Abstract
The use of cardiopulmonary bypass (CPB) during cardiac surgery causes regional ventilation-perfusion mismatch, contributing to regional disturbances in antibiotic penetration into lung tissue. Ventilation-perfusion mismatch is associated with postoperative pneumonia, a frequent and devastating complication after cardiac surgery. In this prospective clinical animal study, we performed in vivo microdialysis to determine the effect of CPB on regional penetration of levofloxacin (LVX) into lung tissue. Six pigs underwent surgery with CPB (CPB group), and another six pigs underwent surgery without CPB (off-pump coronary artery bypass grafting; OPCAB group). LVX (750 mg) was administered intravenously to all pigs immediately after surgery. For regional measurements of LVX in pulmonary concentrations, microdialysis probes were inserted in both lungs of each pig. Pigs were placed in the right lateral position. Time versus concentration profiles of unbound LVX were measured in the upper and lower lung tissue and plasma in all pigs. In all pigs, maximum concentrations (Cmax) of LVX were significantly lower in the upper lung than in the lower lung (OPCAB, P = 0.035; CPB, P < 0.001). Median Cmax of LVX showed a significant difference in the upper versus lower lung in the CPB group (P < 0.05). No significant difference was found in the median Cmax of LVX in the upper and the lower lung in the OPCAB group (P = 0.32). Our data indicate that CPB affects perioperative regional antibiotic penetration into lung tissue. Common clinical antibiotic dosing schemes should be reevaluated in patients undergoing coronary artery bypass grafting with CPB.
INTRODUCTION
Cardiopulmonary bypass (CPB) causes ventilation-perfusion mismatch with subsequent occurrence of pneumonia in patients in the intensive care unit (ICU) after cardiac surgery (1, 2). Several studies in patients undergoing cardiac surgery have reported postoperative pneumonia as a severe infectious complication with an incidence of up to 12% (2, 3). Postoperative pneumonia is associated with increased rates of ICU morbidity and mortality (4). Furthermore, a prolonged duration on respiratory support causes significant additional costs (1, 5).
In cardiac surgery patients, the use of CPB may play an important role in regional antibiotic penetration and distribution into lung tissue. During cardiac surgery with CPB, the patient's oxygenation is provided by CPB, and respiratory support is paused during extracorporeal circuit. For this reason, patients develop severe changes in ventilation-perfusion characteristics of the lungs after CPB. Ventilation-perfusion mismatch after CPB is associated with intrapulmonary right-to-left shunt caused by atelectasis formation (6). Atelectasis formation is presumed to be the major predisposing risk factor for the postoperative occurrence of pneumonia in ICU patients after cardiac surgery with CPB (6, 7).
In contrast, in off-pump coronary artery bypass grafting (OPCAB), CPB is not used and the lungs are ventilated throughout the surgical procedure. Perioperative atelectasis formation and postoperative changes in lung perfusion can be successfully reduced by means of OPCAB (8, 9).
Previous data from our study group showed that levofloxacin (LVX) concentrations in lung tissue were significantly higher in patients undergoing OPCAB than in patients undergoing surgery with CPB (10), suggesting that the ventilation-perfusion mismatch after CPB affects antibiotic distribution into lung tissue.
Penetration of antibiotics into lung tissue is further impaired by severe and prolonged alterations in the microcirculation after CPB (11). Alterations of local microcirculation arise from the ventilation-perfusion mismatch due to supine positioning during CPB and are responsible for the development of pneumonia within the first postoperative days (9). However, relatively increased perfusion in dependent lung tissue should lead to increased antibiotic concentrations in these areas of the lung and should be beneficial for the prevention of pneumonia (10).
In the present study, we hypothesized that CPB affects the penetration of LVX into lung tissue. Therefore, we measured regional concentrations of LVX in different lung areas by in vivo microdialysis (12, 13) in pigs. Our results should help to answer the question of whether ventilation-perfusion disturbances and gravity-induced atelectasis after CPB affect regional antibiotic penetration into lung tissue. We encourage the reevaluation of clinical dosing schemes of antibiotic therapy for cardiac surgery patients operated on with CPB.
