Abstract
In most laboratories, the screening for leishmanicidal compounds is carried out with Leishmania promastigotes or axenic amastigotes. However, the best approach to identify leishmanicidal compounds is the use of amastigotes residing in macrophages. Reporter gene-based assays are relatively new tools in the search for drugs against eucaryotic protozoa, permitting the development of faster, more automated assays. In this paper, we report on the establishment of a rapid screening assay in a 96-well format. A luciferase-transgenic (Luc-tg) Leishmania major strain was generated and used to infect bone marrow-derived macrophages (BMDM). Amastigote-infected BMDM were treated with different compound concentrations. Cells were lysed with a luciferin-containing buffer, and the resulting luminescence was measured to determine the half-maximal inhibitory concentration (IC50). To validate this new amastigote screening assay, a library of a new class of quinolinium salts was synthesized and tested for leishmanicidal activity. Some of the quinolinium salts showed very promising activities, with IC50s against intracellular amastigotes (IC50 < 1 μg/ml) and selectivity indices (SI > 20) that match the criteria of World Health Organization (WHO) for hits. Compound 21c (IC50 = 0.03 μg/ml; SI = 358) could become a new lead structure for the development of improved chemotherapeutic drugs against L. major. In summary, we describe the establishment of a new 96-well format assay with Luc-transgenic L. major for the rapid screening of compounds for leishmanicidal activity against intracellular amastigotes and its application to the identification of a new class of quinolinium salts with most promising leishmanicidal activity.
INTRODUCTION
Leishmaniasis is an infectious disease caused by protozoa of the genus Leishmania, which occurs in the subtropical and tropical regions of the world. It belongs to the 13 most important tropical diseases listed by the WHO (http://www.who.int/neglected_diseases/en/). There are three clinical main forms of this disease—the cutaneous, the mucocutaneous, and the visceral forms—depending on the infecting Leishmania species and on the host immune response. Nonetheless, there are presently only six drugs available to treat human leishmaniasis: the pentavalent-antimony agents sodium stibogluconate and meglumine antimoniate, as well as amphotericin B (compound 1) (Fig. 1), pentamidine, paromomycine, and miltefosine (compound 2) (Fig. 1), are used to treat the different clinical forms of this infectious disease (1–3). Unfortunately, all of these drugs have serious side effects, and there is a high level of resistance development (4–6). Clinical trials with therapeutic or prophylactic vaccinations against leishmaniasis have not been successful yet (7, 8). Therefore, new drugs to combat this infectious disease are urgently required. Screening of compound libraries against Leishmania major—the relatively harmless pathogen causing Old World cutaneous leishmaniasis—also opens the possibility of identifying new compounds to treat more severe forms of leishmaniasis, such as, for instance, non-self-healing mucocutaneous and visceral forms, since most leishmanicidal drugs are active against different Leishmania species.
Fig 1.
Structures of the tested leishmanicidal reference compounds.
Although promastigotes are not the clinically relevant form of Leishmania species, this life stage is addressed in most drug screening assays against Leishmania (9, 10). Cultivation of promastigotes and therefore the realization of screening assays with the insect form of Leishmania are relatively easy and cheap. We and other groups were able to identify new leishmanicidal compounds using promastigotes for drug screening (11–13). Sometimes axenic amastigotes are applied in drug screening assays. However, the generation of completely differentiated axenic amastigotes by temperature and pH switch is not feasible for L. major, in contrast to many other Leishmania species (14, 15). On the other hand, drugs for successful treatment of leishmaniasis should be able to penetrate the plasma membrane of macrophages in order to act on intracellular amastigotes. Sometimes additional host cell-mediated effector mechanisms contribute to intracellular parasite killing. This scenario cannot be tested with axenic amastigotes, which grow outside a host cell. Ideally, to be efficient and exhaustive, a drug screening procedure requires conditions that imitate the environment in the parasitophorous vacuole of the target cell. Thus, testing of compounds against amastigotes residing in macrophages is one of the most important steps in drug screening. Therefore, the most interesting compounds with low half-maximal inhibitory concentrations (IC50s) and good selectivity indices (SI) against L. major promastigotes detected by alamarBlue assay in our laboratory were further tested against intracellular amastigotes. We applied ethidium bromide-acridine orange (EB-AO) staining to determine the rate of infection of peritoneal macrophages with L. major amastigotes after treatment with different concentrations of drugs of interest (12, 13, 16). However, EB-AO staining is very labor-intensive, time-consuming, and expensive. Cell culture in chamber slides has to be performed, and the number of amastigotes in macrophages has to be determined by fluorescence microscopy. Therefore, EB-AO staining is not applicable to the screening of high numbers of compounds for leishmanicidal activity.
Lang and coworkers have recently reported bioluminescence screening to be a powerful new method to analyze drug efficiency against Leishmania amazonensis amastigote-infected macrophages (17). This assay was designed to closely reflect the in vivo situation of Leishmania infections and to allow a high throughput compared to conventional assays with EB-AO or Giemsa staining. In the present paper, we describe the adaption of the amastigote assay to L. major. Several control experiments using the reference drugs against Leishmania—amphotericin B (compound 1) and miltefosine (compound 2) (Fig. 1)—for testing the applicability of the newly established drug screening assay in a 96-well format were performed.
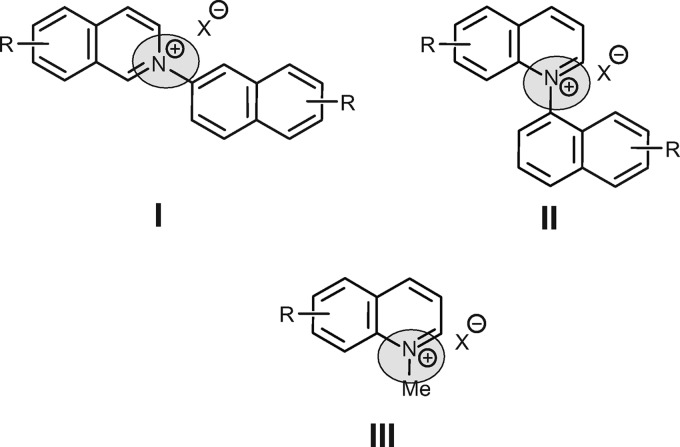
With the new screening assay, the best candidates of a newly designed library of novel quinolinium salts, selected after primary screening against L. major promastigotes and the J774.1 macrophage line, were tested for leishmanicidal activity against amastigotes residing in macrophages. These quinolinium salts are analogs of the N,C-coupled naphthylisoquinolines (18, 19) representing a subclass of the naphthylisoquinoline alkaloids (20–22). After the discovery of ancisheynine, the first cationic (since N,C-coupled) naphthylisoquinoline, in 2003 by Yang and coworkers (18), these secondary metabolites have aroused much stereochemical and synthetic interest (23–25) because of their promising biological activities, e.g., against Leishmania, Plasmodium, and Trypanosoma (16, 26). Based on structure-activity relationship (SAR) studies of the naturally occurring N,C-coupled naphthylisoquinolines, several derivatives with excellent activities have been synthesized in recent years. For an investigation of the importance of the position of the positively charged nitrogen on the biological activity, quinolinium salts were synthesized (Fig. 2). Different from the successful synthesis of the N,C-coupled N-arylisoquinolines (I) (27) from the respective benzopyrylium salts and amines, the analogous preparation of the corresponding N-arylquinolinium salts (II) is not possible. Furthermore, the direct quaternization of quinolines with aryl halides is limited to activated substrates (28). In this initial study, the structural modification was therefore restricted to N-methyl instead of N-aryl analogs. Within the library of 49 novel analogs thus prepared, some showed very promising activity against both stages of L. major.
