kemp D. J., Stark G. R. Method for detection of specific RNAs in agarose gels by transfer to diazobenzyloxymethyl-paper and hybridization with DNA probes. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5350–5354. doi: 10.1073/pnas.74.12.5350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amin M., Mirza A., Weber J. Genetic analysis of adenovirus type 2. VII. Cleavage-modified affinity for DNA of internal virion proteins. Virology. 1977 Jul 1;80(1):83–97. doi: 10.1016/0042-6822(77)90382-8. [DOI] [PubMed] [Google Scholar]
- Anderson C. W., Baum P. R., Gesteland R. F. Processing of adenovirus 2-induced proteins. J Virol. 1973 Aug;12(2):241–252. doi: 10.1128/jvi.12.2.241-252.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Axel R., Melchior W., Jr, Sollner-Webb B., Felsenfeld G. Specific sites of interaction between histones and DNA in chromatin. Proc Natl Acad Sci U S A. 1974 Oct;71(10):4101–4105. doi: 10.1073/pnas.71.10.4101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black B. C., Center M. S. DNA-binding properties of the major core protein of adenovirus 2. Nucleic Acids Res. 1979;6(6):2339–2353. doi: 10.1093/nar/6.6.2339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brison O., Kedinger C., Wilhelm J. Enzymatic properties of viral replication complexes isolated from adenovirus type 2-infected HeLa cell nuclei. J Virol. 1977 Nov;24(2):423–435. doi: 10.1128/jvi.24.2.423-435.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown D. T., Westphal M., Burlingham B. T., Winterhoff U., Doerfler W. Structure and composition of the adenovirus type 2 core. J Virol. 1975 Aug;16(2):366–387. doi: 10.1128/jvi.16.2.366-387.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown M., Weber J. Virion core-like organization of intranuclear adenovirus chromatin late in infection. Virology. 1980 Nov;107(1):306–310. doi: 10.1016/0042-6822(80)90297-4. [DOI] [PubMed] [Google Scholar]
- Caron F., Jacq C., Rouvière-Yaniv J. Characterization of a histone-like protein extracted from yeast mitochondria. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4265–4269. doi: 10.1073/pnas.76.9.4265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corden J., Engelking H. M., Pearson G. D. Chromatin-like organization of the adenovirus chromosome. Proc Natl Acad Sci U S A. 1976 Feb;73(2):401–404. doi: 10.1073/pnas.73.2.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniell E. Cells inhibited by n-butyrate support adenovirus replication. Virology. 1980 Dec;107(2):514–519. doi: 10.1016/0042-6822(80)90318-9. [DOI] [PubMed] [Google Scholar]
- Daniell E. Genome structure of incomplete particles of adenovirus. J Virol. 1976 Aug;19(2):685–708. doi: 10.1128/jvi.19.2.685-708.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniell E., Mullenbach T. Synthesis of defective viral DNA in HeLa cells infected with adenovirus type 3. J Virol. 1978 Apr;26(1):61–70. doi: 10.1128/jvi.26.1.61-70.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniell E. Subgenomic viral DNA species synthesized in simian cells by human and simian adenoviruses. J Virol. 1981 Feb;37(2):620–627. doi: 10.1128/jvi.37.2.620-627.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edvardsson B., Ustacelebi S., Williams J., Philipson L. Assembly intermediates among adenovirus type 5 temperature-sensitive mutants. J Virol. 1978 Feb;25(2):641–651. doi: 10.1128/jvi.25.2.641-651.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everitt E., Lutter L., Philipson L. Structural proteins of adenoviruses. XII. Location and neighbor relationship among proteins of adenovirion type 2 as revealed by enzymatic iodination, immunoprecipitation and chemical cross-linking. Virology. 1975 Sep;67(1):197–208. doi: 10.1016/0042-6822(75)90417-1. [DOI] [PubMed] [Google Scholar]
- Everitt E., Meador S. A., Levine A. S. Synthesis and processing of the precursor to the major core protein of adenovirus type 2. J Virol. 1977 Jan;21(1):199–214. doi: 10.1128/jvi.21.1.199-214.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fareed G. C., Davoli D. Molecular biology of papovaviruses. Annu Rev Biochem. 1977;46:471–522. doi: 10.1146/annurev.bi.46.070177.002351. [DOI] [PubMed] [Google Scholar]
- Felsenfeld G. Chromatin. Nature. 1978 Jan 12;271(5641):115–122. doi: 10.1038/271115a0. [DOI] [PubMed] [Google Scholar]
- Flint S. J., Berget S. M., Sharp P. A. Characterization of single-stranded viral DNA sequences present during replication of adenovirus types 2 and 5. Cell. 1976 Dec;9(4 Pt 1):559–571. doi: 10.1016/0092-8674(76)90038-6. [DOI] [PubMed] [Google Scholar]
- Germond J. E., Hirt B., Oudet P., Gross-Bellark M., Chambon P. Folding of the DNA double helix in chromatin-like structures from simian virus 40. Proc Natl Acad Sci U S A. 1975 May;72(5):1843–1847. doi: 10.1073/pnas.72.5.1843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green M., Piña M., Kimes R. C. Biochemical studies on adenovirus multiplication. XII. Plaquing efficiencies of purified human adenoviruses. Virology. 1967 Mar;31(3):562–565. doi: 10.1016/0042-6822(67)90241-3. [DOI] [PubMed] [Google Scholar]