MATERIALS AND METHODS
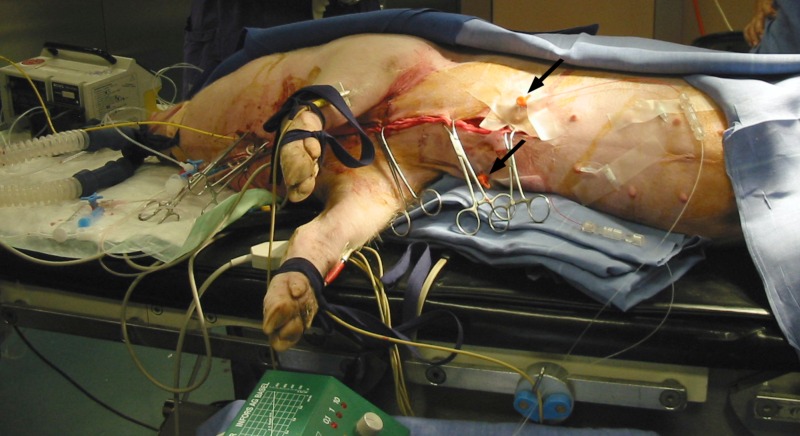
In this prospective comparative study, we examined 12 pigs after cardiac surgery. We chose the pig model for ethical reasons. Pigs were studied in the operation room in the Department of Biomedical Research, Medical University of Vienna. All pigs were placed in the right lateral position throughout the study period (Fig. 1). Six pigs underwent cardiac surgery with CPB (CPB group), and six pigs were operated on with OPCAB (OPCAB group). We measured the unbound concentrations of LVX in plasma and in lung tissue regionally by means of in vivo microdialysis. Regional concentrations of LVX were measured in upper and lower lung areas in all pigs (Fig. 2). Regional differences in antibiotic penetration were measured, with each animal serving as its own control. Demographic, hemodynamic, and laboratory data for all pigs are presented in Table 1.
Fig 1.
Perioperative positioning (right lateral position) of pigs during measurements. Microdialysis probes were inserted into the upper lung and the lower lung in both treatment groups. Black arrows indicate the localization of microdialysis probes in the lungs.
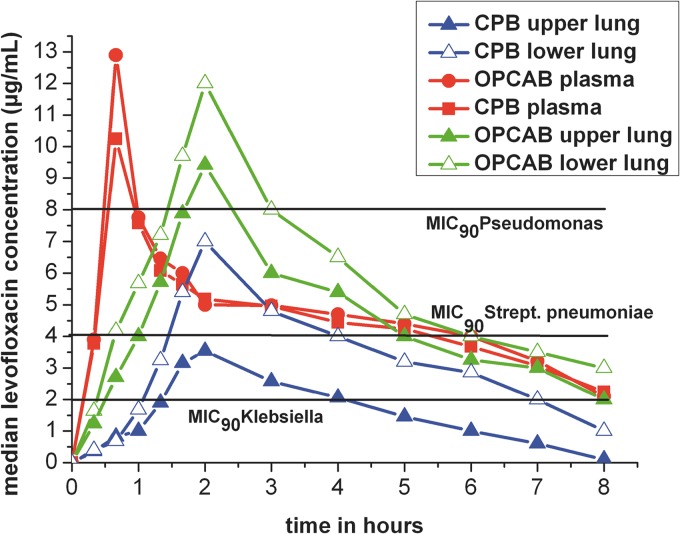
Fig 2.
Time versus median concentration profiles in plasma and in lung tissue (upper and lower lung) in the two treatment groups. Red line, plasma in both groups; blue line, OPCAB group; green line, CPB group. Filled triangles, LVX concentrations in the upper lung; open triangles, LVX concentrations in the lower lung; black horizontal lines, MICs of pathogens.
Table 1.
Demographic, laboratory, hemodynamic, and intraoperative treatment dataa
| Parameter (unit) | CPB group (n = 5) | OPCAB group (n = 5) | P value |
|---|---|---|---|
| Sex (M/F) | 2/3 | 3/2 | |
| Body wt (kg) | 41.0 (35–42)/39.4 ± 1.2 | 38.0 (36–44)/39.4 ± 1.4 | 1.0 |
| Creatinine (mg/dl) | 0.9 (0.9–1.2)/0.95 ± 0.12 | 1.0 (0.7–1.3)/1.0 ± 0.2 | 0.55 |
| BUN (mg/dl) | 10 (8–12)/9.8 ± 2 | 8 (8–10)/8.8 ± 1.8 | 0.26 |
| Lactate max (mmol/liter) | 4.7 (3–6.2)/4.5 ± 1.6 | 3.5 (1.5–4.5)/3.0 ± 1.4 | 0.1 |
| CO (liter/min) | 5.8 (5.5–7.8)/6.2 ± 0.7 | 6.2 (5.1–7.0)/6.2 ± 0.5 | 0.97 |
| Svo2 (%) | 50 (40–60)/51 ± 7.2 | 62 (54–75)/63 ± 7.6 | 0.056 |
| Do2 (ml/min) | 734 (627–910)/756 ± 83.4 | 790 (700–960)/803 ± 90 | 0.25 |
| Vo2 (ml/min) | 245 (240–300)/250 ± 21.1 | 255 (235–311)/261 ± 18.9 | 0.73 |
| Sao2 (%) | 98 (95–99)/98 ± 1.5 | 98 (94–100)/98 ± 1.5 | 0.74 |
| Fluid balance (ml) | 2,050 (2,300–4,500)/2,010 ± 620 | 1,800 (1,050–1,850)/1,430 ± 250 | 0.06 |
| Norepinephrine intraop. (μg/kg/min) | 0.09 (0.06–0.2)/0.11 ± 0.04 | 0.02 (0–0.05)/0.02 ± 0.01 | 0.01a |
| Norepinephrine postop. (μg/kg/min) | 0.02 (0–0.1)/0.07 ± 0.02 | 0.01 (0–0.05)/0.01 ± 0.01 | 0.073 |
| Dobutamine (μg/kg/min) | 4 (2–5)/3.7 ± 0.9 | 0 (0–1)/0.2 ± 0.2 | <0.001a |
| Duration of surgery (min) | 210 (172–244)/196 ± 11.9 | 189 (139–215)/182 ± 15.6 | 0.79 |
| Albumin (g/dl) | 3.0 (2.8–3.2)/3.0 ± 0.3 | 3.2 (3.1–3.7)/3.3 ± 0.4 | 0.1 |
| Total protein (g/dl) | 5.1 (4.9–5.9)/5.3 ± 0.9 | 5.3 (4.7–5.7)/5.4 ± 0.8 | 0.82 |
| Protein binding of LVX (%) | 32.2 (30–35.9)/32.8 ± 1.4 | 36.8 (33.9–40.3)/37 ± 1.9 | 0.06 |
P values of <0.05 indicate a significant difference. Except for P values, values are given as median (range)/mean ± SD. Abbreviations: BUN, blood urea nitrogen; lactate max, intraoperative maximum value of lactate; CO, cardiac output; Svo2, mixed venous blood oxygen saturation; Do2, oxygen delivery; Vo2, oxygen consumption; Sao2, arterial blood oxygen saturation; intraop., intraoperative; postop., postoperative.
The study was approved by the local ethics committee for animals. All animals received humane care in compliance with the Guide for the Care and Use of Laboratory Animals (14).
In vivo measurements and probe calibration.