Fig 2.
Structures of the N-naphthylisoquinolines (I) and the regioisomeric N-naphthylquinolines (II). The first step of the structural modification is N-methylquinolinium salts of type III.
MATERIALS AND METHODS
Leishmania strain and maintenance of wild-type (WT) parasites.
The cloned virulent L. major isolate (MHOM/IL/81/FE/BNI) was maintained by passaging in female BALB/c mice. Promastigotes were grown in vitro in blood agar cultures at 27°C, 5% CO2, and 95% humidity.
Generation of the Luc-tg L. major strain.
A 1.801-kbp fragment of the firefly luciferase (Luc)-coding region was cut from pGL4.13 (Promega, Mannheim, Germany) by NcoI-XbaI and cloned into the NcoI-NheI-restricted Leishmania expression vector pLEXSY-hyg2 (Jena Bioscience, Jena, Germany), containing a marker gene for selection with hygromycin B (HYG). After linearization by SwaI, Luc and HYG were integrated into the 18S rRNA locus of L. major by homologous recombination. The virulence of Luc-transgenic (Luc-tg) L. major was maintained by passage in female BALB/c mice. Luc-tg promastigotes were grown in blood agar cultures with addition of 50 μg/ml HYG at 27°C, 5% CO2, and 95% humidity.
Media.
Two different media were used in the experiments described below. Five hundred milliliters phenol red-free RPMI 1640 medium (Invitrogen, Darmstadt, Germany) was supplemented with 50 ml heat-inactivated fetal calf serum (FCS; PAA, Pasching, Austria), 5 ml 200 mM l-glutamine (Biochrom AG, Berlin, Germany), 5 ml 1 M HEPES (pH 7.2; Invitrogen), 1 ml 100-U/ml penicillin G (Sigma-Aldrich, Taufkirchen, Germany), 2.5 ml 10-mg/ml gentamicin (Sigma-Aldrich), and 2.5 ml 10 mM 2-mercaptoethanol (Sigma-Aldrich). Conditioned medium for generation of bone marrow-derived macrophages (BMDM) contained 50 ml Dulbecco modified Eagle medium (DMEM; Invitrogen), 0.25 ml 10 mM 2-mercaptoethanol (Sigma-Aldrich), 0.5 ml nonessential amino acids (Invitrogen), 5 ml heat-inactivated FCS (PAA), 2.5 ml heat-inactivated horse serum (Invitrogen), 0.5 ml 1 M HEPES (Invitrogen), 1 ml 200 mM l-glutamine (Biochrom AG), and 7.5 ml L929 supernatant (29).
Isolation of peritoneal macrophages and generation of BMDM.
Peritoneal macrophages were isolated as previously described (12, 13). The method for generating BMDM was adapted from a publication by Schleicher and Bogdan (29). Female BALB/c mice (6 to 12 weeks old) were sacrificed by cervical dislocation and disinfected with 70% ethanol. Femurs and tibiae of the mice were removed and purified from the surrounding muscles and connective tissue. The bones were opened at the epiphyses under sterile conditions. The bone marrow was flushed with a 23-gauge needle with ice-cold phenol red-free RPMI 1640 medium with 10% FCS into a 50-ml Falcon tube. The cells were centrifuged (300 × g, 4°C, 10 min) and washed with cold phosphate-buffered saline (PBS). After a further centrifugation (300 × g, 4°C, 10 min), the cells were resuspended in conditioned medium for macrophage generation containing L929 supernatant (medium composition above). The number of nucleated cells in bone marrow was determined with Tuerk solution; 6 × 106 bone marrow cells in a total volume of 50 ml conditioned medium were transferred to hydrophobic cell culture plates for suspension cultures to ensure low adherence of the generated macrophages (8 ml per plate; Sarstedt, Nümbrecht, Germany). Cells were incubated at 37°C and 5% CO2, and BMDM were harvested after 6 days. The old medium was replaced with 10 ml ice-cold phenol red-free RPMI 1640 medium with 10% FCS, thus removing nonadherent cells and debris. The culture plates were incubated for 10 min on ice to alleviate the detachment with rubber policemen. Detached BMDM were collected in precooled 50-ml Falcon tubes and centrifuged (300 × g, 4°C, 10 min). BMDM were washed with 20 ml ice-cold RPMI 1640 medium with 10% FCS and centrifuged (300 × g, 4°C, 10 min). Finally, BMDM were resuspended in 1 ml ice-cold complete phenol red-free RPMI 1640 medium with 10% FCS. The cell number was determined after staining with trypan blue.
Tested compounds.
Compound 1 (amphotericin B; Sigma-Aldrich) and compound 2 (miltefosine [1-hexadecylphosphorylcholine]; Cayman Chemical Company, Ann Arbor, MI) were tested as leishmanicidal reference compounds (Fig. 1). IC50s for all compounds against L. major promastigotes and amastigotes determined in our laboratory have been published previously (12, 13, 16, 30).
The compounds were dissolved in dimethyl sulfoxide (DMSO). The final concentration of DMSO in each tested well was 1%. All control assays (blanks and growth controls) also contained 1% concentrations of solvents.
Chemical materials.
Starting materials for the synthesis of compounds were purchased from Sigma-Aldrich and used without further purification. The organic solvents were distilled and dried prior to use, using standard procedures.
General experimental procedures.
Melting points were determined on a Reichert-Jung Thermovar hot plate (Reichert Optische Werke, Vienna, Austria) and are uncorrected. The nuclear magnetic resonance (NMR) spectra (1H, 250 MHz/400 MHz, and 13C, 100 MHz/150 MHz) were recorded on Bruker DMX 600, Avance 400, or Avance 250 (Bruker BioSpin, Rheinstetten, Germany), using deuterochloroform (δ 7.24 and 77.23; Roth, Karlsruhe, Germany) and methanol-d4 (δ 3.31 and 49.15; Roth, Karlsruhe, Germany) as the solvents and internal 1H and 13C standards. Electron ionization mass spectra were determined on a Finnigan MAT 8200 mass spectrometer (Thermo Electron Corporation, Whatman) in the positive mode (70 eV). Electrospray ionization (ESI) spectra were determined on a Bruker Daltonics micrOTOF-focus. Combustion analyses were performed on a CHNS 932 analyzer (Leco Instruments, St. Joseph, MI).