- Groff D. E., Daniell E. Early RNA of adenovirus type 3 in permissive and abortive infections. J Virol. 1981 Nov;40(2):620–624. doi: 10.1128/jvi.40.2.620-624.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagopian H. K., Riggs M. G., Swartz L. A., Ingram V. M. Effect of n-butyrate on DNA synthesis in chick fibroblasts and HeLa cells. Cell. 1977 Nov;12(3):855–860. doi: 10.1016/0092-8674(77)90284-7. [DOI] [PubMed] [Google Scholar]
- Harpst J. A., Ennever J. F., Russell W. C. Physical properties of nucleoprotein cores from adenovirus type 5. Nucleic Acids Res. 1977 Feb;4(2):477–490. doi: 10.1093/nar/4.2.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honda B. M., Baillie D. L., Candido E. P. Properties of chromatin subunits from developing trout testis. J Biol Chem. 1975 Jun 25;250(12):4643–4647. [PubMed] [Google Scholar]
- Javaherian K., Liu J. F., Wang J. C. Nonhistone proteins HMG1 and HMG2 change the DNA helical structure. Science. 1978 Mar 24;199(4335):1345–1346. doi: 10.1126/science.628842. [DOI] [PubMed] [Google Scholar]
- Kedinger C., Brison O., Perrin F., Wilhelm J. Structural analysis of viral replicative intermediates isolated from adenovirus type 2-infected HeLa cell nuclei. J Virol. 1978 May;26(2):364–379. doi: 10.1128/jvi.26.2.364-379.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kit G., Daniell E. Adenovirus core protein synthesis in the absence of viral DNA synthesis late in infection. J Virol. 1978 Jul;27(1):74–80. doi: 10.1128/jvi.27.1.74-80.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornberg R. D. Structure of chromatin. Annu Rev Biochem. 1977;46:931–954. doi: 10.1146/annurev.bi.46.070177.004435. [DOI] [PubMed] [Google Scholar]
- Maniatis T., Jeffrey A., van deSande H. Chain length determination of small double- and single-stranded DNA molecules by polyacrylamide gel electrophoresis. Biochemistry. 1975 Aug 26;14(17):3787–3794. doi: 10.1021/bi00688a010. [DOI] [PubMed] [Google Scholar]
- Mirza M. A., Weber J. Uncoating of adenovirus type 2. J Virol. 1979 May;30(2):462–471. doi: 10.1128/jvi.30.2.462-471.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pignatti P. F., Cassai E. Analysis of herpes simplex virus nucleoprotein complexes extracted from infected cells. J Virol. 1980 Dec;36(3):816–828. doi: 10.1128/jvi.36.3.816-828.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prage L., Pettersson U. Structural proteins of adenoviruses. VII. Purification and properties of an arginine-rich core protein from adenovirus type 2 and type 3. Virology. 1971 Aug;45(2):364–373. doi: 10.1016/0042-6822(71)90337-0. [DOI] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Rouvière-Yaniv J., Yaniv M., Germond J. E. E. coli DNA binding protein HU forms nucleosomelike structure with circular double-stranded DNA. Cell. 1979 Jun;17(2):265–274. doi: 10.1016/0092-8674(79)90152-1. [DOI] [PubMed] [Google Scholar]
- Russell W. C., McIntosh K., Skehel J. J. The preparation and properties of adenovirus cores. J Gen Virol. 1971 Apr;11(1):35–46. doi: 10.1099/0022-1317-11-1-35. [DOI] [PubMed] [Google Scholar]
- Sergeant A., Tigges M. A., Raskas H. J. Nucleosome-like structural subunits of intranuclear parental adenovirus type 2 DNA. J Virol. 1979 Mar;29(3):888–898. doi: 10.1128/jvi.29.3.888-898.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw J. E., Levinger L. F., Carter C. W., Jr Nucleosomal structure of Epstein-Barr virus DNA in transformed cell lines. J Virol. 1979 Feb;29(2):657–665. doi: 10.1128/jvi.29.2.657-665.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimojo H., Yamashita T. Induction of DNA synthesis by adenoviruses in contact-inhibited hamster cells. Virology. 1968 Nov;36(3):422–433. doi: 10.1016/0042-6822(68)90167-0. [DOI] [PubMed] [Google Scholar]
- Simpson R. T. Structure of chromatin containing extensively acetylated H3 and H4. Cell. 1978 Apr;13(4):691–699. doi: 10.1016/0092-8674(78)90219-2. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Sung M. T., Lischwe M. A., Richards J. C., Hosokawa K. Adenovirus chromatin I. Isolation and characterization of the major core protein VII and precursor Pro-VII. J Biol Chem. 1977 Jul 25;252(14):4981–4987. [PubMed] [Google Scholar]
- Tate V. E., Philipson L. Parental adenovirus DNA accumulates in nucleosome-like structures in infected cells. Nucleic Acids Res. 1979 Jun 25;6(8):2769–2785. doi: 10.1093/nar/6.8.2769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahl G. M., Stern M., Stark G. R. Efficient transfer of large DNA fragments from agarose gels to diazobenzyloxymethyl-paper and rapid hybridization by using dextran sulfate. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3683–3687. doi: 10.1073/pnas.76.8.3683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinmann R., Raskas H. J., Roeder R. G. Role of DNA-dependent RNA polymerases II and III in transcription of the adenovirus genome late in productive infection. Proc Natl Acad Sci U S A. 1974 Sep;71(9):3426–3439. doi: 10.1073/pnas.71.9.3426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weintraub H., Van Lente F. Dissection of chromosome structure with trypsin and nucleases. Proc Natl Acad Sci U S A. 1974 Oct;71(10):4249–4253. doi: 10.1073/pnas.71.10.4249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winnacker E. L. Adenovirus DNA: structure and function of a novel replicon. Cell. 1978 Aug;14(4):761–773. doi: 10.1016/0092-8674(78)90332-x. [DOI] [PubMed] [Google Scholar]