In vivo microdialysis is a minimally invasive technique that measures unbound molecules in the interstitial space fluid (12). We used a custom-made flexible microdialysis probe (CMA 60; Metalant AB, Stockholm, Sweden). To obtain the absolute extracellular concentrations from the dialysate concentrations, we performed microdialysis probe calibration in each animal according to the retrodialysis method (12).
The principle of the retrodialysis method relies on the assumption that the diffusion process is quantitatively equal in both directions through the semipermeable membrane. Therefore, we added levofloxacin to the perfusion medium, and the disappearance rate (delivery) through the membrane was taken as the in vivo recovery. The in vivo recovery value was calculated as follows: recovery (%) = 100 − (100 × levofloxacin(out)/levofloxacin (in)). The in vivo recovery was assessed for each individual microdialysis probe by dialyzing the lung tissue with a perfusion medium containing 2 μg/ml of levofloxacin and a flow rate of 1.5 μl/min using a microinfusion pump (CMA Microdialysis AB) for 60 min.
Interstitial, extracellular concentrations were calculated according to the following equation: interstitial concentration = 100 × (sample concentration/in vivo recovery), where the concentrations are in μg/ml and the in vivo recovery is given as a percentage.
Blood samples were kept on ice for a maximum of 60 min and were centrifuged at 4°C and 4,000 rpm for 10 min; cells were then discharged, and plasma was obtained. Plasma samples and dialysate samples were snap-frozen at −20°C and thereafter stored at −80°C until analysis.
Determination of LVX concentration.
Total LVX levels in plasma and free (unbound) concentrations in microdialysates were measured by a validated reversed-phase high-performance liquid chromatography (HPLC) method (15) using a Dionex “UltiMate 3000” System (Dionex Corp., Sunnyvale, CA). Briefly, plasma samples containing protein were deproteinized by methanol precipitation. Ultrafiltrates were analyzed without further precipitation. The injection volume was 10 μl. Chromatographic separation of LVX was performed at 45°C on a Hypersil BDS-C18 column (5 μm; inner diameter [i.d.], 250 by 4.6 mm; Astmoor, England), preceded by a Hypersil BDS-C18 precolumn (5 μm; i.d., 10 by 4.6 mm), at a flow rate of 1 ml/min. The mobile phase was prepared as follows: 1.1 ml orthophosphoric acid (85%; Merck, Darmstadt, Germany) was added to water to a volume of 1,000 ml. This solution was adjusted to pH 3.0 with 1 M tetrabutylammonium hydroxide solution in water (Fluka, Buchs, Switzerland). Afterwards, 15 ml HPLC grade acetonitrile was mixed into this solution to yield a total of 1,000 ml. After degassing by ultrasonication, the final pH of the mobile phase was 3.1. LVX was measured by fluorescence excitation and emission wavelengths of 310 nm and 476 nm, respectively. Calibration curves were calculated from the peak areas of LVX compared with the external standard by spiking drug-free human plasma and ultrafiltrate with standard solutions of LVX (average correlation coefficients, >0.99). The concentrations of the calibration samples were 0.005, 0.01, 0.05, 0.1, 0.5, 1, and 5 μg/ml of LVX. The limit of quantification was 0.005 μg/ml. The intrarun and interrun precision and accuracy were calculated by analyzing six replicated of quality control (QC) samples (spiked drug-free plasma and ultrafiltrate, respectively) at LVX concentrations of 0.5 and 5 μg/ml, in three different days. The intraday relative standard deviation (RSD) for LVX ranged from 2.12 to 5.32% and the accuracy from −3.17 to 2.45%. The interday RSD for LVX varied from 3.70 to 7.47% and the accuracy from −4.89 to 3.76%. Based on the selectivity of the fluorescence assay, no interaction with coadministered drugs was observed (data not shown).