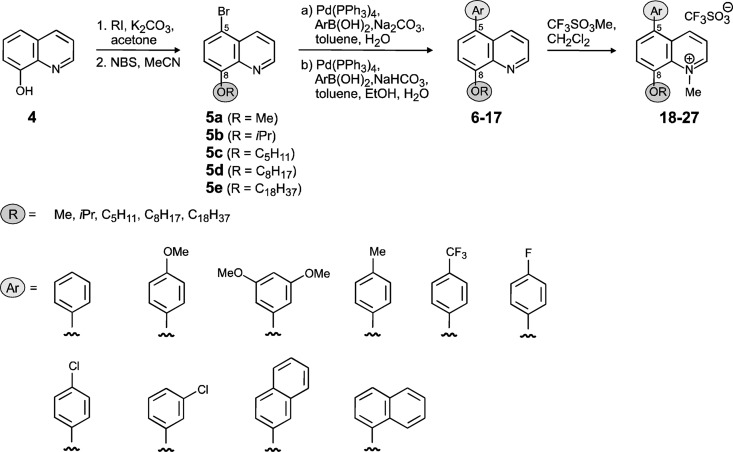
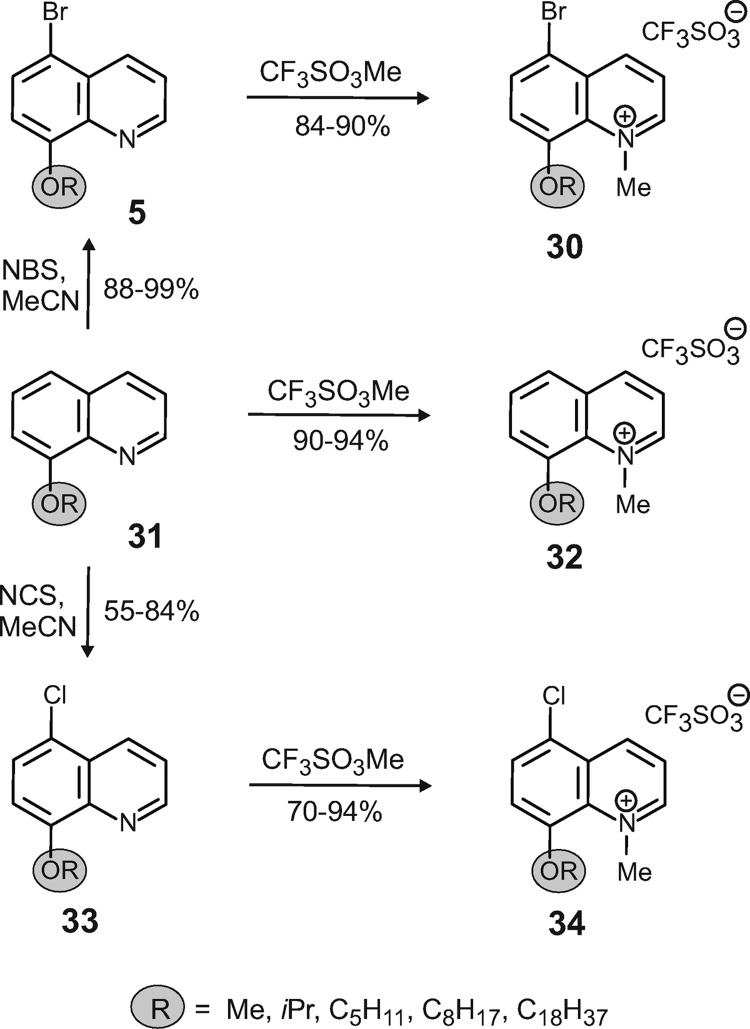
Synthesis of the 8-alkyloxyquinolines 31a to 31e.
(Fig. 3 and 4) To a solution of 8-hydroxyquinoline (compound 4; 10.0 g, 68.9 mmol) and K2CO3 (23.8 g, 172 mmol) in acetone (300 ml) the corresponding alkyl halide (172 mmol) was added, and the reaction mixture was refluxed for 48 h. After extraction of the aqueous phase with CH2Cl2, the organic phase was washed with a saturated aqueous solution of K2CO3 and dried (MgSO4). After removal of the solvent by evaporation, the residue was purified on a flash column (eluent was petroleum ether-ethyl acetate, 5:1), giving the 8-alkyloxyquinolines in good yields (75 to 99%).
Fig 3.
Synthesis of the N-methylquinolinium salts.
Fig 4.
Extension of the compound library: synthesis of 8-bromo- and 8-chloroquinolinium salts.
Synthesis of 8-alkyloxy-5-bromoquinolines 5a to 5e and 8-alkyloxy-5-chloroquinolines 33a to 33d (Fig. 3 and 4).
To a solution of 8-Alkyloxyquinolines (5.00 mmol) in acetonitrile (35 ml), N-bromosuccinimide (5.00 mmol) was added. After stirring for 18 h at room temperature (RT), the solvent was evaporated and the residue extracted with water and CH2Cl2. The organic layer was dried (MgSO4) and the residue recrystallized from CH2Cl2/petroleum ether, yielding the desired 8-alkoxy-5-bromoquinolines compounds as bright yellow crystals (88 to 99%). For the synthesis of the 8-alkyloxy-5-chloroquinolines, N-chlorosuccinimide instead of N-bromosuccinimide was used.
Synthesis of 8-alkyloxy-5-arylquinolines 6 to 17.
(Fig. 3)A degassed mixture of EtOH (4 ml), H2O (4 ml), and toluene (20 ml) was added to a mixture of 5-bromo-8-alkoxyquinoline (0.84 mmol), NaHCO3 (2.02 mmol), boronic acid (1.01 mmol), and Pd(PPh3). The mixture was heated at 90°C overnight. After cooling to RT, H2O (10 ml) was added and the aqueous phase was extracted with CH2Cl2. Drying (MgSO4) and evaporation of the combined organic extracts gave a residue which was purified on a flash column (eluent was petroleum ether-ethyl acetate, 4:1), providing the 8-alkyloxy-5-arylquinolines as white solids (74 to 95%).
Synthesis of 8-alkyloxy-1-methylquinolinium salts 18 to 30, 32, and 34.
(Fig. 3 and 4)To a solution of 8-alkyloxy-5-arylquinolines (100 mg) in dry CH2Cl2 (10 ml) methyl trifluoromethanesulfonate (1.3 eq) was added. After stirring at RT overnight, the solvent was evaporated and the residue was purified on a flash column (eluent was CH2Cl2-MeOH, 15:1), yielding the desired products as yellow crystals (72 to 88%).
The full experimental data for compounds 5 to 34 can be found in the supplemental material.
Determination of IC50s for L. major promastigotes and macrophages.
Antiparasitic activities against WT and Luc-tg L. major promastigotes with a final cell density of 1 × 107 per ml were tested at 27°C using the alamarBlue assay as described previously (12, 13, 16).
Cytotoxicities of compounds against J774.1 macrophages and BMDM were determined using the same protocol for the alamarBlue assay as for promastigotes, with two modifications. The final cell concentrations were 1 × 105 per ml for J774.1 macrophages and 2 × 105 per ml for BMDM. Plates with macrophages were incubated at 37°C.
Each compound was tested twice in two independent experiments against L. major and macrophages, and the result was expressed as the mean value.
Determination of IC50 for intracellular Luc-tg L. major amastigotes.
The method of screening of drugs against Luc-tg L. major amastigotes residing in macrophages was adapted from that of Lang and coworkers (17). BMDM or peritoneal macrophages were seeded in white 96-well plates with clear bottoms (Greiner Bio-One, Frickenhausen, Germany) at a final concentration of 2 × 105 cells per ml (final culture volume per well: 200 μl). BMDM were incubated for 4 h at 37°C to promote cell adhesion. Macrophages were infected with Luc-tg L. major promastigotes at a ratio of 1:15. For this purpose, a promastigote solution of 3 × 106 per ml in RPMI 1640 medium with 10% FCS was prepared. Medium was removed from the macrophages, and 200 μl Luc-tg promastigote solution was added. Cocultures were performed for 24 h at 37°C to allow infection and amastigote differentiation. The amastigote-infected macrophages were washed three times with RPMI 1640 medium with 10% FCS to remove remaining extracellular promastigotes. Finally, 200 μl phenol red-free RPMI 1640 medium with 10% FCS containing decreasing concentrations of compounds (100 μM, 20 μM, 4 μM, 0.8 μM, 0.16 μM, 0.032 μM, etc.) was added (for plate setup, see Fig. S1 in the supplemental material). Notably, the outer positions of the 96-well plate were filled with distilled water to avoid edge effects by evaporation. Drug-treated infected macrophages were incubated for a further 24 h. Finally, 50 μl britelite plus (PerkinElmer, Waltham, MA) was added to each well. britelite plus is a commercially available lysis buffer, containing ATP and luciferin, which initiates the enzymatic reaction of luciferin to oxyluciferin catalyzed by luciferase expressed by living L. major amastigotes. After 5 min of incubation, the resulting luminescence was measured with a Victor X Light 2030 luminometer (PerkinElmer). The intensity of light emission after cell lysis is proportional to the number of intracellular amastigotes in macrophages (data not shown). The luminescence was reduced after treatment with a leishmanicidal compound compared to the controls without compound (growth control). The measured luminescence values were corrected for samples with 100 μM by subtraction of blank 1 (100 μM compound + medium + solvent) and for samples with lower compound concentrations and the growth control by subtraction by blank 2 (medium + solvent) (see Fig. S1 in the supplemental material). The IC50s were calculated by linear interpolation as previously described (31). Each assay was performed in two independent experiments, and the results were expressed as the means.
The Z′ factor characterizes quantitatively the robustness of a screening assay (32). This statistical factor was calculated for each 96-well plate used to screen the compound library for the positive controls with amphotericin B (compound 1) and for the negative controls (growth controls) according to the method of Zhang et al. (32) and expressed as the mean.
Transmission electron microscopy.
Transmission electron microscopy using contrastive agents OsO4 and uranyl acetate was performed as previously described (30).
Cysteine cathepsin fluorescence activity assay.
Proteins were isolated from BMDM after two washings with PBS and pelleted by centrifugation at 300 × g for 10 min. The pellet was redissolved in acidic sodium acetate buffer (200 mM sodium acetate [CH3COONa], 1 mM EDTA, 0.05% polyoxyethyleneglycol dodecyl ether [Brij 35], 0.5 mM dithiothreitol [DTT] [pH 5.5]). Cells were disrupted by repeated freezing in liquid nitrogen and thawing at 37°C (3 cycles), followed by centrifugation (700 × g, 4°C, 15 min). Supernatant containing the lysosomal cysteine cathepsins was transferred to fresh tubes and stored at −20°C until use. Final protein concentrations of these lysates were determined with bicinchoninic acid (BCA) protein assay kit (Pierce Biotechnology, Inc., Pittsburgh, PA). Yields were approximately 0.5 to 1 μg/μl. Proteolytic activities were determined by degradation of the fluoropeptide Z-Phe-Arg-4-methyl-coumarin-7-amide (Z-Phe-Arg-AMC; Bachem, Bubendorf, Switzerland), a specific substrate of cathepsins B and L, using fluorescence proteinase activity assays. These assays were carried out in black 96-well microtiter plates (Nunc GmbH, Langenselbold, Germany). A volume of 5 μl protein lysate was mixed with 89 μl acidic sodium acetate buffer for each sample, followed by addition of 1 μl DMSO, 1 μl 10 mM epoxysuccinyl peptide l-trans-epoxysuccinyl-Leu-4-guanidinobutylamide (E64; Bachem), or 1 μl 5 mM l-trans-epoxysuccinyl-Ile-Pro-OH propylamide (CA074; Bachem). These samples were preincubated for 15 min at 37°C. Finally, the proteolytic reaction was initiated by addition of 5 μl 500 μM Z-Phe-Arg-AMC solution. Proteolytic release of 7-amino-4-methyl-coumarin (AMC) was continuously monitored for 45 min by spectrofluorometry at an excitation wavelength of 355 nm and an emission wavelength of 460 nm using a Fluoroscan Ascent fluorescence reader (Thermo Electron Corporation, Langenselbold, Germany). Standard curves were prepared with the fluorochrome AMC (Bachem). Proteinase activities were calculated using the linear range of reaction curves.
Flow cytometric analysis.
Cell suspensions (1 ml) of bone marrow cells and BMDM (1 × 106 per ml) were pipetted into tubes and centrifuged (750 × g, 4°C, 5 min). The resulting cell pellets were resuspended in 1 ml buffer (0.1% sodium azide, 5% FCS in PBS) and again centrifuged (750 × g, 4°C, 5 min). The cell pellet was dissolved in 50 μl buffer. Antibodies against CD16/CD32 (1 μl; BD Biosciences, Pharmingen, San Diego, CA) to block unspecific binding of the specific antibodies to Fc receptors were added and incubated on ice for 10 min. After cells were washed with 1 ml buffer, they were stained in 50 μl buffer with 1 μl anti-mouse F4/80-fluorescein isothiocyanate (FITC)-labeled antibody (BioLegend, San Diego, CA) and incubated on ice in the dark for 30 min. Cells were washed with 2 ml buffer and centrifuged (750 × g, 4°C, 5 min). Finally, the pellet was resolved in 300 μl buffer. The control samples contained unstained cells or cells stained with a FITC-labeled IgG2a kappa isotype control antibody (BD Biosciences, Pharmingen). The data were recorded with a MACSQuantAnalyzer (Miltenyi Biotec, Bergisch Gladbach, Germany).
RESULTS
In vitro-generated BMDM can be successfully infected with L. major but are not feasible for EB-AO staining.