Determination of LVX-protein binding.
Plasma samples were thawed to room temperature, mixed, and centrifuged at 13,000 × g for 5 min. A 200-μl aliquot was transferred to a Vivaspin 500 ultrafiltration device (Sartorius Stedim Biotech, France) and centrifuged at 13,000 × g for 10 min at room temperature. The recovered ultrafiltrate was analyzed directly by reversed-phase high-performance liquid chromatography without extraction to determine the concentration of unbound drug in the plasma. Samples that failed ultrafiltration were assayed to determine total (bound and unbound) drug concentration. Protein binding of LVX was determined according to the following formula: protein binding (%) = (total drug − unbound drug/total drug) × 100. Nonspecific binding (NBS) to the ultrafiltration device was evaluated by replacing plasma with phosphate-buffered saline (PBS) (pH 7.4). LVX showed a very low NBS value of only 1.67% at a concentration of 1 μg/ml.
Experimental design.
Before surgery, pigs were administered 10 mg/kg of body weight ketamine intramuscularly. Ringer's lactate solution (10 ml/kg) was given to all pigs during induction of anesthesia. General anesthesia was performed using midazolam (0.1 mg/kg), thiopental (0.25 mg/kg), fentanyl (0.01 mg/kg), and pancuronium (0.15 mg/kg). For maintenance of anesthesia, pigs were continuously administered fentanyl (0.5 mg/h), propofol 1% (10 to 20 mg/kg per h), and pancuronium (0.1 mg/kg per h). Electrocardiography, pulse oximetry, and continuous arterial blood pressure monitoring were performed in all pigs after induction of anesthesia.
Mechanical ventilation was performed by using 45% O2 in air, tidal volume (Vt) of 7 ml/kg body weight, respiratory frequency keeping Paco2 in the normal range, and positive end-expiratory pressure (PEEP) of 5 to 7 cm H2O in all pigs.
For study purposes, two microdialysis probes were inserted into lung tissue. The locations of the probes were standardized in all pigs: one into the ventral right upper lobe (segment 2) and one into the ventral left upper lobe (segment 3) (Fig. 1). With a gutter-like 1.4-mm slit needle (supplied in the CMA-60 microdialysis set; CMA Microdialysis AB, Solna, Sweden), the tip of the probe was inserted into the reinflated lung under visual control. After surgery, the microdialysis probes were connected and perfused with Ringer's solution at a flow rate of 1.5 μl/min using a microinfusion pump (CMA Microdialysis AB). After a 30-min baseline sampling period, we administered LVX continously over a perfusion pump for 30 min. Blood sampling and microdialysis sampling were performed at 30-min intervals for the first 2 h. After 2 h, we prolonged the sampling intervals to 60 min for the next 6 h. The sampling period was in total 8 h. The start of LVX administration was defined as the time point 0 min. In vivo probe calibration (60 min) was performed at the end of sampling. Microdialysis probes were removed immediately after probe calibration.
Surgical procedure and extracorporeal circulation (CPB).
A median sternotomy was performed in all pigs. We renounced to perform surgical aortocoronary bypass grafting in all pigs of both treatment groups.
Animals of the two groups were treated differently due to surgical procedure.
In all pigs of the CPB group, the cardiac procedure includes median sternotomy and additionally cannulation for cardiopulmonary bypass (CPB). CPB was performed for 120 min in the CPB group. For CPB, all pigs in the CPB group received heparin sodium (400 IU/kg) for CPB, and activated clotting time was kept above 500 s (Hemochrom 400; International Technidyne, Edison, NJ). Ringer's lactate solution (1,000 ml), 20% mannitol (100 ml), and heparin sodium (8,000 IU) were used to prime the extracorporeal circuit. Mechanical ventilation was terminated after cardioplegic arrest during the entire time of CPB (120 min). The recruitment maneuver by Lachmann was performed after cardioplegic arrest to reexpand the lungs.
The pigs in the OPCAB group were treated in the same way as the pigs in the CPB group regarding monitoring, ventilation, and anesthesiologic management. In pigs undergoing OPCAB surgery, mechanical ventilation was performed throughout the procedure. The insertion and calibration of the microdialysis probes and the drawing of plasma and microdialysis samples were performed in the same manner as described for the CPB group.