In our previous experiments, peritoneal macrophages isolated from naive mice had been applied to EB-AO staining to determine IC50s against intracellular amastigotes (12, 13, 16). Up to three mice were required to determine the IC50s of one compound for L. major amastigotes by EB-AO staining. Therefore, macrophages generated from bone marrow should be alternatively applied in our amastigote screening assay to reduce the costs and preparation time, and to follow the recommendations for animal welfare (33, 34). The BMDM were generated for 6 days from bone marrow cells in medium containing L929 supernatant as described in Materials and Methods. The resulting cell population was phenotypically characterized to ensure that macrophages had been generated successfully. The phenotypical analysis by transmission electron microscopy revealed that generated BMDM had the typical morphology of macrophages with pseudopods (Fig. 5A).
Fig 5.
Phenotypical characterization of BMDM. Macrophages were generated from bone marrow cells isolated from BALB/c mice as described in Materials and Methods. Generated cells were phenotypically characterized. (A) Transmission electron microscopic image of generated BMDM (magnification, ×5,000). (B) Flow cytometric analysis of generated cells with macrophage-specific antibody against F4/80 (black) and isotype control (gray). (C) Cysteine cathepsin fluorescence activity assay using substrate Z-Phe-Arg-AMC and inhibitors E64 and CA074. (D) Transmission electron microscopic image of L. major amastigotes inside BMDM 24 h after infection (magnification, ×16,000). Am, amastigote; N, nucleus of macrophage; P, pseudopod of macrophage.
Furthermore, flow cytometric analysis demonstrated that 87% of the cells were positive for the macrophage-specific marker F4/80 (Fig. 5B) (29). In contrast, bone marrow cells from BALB/c mice were negative for F4/80 (data not shown). High cysteine cathepsin activity is a further hallmark of macrophages (35). Therefore, activities of cysteine cathepsins in bone marrow stem cells and generated BMDM were measured and the activities were compared. The results of the fluorescence activity assay using the specific substrate Z-Phe-Arg-AMC revealed that BMDM contained high cysteine cathepsin activity after 6 days of in vitro generation (Fig. 5C). In contrast, the cysteine cathepsin activity in bone marrow cells was very low (Fig. 5C). The protease activity was specifically inhibited by E64, a broad-spectrum inhibitor for cysteine cathepsins, and by CA074, a cathepsin B-specific inhibitor. Furthermore, our analysis verified that 6-day-old BMDM were successfully infected with L. major promastigotes. Intracellular unflagellated roundish amastigotes were detected in BMDM by transmission electron microscopy 24 h after incubation of the coculture (Fig. 5D).
The phenotypical analyses suggested not only that BMDM were successfully generated and infected with L. major but also that intracellular differentiation from promastigotes to amastigotes took place within 24 h (Fig. 5D).
However, detection of amastigotes by EB/AO staining was not practicable with amastigote-infected BMDM. In contrast to peritoneal macrophages, BMDM have numerous acidic vacuoles. Therefore, “unspecific” staining of acidic compartments (e.g., lysosomes) in infected BMDM by AO overlaid amastigote-specific signals (data not shown).
Screening assay in a 96-well format with Luc-tg L. major is applicable to determine IC50s against intracellular amastigotes.
In order to accelerate the screening of compounds against the clinically relevant amastigotic form of L. major and to establish a drug screening protocol with BMDM, we modified the protocol recently published by Lang and coworkers for drug screening with Luc-tg L. amazonensis (17). The plate setup used for our developed amastigote screening assay is shown in Fig. S1 in the supplemental material. BMDM were generated from bone marrow of BALB/c mice and infected with Luc-tg L. major promastigotes. The Luc-tg L. major strain originated from WT L. major strain MHOM/IL/81/FE/BNI. The newly developed assay was set up in a 96-well format, and some wells with control cultures (blanks 1 and 2, growth control) were included (see Fig. S1 in the supplemental material). Several experiments were performed to verify the new assay. The clinically used reference drugs 1 and 2 were applied for the control experiments (12, 13).
In the first experiment, the susceptibilities of WT and Luc-tg L. major promastigotes to different leishmanicidal compounds were compared. The IC50s recently published for compounds 1 and 2 (12, 13, 30) were confirmed for WT L. major promastigotes with the alamarBlue assay (Table 1). The IC50s for all tested compounds for Luc-tg L. major promastigotes were in the same range and did not show any significant differences (Table 1). Additionally, the IC50s for all compounds were also determined for the Luc-tg L. major strain by measurement of luminescence after addition of britelite plus, a lysis buffer containing luciferin and ATP (Table 1). There were no significant differences in the IC50s for Luc-tg L. major promastigotes detected by the alamarBlue assay or by luminescence readout (Table 1). The comparison of the WT and Luc-tg L. major strains and of the two different readouts suggested that the sensitivities of the two strains of L. major to drugs and the two readouts were highly comparable.
Table 1.
Comparison of the IC50s determined by the alamarBlue assay for WT and Luc-tg L. major promastigotes and by luminescence for Luc-tg promastigotes
| Compound | IC50 (μM) |
||
|---|---|---|---|
| WT L. major, alamarBlue | Luc-tg L. major |
||
| alamarBlue | Luminescence | ||
| 1 | 0.2 | 0.2 | 0.4 |
| 2 | 31.5 | 28.6 | 33.5 |
Furthermore, we were interested in determining whether the IC50s determined by EB-AO staining would be comparable with IC50s of the new amastigote assay. The IC50 determined by the luminescence assay in the 96-well format with Luc-tg L. major infecting peritoneal macrophages (Table 2) was highly comparable with the IC50 of 0.075 μM for amphotericin B (compound 1) recently determined by EB-AO staining in our lab (12, 13).
Table 2.
Determination of the IC50s of compounds 1 and 2 for amastigotes infecting peritoneal macrophages or BMDM by the luminescence assay in 96-well plates after 24 h of incubation
| Compound | IC50 (μM) for L. major infecting: |
|
|---|---|---|
| Peritoneal macrophages | BMDM | |
| 1 | 0.04 | 0.05 |
| 2 | 42.2 | 33.0 |
Finally, the IC50s of the clinically used drugs against amastigotes were compared for Luc-tg L. major infecting peritoneal macrophages and BMDM (Table 2). For reference compounds 1 and 2, no significant differences of the IC50 were detected.
The Z′ factor, calculated to determine the robustness of the new screening assay in the 96-well format with Luc-tg L. major, was 0.63. Therefore, the newly established assay is optimal to determine IC50s against intracellular amastigotes (32).
In summary, the results show that the new assay in the 96-well format with Luc-tg L. major parasites infecting BMDM to screen compounds more rapidly against intracellular amastigotes was established successfully.
Identification of new leishmanicidal quinolinium salts active against intracellular amastigotes with high selectivity indices.
A library of 49 synthetic quinolinium salts, 18 to 30, 32, and 34, was tested against L. major promastigotes. Additionally, the cytotoxicities of these compounds against J774.1 macrophages were determined by the alamarBlue assay.