All pigs in both groups were placed in the right lateral position before sampling (Fig. 1).
At the end of the study period, all pigs were euthanized by the administration of midazolam (bolus of 25 mg intravenously [i.v.]), thiopental (bolus of 20 mg i.v.), and potassium chloride (bolus of 100 mmol i.v.).
Statistical analysis.
Calculations and data analysis were performed using Statistica (StatSoft, Inc.) and SigmaStat 3.5 (Systat Software Inc., Erkrath, Germany). Data are presented as medians and means ± standard deviations (SD). Student's t test was used to compare the two groups (demographic parameters, time to maximum concentration of drug in serum [Tmax], maximum concentration of drug in serum [Cmax], LVX concentrations in the upper versus lower lung areas, and terminal half-life [t1/2]). When normality assumption failed, the Wilcoxon signed-rank test was used (various area under the concentration-time curves [AUCs], AUC/MIC90, Cpeak/MIC90). A P value of <0.05 was considered the level of significance.
To check the significance of values, we performed multiple testing with summary measurements, linear regression, and one-way analysis of variance (ANOVA).
Pharmacokinetic data were fitted by a commercially available computer program (Kinetica 2.0.2; Thermo Fisher Scientific, Philadelphia, PA). The time versus LVX concentration profiles for plasma and interstitial lung tissue were measured. The following pharmacokinetic parameters were determined: Cmax, Tmax, and AUC from 0 to 8 h (AUC0–8). We also calculated AUC from 0 h to infinity (AUC0-∞). The kinetics was based on a noncompartmental model. AUC0-∞ was calculated by analytical integration. t1/2 was obtained by using the elimination rate based on the slope of the terminal-phase linear regression.
We calculated the AUCplasma/AUCtissue (0 to 8 h) ratio as a value for drug penetration into the interstitial compartment. We calculated the Cmax/MIC90 ratio (maximum concentration) for LVX, an important parameter for concentration-dependent antibiotics.
For fluoroquinolones, the pharmacodynamic value that best correlates with the outcome is the ratio of AUC in lung tissue to MIC (AUCtissue/MIC). We calculated the AUC0–8/MIC90 of respiratory pathogens (MIC90 for Pseudomonas aeroginosa, 8 μg/ml (16); for Streptococcus pneumoniae, 4 μg/ml (16); and for Klebsiella species, 2 μg/ml (16). Since we performed drug concentration measurements for only 8 h after fluoroquinolone administration, AUC0-∞/MIC90 had to be extrapolated to 24 h.
RESULTS
Six pigs underwent surgery with CPB, and six pigs underwent surgery with OPCAB. The insertion of microdialysis probes into lung tissue was successful in all pigs. No adverse events or clinical complications related to the microdialysis procedure were observed.
Two pigs (one in each group) died before the start of the study, before study medication was administered: one pig died from major bleeding during arterial cannulation for CPB; the other pig died from therapy-resistant hemodynamic instability immediately after introduction of anesthesia. Data for these two pigs were not included in the calculations.
Demographic, laboratory, hemodynamic, and perioperative treatment data are given in Table 1.
After administration of LVX (750 mg i.v.), Cmax in plasma was reached after 32 min in the CPB group (Tmax range, 20 to 60 min) and after 30 min in the OPCAB group (Tmax range, 20 to 40 min) (P = 0.94) (Fig. 2 and Tables 1 and 2). No significant difference in Cmax of LVX in plasma was found between treatment groups (P = 0.89) (for the CPB group, median Cmax, 10.6 μg/ml, and range, 2.2 to 12.1 μg/ml; for the OPCAB group, median, 13.2 μg/ml, and range, 2.1 to 15.6 μg/ml) (Fig. 2). We did not find significant differences in AUC0–8 (P = 0.75), AUCtot (P = 0.64), and t1/2 in plasma (P = 0.69) between the CPB group and the OPCAP group.
Table 2.
Main pharmacokinetic parameters of levofloxacin in the upper lung, lower lung, and plasma (CPB group)a
| Parameter (unit) | Upper lung | Lower lung | P value | Plasma |
|---|---|---|---|---|
| Tmax (min) | 109 (60–120)/110 ± 21 | 107 (60–120)/108 ± 20 | 1c | 40 (20–60)/32 ± 15 |
| Cmax (μg/ml) | 3.6 (2.0–7.9)/3.9 ± 2.2 | 6.7 (5.6–10)/7.3 ± 2 | 0.05b | 10.6 (2.2–12.1)/10.2 ± 4.3 |
| AUC0–8 (μg/h/ml) | 20.9 (6.7–24.4)/18.3 ± 7.2 | 28.9 (24.0–49.9)/32.1 ± 5.6 | 0.04b | 35.7 (33.3–40.3)/36 ± 4.8 |
| AUC0-∞ (μg/h/ml) | 22 (7.6–34.7)/20.9 ± 8.7 | 34.9 (30.2–55.1)/37.4 ± 5 | 0.04b | 46.8 (41.4–48.2)/45.2 ± 1.9 |
| AUCtissue/AUCplasma | 0.5 (0.2–0.64)/0.3 ± 0.3 | 0.9 (0.6–2)/0.7 ± 0.9 | 0.05b | |
| Cmax/MIC90 (Klebsiella sp.) | 1.9 (1–3.4)/2.2 ± 0.8 | 0.8 (0.7–1.3)/0.9 ± 0.2 | 0.12b | 5.3 (2–6.1)/5 ± 0.6 |
| Cmax/MIC90 (S. pneumoniae) | 0.9 (0.5–2)/1 ± 0.6 | 1.7 (1.4–2.6)/1.8 ± 0.5 | 0.14b | 8.9 (8.2–10.1)/9 ± 0.8 |
| Cmax/MIC90 (P. aeruginosa) | 0.7 (0.3–1)/0.8 ± 0.1 | 0.8 (0.6–1)/0.9 ± 0.1 | 0.13b | 4.5 (4.2–5)/4.3 ± 0.2 |
| AUC0–8/MIC90 (Klebsiella sp.) extrapolated 24 h | 9.1 (4.2–12.8)/9.4 ± 2.6 | 15.6 (9.6–21.3)/15.8 ± 4.3 | 0,46c | 17.9 (16.7–20.2)/18 ± 2.3 |
| AUC0–8/MIC90 (S. pneumoniae) extrapolated 24 h | 5.1 (1.7–8.5)/4.9 ± 3.1 | 7.1 (5.8–11)/7.8 ± 2.1 | 0.45c | 8.9 (8.3–10.1)/9 ± 1 |
| AUC0–8/MIC90 (P. aeruginosa) extrapolated 24 h | 2.3 (1.1–3.9)/2.6 ± 1.3 | 3.8 (2.7–5.8)/3.9 ± 0.8 | 0.45c | 4.5 (4.1–5)/4.2 ± 0.3 |
| t1/2 (h) | 2.2 (1.2–4.8)/2.6 ± 0.9 | 2.0 (1.4–3.1)/2.2 ± 0.4 | 0.56b | 2.9 (2.5–4.1)/3.0 ± 1.1 |
P values of <0.05 indicate a significant difference. Except for P values, values are given as median (range)/mean ± SD. AUC0–8, AUC from 0 to 8 h; AUC0-∞, AUC from 0 to infinity; AUCtissue/AUCplasma ratio, ratio of AUC in tissue to AUC in plasma from 0 to 8 h; AUC0–8/MIC90, ratio of AUC from 0 to 8 h to MIC90 (extrapolated over 24 h); Cmax, peak drug concentration; Cmax/MIC90, peak concentration of LVX to MIC90; Tmax, time until maximum drug concentration was observed; t1/2, half-life.
Statisical analysis using Student's t test.
Statisical analysis using the Wilcoxon signed-rank test.
The time versus concentration profiles of LVX in the upper and lower lung areas of both groups and the MIC90 for pathogens (MIC90 for Pseudomonas aeruginosa, 8 μg/ml [16]; for Streptococcus pneumoniae, 4 μg/ml [16]; and for Klebsiella species, 2 μg/ml [16]) are shown in Fig. 2.
Median Cmax of LVX showed a significant difference between the upper and lower lung areas in the CPB group (P < 0.05). No significant difference was found in the median Cmax of LVX between the upper and lower lung areas in the OPCAB group (P = 0.32) (Fig. 3).
Fig 3.
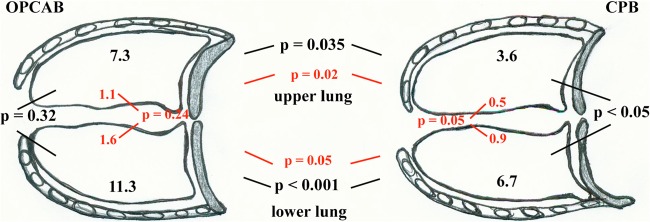
Regional LVX distribution into lung tissue in the two treatment groups. Values represent median Cmax of LVX concentrations (μg/ml) in the upper lung and the lower lung in both treatment groups (black letters). Values in red represent AUCtissue/AUCplasma ratios in the upper lung and the lower lung in both treatment groups. P values (black or red letters) show the differences between LVX concentrations into the upper and lower lung in each group. P values (black or red letters) additionally show the differences between regional LVX concentrations in lung tissue and AUCtissue/AUCplasma ratios in both treatment groups.
Median Cmax of LVX in the upper lung tissue was significantly higher (P = 0.035) in the OPCAB group than in the CPB group (Tables 2 and 3). Median Cmax of LVX in the lower lung tissue showed a significant difference (P < 0.001) between treatment groups (for the OPCAB group, median, 11.3 ± 6, range, 8.3 to 24.7; for the CPB group, median, 6.7 ± 2.8, range, 5.6 to 10) (Tables 2 and 3).