Only 19 of the tested compounds showed no leishmanicidal activity against promastigotes (IC50 > 100 μM) (Table 3). Ten compounds showed moderate activity against L. major promastigotes (100 μM > IC50 ≥ 10 μM) (Table 3). Finally, 20 tested quinolinium salts revealed good antileishmanial activity (IC50 < 10 μM) (Table 3). Structure-activity relationships demonstrated that compounds with a methoxy or an isopropoxy function at C-8 showed neither an antileishmanial effect nor any cytotoxicity. Structures with a longer alkyl chain on the C-8 oxygen, like, e.g., n-pentyl or n-octyl, however, had very promising activity against L. major. Furthermore, the substituent at C-5 had an influence on the leishmanicidal activity, too. The best activities against L. major were shown by structures with an electron-donating substituent on the aryl residue at C-5. The most auspicious compounds, 19d, 21c, and 21d, have very high selectivity indices, between 15.9 and 19.8. The quinolinium salts with an aryl part with an electron-withdrawing substituent at C-5, in contrast, had only low leishmanicidal properties and therefore bad selectivity indices, of about 1.
Table 3.
IC50s of synthetic quinolinium salts 18 to 30, 32, and 34 for L. major promastigotes and J774.1 macrophages determined by the alamarBlue assay and calculated SI

a SI = IC50 for J774.1/IC50 for L. major.
b ND, not determined.
Out of these 20 compounds, 7 were further tested against L. major amastigotes infecting BMDM with the newly developed luminescence assay in the 96-well format. Of these, compounds 19d, 21c, and 21d, which had low IC50s (<1.5 μM) against L. major promastigotes and very good SI (≥10), were selected for further tests. The additionally selected compounds 18d, 22d, 26d, and 27d, however, which had IC50s of <1.5 μM, were characterized by a bad or moderate SI (<10) (Table 3). All selected compounds revealed activities against amastigotes, with IC50s lower than the corresponding IC50s against promastigotes. Out of the 7 compounds, 6 revealed activities against intracellular amastigotes, with an IC50 of <1 μg/ml and an SI of >20, matching the criteria of the WHO for hits (Table 4) (36, 37). Out of all compounds tested, 21c turned out to be the best one, with an IC50 of 0.06 μM against amastigotes and an SI of 358. Therefore, this quinolinium salt should become a new lead for the development of new drugs against L. major (Table 4).
Table 4.
IC50s of synthetic quinolinium salts against Luc-tg L. major amastigotes, determined by the luminescence assay, and against BMDM, determined by the alamarBlue assay, and calculated SI
| Compound | IC50 for: |
SIa | ||
|---|---|---|---|---|
| Amastigotes |
BMDM, μM | |||
| μM | μg/ml | |||
| 1 | 0.05 | 0.05 | >20 | >400 |
| 18d | 0.05 | 0.03 | 7.4 | 148 |
| 19d | 0.03 | 0.01 | 2.30 | 76.7 |
| 21c | 0.06 | 0.03 | 21.5 | 358 |
| 21d | 0.04 | 0.02 | 2.80 | 70.0 |
| 22d | 0.26 | 0.14 | 5.30 | 20.4 |
| 26d | 0.04 | 0.02 | 1.49 | 37.3 |
| 27d | 0.09 | 0.06 | 1.65 | 18.3 |
SI = IC50 for BMDM/IC50 for L. major.
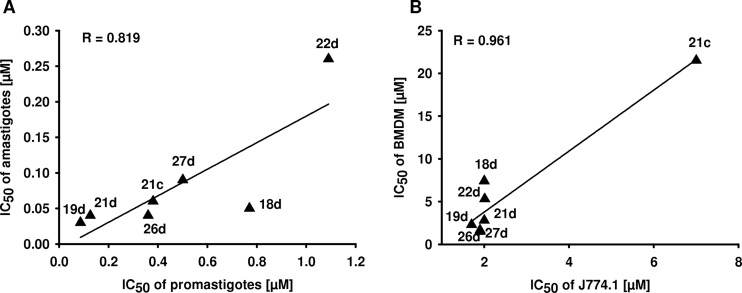
Interestingly, there was a positive correlation between the IC50s of the quinolinium salts for L. major promastigotes determined in the alamarBlue assay and the IC50s against amastigotes (Fig. 6A). The only outlier was compound 18d, which had a lower IC50 for L. major amastigotes than expected. We assume that this compound interacts additionally with host cell molecules that are essential for Leishmania-host cell interaction. There was also a correlation of the IC50s of compounds for J774.1 macrophages and BMDM (Fig. 6B).
Fig 6.
(A) Correlation of IC50s of compounds against promastigotes and amastigotes. (B) Correlation of IC50s of compounds for J774.1 macrophages and BMDM.
In summary, the new amastigote assay with Luc-tg L. major infecting BMDM successfully identified the antiamastigotic activity of a family of new synthetic quinolinium salts.
DISCUSSION
Different screening methods to determine IC50s against Leishmania amastigotes have been described in the literature (9, 38–42). Most of these assays, however, are very time-consuming or expensive or need highly specialized equipment (39, 42).
Over the past years, the ability to select transgenic Leishmania, expressing reporter proteins such as green fluorescent protein, β-lactamase, or luciferase, opened up new possibilities for the development of time-saving drug screening tests against the clinically relevant amastigote stage (17, 40, 41). Recently, bioluminescent L. amazonensis parasites expressing luciferase were applied for the high-throughput screening of drugs acting on amastigote-harboring macrophages (17). In the present paper, we describe the successful transfer of this method to a second Leishmania species. We developed an amastigote assay with BMDM and a Luc-tg L. major strain in a 96-well format for the fast and economically priced screening of compounds. Only a luminescence reader for 96-well plates is additionally necessary to perform this screening assay in a standard equipped cell culture laboratory. It is now possible to measure the killing of L. major amastigotes inside their host macrophages in a quantitative way. In comparison to the microscopic counting of EB-AO-stained amastigotes infecting macrophages, this method is significantly faster and cheaper, and the determination of IC50s is highly objective. The developed plate setup of our new amastigote assay permits testing of up to three compounds per 96-well plate (see Fig. S1 in the supplemental material).
The first successful application of the new amastigote assay was performed with seven selected members of a library of novel synthetic quinolinium salts, which had first been tested against L. major promastigotes in the alamarBlue assay (compounds 18 to 30, 32, and 34). All of these selected quinolinium salts were active against intracellular amastigotes. Six compounds revealed activities with IC50s of <1 μg/ml and SI of >20, matching the criteria of the WHO for new hits (36, 37). Compound 21c, with an SI of 358, turned out to be the best derivative of the quinolinium salts and thus may become a lead structure for further improvement of the new antileishmanial compound family. It should be further tested in vivo in the L. major infection model.
Interestingly, there was a significant correlation of IC50s against L. major promastigotes and amastigotes, suggesting a direct antiparasitic mechanism for most of the analyzed quinolinium salts without additional contribution of antileishmanial host cell factors and promising that the alamarBlue promastigote assay is sufficient to screen compound libraries. However, potential leishmanicidal compounds targeting strongly or exclusively the parasite-macrophage interaction and amastigote-specific gene products could be not identified with promastigote assays. Therefore, drug screening against intracellular amastigotes is highly recommended in literature (39, 43). The newly developed Luc-tg L. major amastigote assay with BMDM in the 96-well format is a good tool for drug screening to avoid false-negative results during screening for leishmanicidal compounds.
In summary, we report the successful establishment of a compound screening assay in a 96-well format against the amastigote stage of L. major. The use of luciferase as a reporter gene permits a fast, economically priced, and objective screening of new potential drug candidates against L. major amastigotes even in a moderately equipped laboratory, which can be applied as primary assay to detect leishmanicidal compounds. We found new synthetic aminoquinolinium salts revealing strong antiamastigotic activities. Especially compound 21c (IC50 = 0.03 μg/ml; SI = 358) could become a lead structure for the development of new chemotherapeutic drugs against L. major.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by a grant of the German Research Foundation (DFG), Collaborative Research Center 630 (SFB 630), “Recognition, Preparation, and Functional Analysis of Agents against Infectious Diseases” (projects A2, B3, and Z1).
We thank Heike Bruhn (Quality Manager of SFB 630) for fruitful discussions and advice, Angela Bruder for characterization of the Luc-tg L. major strain, and Christina Daumberger for technical assistance.
Footnotes
Published ahead of print 15 April 2013
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AAC.02201-12.
REFERENCES
- 1. Ameen M. 2010. Cutaneous leishmaniasis: advances in disease pathogenesis, diagnostics and therapeutics. Clin. Exp. Dermatol. 35:699–705 [DOI] [PubMed] [Google Scholar]
- 2. den Boer ML, Alvar J, Davidson RN, Ritmeijer K, Balasegaram M. 2009. Developments in the treatment of visceral leishmaniasis. Expert Opin. Emerg. Drugs 14:395–410 [DOI] [PubMed] [Google Scholar]
- 3. Goto H, Lindoso JA. 2010. Current diagnosis and treatment of cutaneous and mucocutaneous leishmaniasis. Expert Rev. Anti Infect. Ther. 8:419–433 [DOI] [PubMed] [Google Scholar]
- 4. Ait-Oudhia K, Gazanion E, Vergnes B, Oury B, Sereno D. 2011. Leishmania antimony resistance: what we know what we can learn from the field. Parasitol. Res. 109:1225–1232 [DOI] [PubMed] [Google Scholar]
- 5. Choudhury K, Zander D, Kube M, Reinhardt R, Clos J. 2008. Identification of a Leishmania infantum gene mediating resistance to miltefosine and SbIII. Int. J. Parasitol. 38:1411–1423 [DOI] [PubMed] [Google Scholar]
- 6. Srivastava P, Prajapati VK, Rai M, Sundar S. 2011. Unusual case of resistance to amphotericin B in visceral leishmaniasis in a region in India where leishmaniasis is not endemic. J. Clin. Microbiol. 49:3088–3091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Nagill R, Kaur S. 2011. Vaccine candidates for leishmaniasis: a review. Int. Immunopharmacol. 11:1464–1488 [DOI] [PubMed] [Google Scholar]
- 8. Okwor I, Uzonna JE. 2009. Immunotherapy as a strategy for treatment of leishmaniasis: a review of the literature. Immunotherapy 1:765–776 [DOI] [PubMed] [Google Scholar]
- 9. Fumarola L, Spinelli R, Brandonisio O. 2004. In vitro assays for evaluation of drug activity against Leishmania spp. Res. Microbiol. 155:224–230 [DOI] [PubMed] [Google Scholar]
- 10. Siqueira-Neto JL, Song OR, Oh H, Sohn JH, Yang G, Nam J, Jang J, Cechetto J, Lee CB, Moon S, Genovesio A, Chatelain E, Christophe T, Freitas-Junior LH. 2011. Antileishmanial high-throughput drug screening reveals drug candidates with new scaffolds. PLoS Negl. Trop. Dis. 4:e675. 10.1371/journal.pntd.0000675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Patil V, Guerrant W, Chen PC, Gryder B, Benicewicz DB, Khan SI, Tekwani BL, Oyelere AK. 2010. Antimalarial and antileishmanial activities of histone deacetylase inhibitors with triazole-linked cap group. Bioorg. Med. Chem. 18:415–425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ponte-Sucre A, Faber JH, Gulder T, Kajahn I, Pedersen SE, Schultheis M, Bringmann G, Moll H. 2007. Activities of naphthylisoquinoline alkaloids and synthetic analogs against Leishmania major. Antimicrob. Agents Chemother. 51:188–194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ponte-Sucre A, Vicik R, Schultheis M, Schirmeister T, Moll H. 2006. Aziridine-2,3-dicarboxylates, peptidomimetic cysteine protease inhibitors with antileishmanial activity. Antimicrob. Agents Chemother. 50:2439–2447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Debrabant A, Joshi MB, Pimenta PF, Dwyer DM. 2004. Generation of Leishmania donovani axenic amastigotes: their growth and biological characteristics. Int. J. Parasitol. 34:205–217 [DOI] [PubMed] [Google Scholar]
- 15. Sharlow ER, Close D, Shun T, Leimgruber S, Reed R, Mustata G, Wipf P, Johnson J, O'Neil M, Grogl M, Magill AJ, Lazo JS. 2009. Identification of potent chemotypes targeting Leishmania major using a high-throughput, low-stringency, computationally enhanced, small molecule screen. PLoS Negl. Trop. Dis. 3:e540. 10.1371/journal.pntd.0000540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ponte-Sucre A, Gulder T, Wegehaupt A, Albert C, Rikanovic C, Schaeflein L, Frank A, Schultheis M, Unger M, Holzgrabe U, Bringmann G, Moll H. 2009. Structure-activity relationship and studies on the molecular mechanism of leishmanicidal N,C-coupled arylisoquinolinium salts. J. Med. Chem. 52:626–636 [DOI] [PubMed] [Google Scholar]
- 17. Lang T, Goyard S, Lebastard M, Milon G. 2005. Bioluminescent Leishmania expressing luciferase for rapid and high throughput screening of drugs acting on amastigote-harbouring macrophages and for quantitative real-time monitoring of parasitism features in living mice. Cell. Microbiol. 7:383–392 [DOI] [PubMed] [Google Scholar]