Table 3.
Main pharmacokinetic parameters of levofloxacin in the upper lung, lower lung, and plasma (OPCAB group)a
| Parameter (unit) | Upper lung | Lower lung | P value | Plasma |
|---|---|---|---|---|
| Tmax (min) | 101 (60–120)/102 ± 19 | 104 (60–120)/105 ± 18 | 0.38b | 40 (20–40)/30 ± 18 |
| Cmax (μg/ml) | 7.3 (5.2–15.7)/9.4 ± 3.1 | 11.3 (8.3–24.7)/11.7 ± 3.4 | 0.32b | 13.2 (2.1–15.6)/12.9 ± 5.5 |
| AUC0–8 (μg/h/ml) | 40.9 (19.7–73.5)/42.0 ± 11.6 | 58 (47.7–98.8)/59.0 ± 6.7 | 0,19b | 36.7 (33–39.9)/36.5 ± 2.3 |
| AUC0-∞ (μg/h/ml) | 49.2 (22.4–89.6)/48.3 ± 9 | 93.1 (60.2–106.1)/98.4 ± 11.1 | 0.09b | 47.2 (39.3–53.7)/44.7 ± 6.8 |
| AUCtissue/AUCplasma | 1.1 (0.2–1.4)/0.8 ± 0.2 | 1.6 (1.2–4)/1.9 ± 2.4 | 0.24b | |
| Cmax/MIC90 (Klebsiella species) | 3.9 (2.1–8)/4.3 ± 1.1 | 1.4 (1–3.1)/1.5 ± 0.6 | 0.24b | 6.6 (1.1–7.8)/6.5 ± 1 |
| Cmax/MIC90 (S. pneumoniae) | 1.8 (1.3–4)2.4 ± 0.8 | 2.9 (2.1–6.3)/2.9 ± 1.4 | 0.23b | 3.3 (0.5–3.8)/3.2 ± 0.9 |
| Cmax/MIC90 (P. aeruginosa) | 1.2 (0.7–2)/1.4 ± 0.3 | 1.6 (0.25–2.0)/1.7 ± 0.8 | 0.23b | 1.7 (0.3–2)/1.6 ± 0.5 |
| AUC0–8/MIC90 (Klebsiella species) extrapolated 24 h | 21.9 (10.3–27.6)/22.1 ± 3.9 | 44.4 (32.5–52.3)/45.0 ± 5.2 | 0.28c | 18.4 (16.5–20)/18.3 ± 1.6 |
| AUC0–8/MIC90 (S. pneumoniae) extrapolated 24 h | 10.2 (4.9–18.3)/10.4 ± 6.7 | 14.6 (10.6–21.5)/14.7 ± 5.1 | 0.28c | 9.2 (8.3–10)/9.1 ± 0.6 |
| AUC0–8/MIC90 (P. aeruginosa) extrapolated 24 h | 5.5 (3.2–8.1)/5.8 ± 2 | 11.2 (6.7–12.6)/11.5 ± 0.8 | 0.29c | 4.6 (4.1–4.9)/4.5 ± 0.3 |
| t1/2 tissue (h) | 2.1 (0.95–3.0)/2.1 ± 1 | 2.2 (1.6–4.4)/2.6 ± 0.5 | 0.44b | 3.0 (2.5–4.7)/3.1 ± 0.7 |
P values of <0.05 indicate a significant difference. Except for P values, values are given as median (range)/mean ± SD. AUC0–8, AUC from 0 to 8 h; AUC0-∞, AUC from 0 to infinity; AUCtissue/AUCplasma ratio, ratio of AUC in tissue to AUC in plasma from 0 to 8 h; AUC0–8/MIC90, ratio of AUC from 0 to 8 h to MIC90 (extrapolated over 24 h); Cmax, peak drug concentration; Cmax/MIC90, peak concentration of LVX to MIC90; Tmax, time until maximum drug concentration was observed; t1/2, half-life.
Statisical analysis using Student's t test.
Statisical analysis using the Wilcoxon signed-rank test.
Postoperative chest radiography was performed in all pigs. The X rays of all pigs showed atelectasis formation in the lower lung. Opacification of lower lung areas was increased considerably in all pigs in the CPB group.
DISCUSSION
In this prospective, comparative, experimental animal study, we performed in vivo microdialysis to determine the effect of the intraoperative use of CPB on regional penetration of LVX into lung tissue. LVX concentrations were measured in upper and lower lung areas in 12 pigs undergoing cardiac surgery with (CPB group) and without (OPCAB group) CPB. Regional differences in antibiotic penetration were measured, with each animal serving as its own control.
To our knowledge, we are the first group internationally to measure regional antibiotic concentrations in different lung areas in the same individual.