- 18. Yang LK, Glover RP, Yoganathan K, Sarnaik JP, Godboleb AJ, Soejartoc DD, Bussa AD, Butler MS. 2003. Ancisheynine, a novel naphthylisoquinolinium alkaloid from Ancistrocladus heyneanus. Tetrahedron Lett. 44:5827–5829 [Google Scholar]
- 19. Bringmann G, Kajahn I, Reichert M, Pedersen SE, Faber JH, Gulder T, Brun R, Christensen SB, Ponte-Sucre A, Moll H, Heubl G, Mudogo V. 2006. Ancistrocladinium A and B, the first N,C-coupled naphthyldihydroisoquinoline alkaloids, from a Congolese Ancistrocladus species. J. Org. Chem. 71:9348–9356 [DOI] [PubMed] [Google Scholar]
- 20. Bringmann G, Pokorny F. 1995. The naphthylisoquinoline alkaloids, p 127–271 In Cordell GA. (ed), The alkaloids. Academic Press, New York, NY [Google Scholar]
- 21. Bringmann G, Feineis G. 2000. Novel antiparasitic biaryl alkaloids from West African Dioncophyllaceae plants. Actual. Chim. Therapeut. 26:151–171 [Google Scholar]
- 22. Bringmann G, Gunther C, Ochse M, Schupp O, Tasler S. 2001. Biaryls in nature: a multi-facetted class of stereochemically, biosynthetically, and pharmacologically intriguing secondary metabolites. Fortschr. Chem. Org. Naturst. 82:1–249 [DOI] [PubMed] [Google Scholar]
- 23. Bringmann G, Gulder T, Hertlein B, Hemberger Y, Meyer F. 2010. Total synthesis of the N,C-coupled naphthylisoquinoline alkaloids ancistrocladinium A and B and related analogues. J. Am. Chem. Soc. 132:1151–1158 [DOI] [PubMed] [Google Scholar]
- 24. Bringmann G, Gulder T, Reichert M, Meyer F. 2006. Ancisheynine, the first N,C-coupled naphthylisoquinoline alkaloid: total synthesis and stereochemical analysis. Org. Lett. 8:1037–1040 [DOI] [PubMed] [Google Scholar]
- 25. Bringmann G, Hertlein-Amslinger B, Kajahn I, Dreyer M, Brun R, Moll H, Stich A, Ioset KN, Schmitz W, Ngoc LH. 2011. Phenolic analogs of the N,C-coupled naphthylisoquinoline alkaloid ancistrocladinium A, from Ancistrocladus cochinchinensis (Ancistrocladaceae), with improved antiprotozoal activities. Phytochemistry 72:89–93 [DOI] [PubMed] [Google Scholar]
- 26. Ponte-Sucre A, Gulder T, Gulder TA, Vollmers G, Bringmann G, Moll H. 2010. Alterations to the structure of Leishmania major induced by N-arylisoquinolines correlate with compound accumulation and disposition. J. Med. Microbiol. 59:69–75 [DOI] [PubMed] [Google Scholar]
- 27. Dimroth K, Odenwaelder H. 1971. Reaktionen mit Benzo- und Naphthopyryliumsalzen. Chem. Ber. 104:2984–2994 [Google Scholar]
- 28. Reimann E. 1991. 1-Alkyl- und 1-Aryl-chinolinium-Salze. Methoden Org. Chem. (Houben-Weyl) E 7a:542–570 [Google Scholar]
- 29. Schleicher U, Bogdan C. 2009. Generation, culture and flow-cytometric characterization of primary mouse macrophages. Methods Mol. Biol. 531:203–224 [DOI] [PubMed] [Google Scholar]
- 30. Schurigt U, Schad C, Glowa C, Baum U, Thomale K, Schnitzer JK, Schultheis M, Schaschke N, Schirmeister T, Moll H. 2010. Aziridine-2,3-dicarboxylate-based cysteine cathepsin inhibitors induce cell death in Leishmania major associated with accumulation of debris in autophagy-related lysosome-like vacuoles. Antimicrob. Agents Chemother. 54:5028–5041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Huber W, Koella JC. 1993. A comparison of three methods of estimating EC50 in studies of drug resistance of malaria parasites. Acta Trop. 55:257–261 [DOI] [PubMed] [Google Scholar]
- 32. Zhang JH, Chung TD, Oldenburg KR. 1999. A simple statistical parameter for use in evaluation and validation of high throughput screening assays. J. Biomol. Screen. 4:67–73 [DOI] [PubMed] [Google Scholar]
- 33. Liebsch M, Grune B, Seiler A, Butzke D, Oelgeschlager M, Pirow R, Adler S, Riebeling C, Luch A. 2011. Alternatives to animal testing: current status and future perspectives. Arch. Toxicol. 85:841–858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Russel WMS, Burch RL. 1959. The principles of human experimental technique. Methuen & Co Ltd, London, United Kingdom [Google Scholar]
- 35. Schmid H, Sauerbrei R, Schwarz G, Weber E, Kalbacher H, Driessen C. 2002. Modulation of the endosomal and lysosomal distribution of cathepsins B, L and S in human monocytes/macrophages. Biol. Chem. 383:1277–1283 [DOI] [PubMed] [Google Scholar]
- 36. Nwaka S, Hudson A. 2006. Innovative lead discovery strategies for tropical diseases. Nat. Rev. Drug Discov. 5:941–955 [DOI] [PubMed] [Google Scholar]
- 37. Nwaka S, Ramirez B, Brun R, Maes L, Douglas F, Ridley R. 2009. Advancing drug innovation for neglected diseases—criteria for lead progression. PLoS Negl. Trop. Dis. 3:e440. 10.1371/journal.pntd.0000440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Channon JY, Roberts MB, Blackwell JM. 1984. A study of the differential respiratory burst activity elicited by promastigotes and amastigotes of Leishmania donovani in murine resident peritoneal macrophages. Immunology 53:345–355 [PMC free article] [PubMed] [Google Scholar]
- 39. de Muylder G, Ang KK, Chen S, Arkin MR, Engel JC, McKerrow JH. 2011. A screen against Leishmania intracellular amastigotes: comparison to a promastigote screen and identification of a host cell-specific hit. PLoS Negl. Trop. Dis. 5:e1253. 10.1371/journal.pntd.0001253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Mandal S, Maharjan M, Ganguly S, Chatterjee M, Singh S, Buckner FS, Madhubala R. 2009. High-throughput screening of amastigotes of Leishmania donovani clinical isolates against drugs using a colorimetric beta-lactamase assay. Indian J. Exp. Biol. 47:475–479 [PMC free article] [PubMed] [Google Scholar]
- 41. Plock A, Sokolowska-Kohler W, Presber W. 2001. Application of flow cytometry and microscopical methods to characterize the effect of herbal drugs on Leishmania spp. Exp. Parasitol. 97:141–153 [DOI] [PubMed] [Google Scholar]
- 42. Siqueira-Neto JL, Moon S, Jang J, Yang G, Lee C, Moon HK, Chatelain E, Genovesio A, Cechetto J, Freitas-Junior LH. 2012. An image-based high-content screening assay for compounds targeting intracellular Leishmania donovani amastigotes in human macrophages. PLoS Negl. Trop. Dis. 6:e1671. 10.1371/journal.pntd.0001671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Vermeersch M, da Luz RI, Tote K, Timmermans JP, Cos P, Maes L. 2009. In vitro susceptibilities of Leishmania donovani promastigote and amastigote stages to antileishmanial reference drugs: practical relevance of stage-specific differences. Antimicrob. Agents Chemother. 53:3855–3859 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.