Lateral positioning was performed in all pigs to simulate the supine position of ICU patients after cardiac surgery. The gravity-induced ventilation-perfusion mismatch used in our animal model is the same as that observed in postoperative cardiac surgery patients in long-term supine position. In the supine position, gravity-induced perfusion increases in dorsal lung areas (lower lung in the pig model), whereas ventilation increases in ventral lung areas (upper lung in the pig model).
Cefazolin, a first-generation cephalosporin, is used for standard perioperative antibiotic prophylaxis. LVX was administered along with cefazolin for study purposes due to its fast penetration into lung tissue.
In a previous in vivo microdialysis study, we showed that there are significant differences in concentrations of LVX in lung tissue in patients undergoing surgery with CPB versus those operated on with OPCAB (10). Data from that study showed that LVX concentrations in lung tissue were significantly higher in patients undergoing coronary artery bypass grafting with OPCAB than in patients operated on with CPB (10), suggesting that extracorporeal circulation affects antibiotic distribution into lung tissue. However, the limitation of our previous in vivo microdialysis study (10) in humans was the localization of microdialysis probes in lung tissue. Surgical access permitted insertion of microdialysis probes for intrapulmonary measurements of LVX concentrations in ventrally sited lung areas only. We postulated that measurements of pulmonary concentrations of LVX were, in total, too low because of the selective localization of microdialysis probes in ventrally sited lung areas.
In the present study, we measured regional pulmonary concentrations of LVX in the upper lung areas in pigs. The LVX concentrations in pigs were comparable to human pulmonary LVX concentrations (median, 2.5, and range, 2.0 to 2.9 μg/ml, in the CPB group; median, 4.1, and range, 3.7 to 11.9 μg/m, in the OPCAB group). As also shown in the previous study in human patients, concentrations of LVX were significantly different in the porcine OPCAP group versus the porcine CPB group in upper lung areas (P = 0.035) (Tables 2 and 3 and Fig. 3). These differences in LVX concentrations in upper lung areas could be attributed to CPB.
Data from the present study show that CPB has a greater effect on antibiotic penetration into lung tissue than does perioperative positioning. Regional concentrations of LVX in lower and upper lung areas differed significantly between the CPB group and the OPCAB group. However, regional concentrations of LVX were markedly lower and differed significantly in pigs in the CPB group (P = 0.05), whereas concentrations of LVX in pigs in the OPCAB group did not show significant differences between the lower and upper lung areas (P = 0.32) (Fig. 2). Consequently, CPB seems to impair regional LVX penetration into lung tissue.
The reasons for the effect of CPB on antibiotic penetration into lung tissue are multifaceted. Dilution due to pump priming may account for lower antibiotic concentrations both in plasma and in target tissue. Intraoperative fluid load—although the difference was not significant—was higher in the CPB group (Table 1). Changes in microcirculation due to laminar pump flow on CPB may also affect antibiotic distribution.
Perioperative positioning affects ventilation-perfusion mismatch with the development of atelectasis formation (17). To avoid atelectasis formation and improve perfusion disturbances after cardiac surgery, we used the following procedures: fast-track anesthesia, rapid weaning to spontaneous breathing immediately after cardiac surgery (18, 19), and recruitment maneuvers of the lungs before restarting mechanical ventilation after CPB (20). Despite these efforts, the incidence of atelectasis formation did not decrease in the perioperative ICU setting (17). Consequently, it is essential to reevaluate the current clinical dosing schemes of antibiotics, especially in patients undergoing cardiac surgery with CPB.
The AUCtissue/plasma concentration ratio, the gold standard for evaluating drug penetration, characterizes antibiotic distribution into the interstitium. The distribution process and thereby the penetration of antibiotics into the interstitium are highly variable and have been shown to be altered by iatrogenic procedures such as extracorporeal circulation and intensive care therapy (21). We found a median LVX AUCtissue/plasma concentration ratio, which—though the difference was not significant in the OPCAB group—was higher in lower lung areas than in upper lung areas in pigs in both groups (CPB group, P = 0.05; OPCAB group, P = 0.24). These findings imply that perfusion redistribution after CPB leads to critically lower interstitial concentrations of LVX in both upper and lower lung areas.
Despite increasing the dosage of LVX to 750 mg i.v. in our porcine model, concentrations in upper lung areas did not exceed the MIC90 of Klebsiella species in all pigs (n = 3/5) in the CPB group. In lower lung areas, only peak concentrations (Cmax) of LVX exceeded the MIC90 of Klebsiella species in all pigs in the CPB group. However, the increased dosage was not sufficient to inhibit bacterial growth after 6 h. Therefore, we conclude that LVX administered at the increased dose is not associated with sufficient lung tissue concentrations to be effective against Klebsiella species immediately after cardiac surgery with CPB.
As in our human study, tissue concentrations were insufficient to treat perioperative infections caused by P. aeruginosa in both groups. Interstitial concentrations in upper and lower lung areas of both treatment groups were far below the MIC90 of P. aeruginosa. The efficacy of therapy with LVX (and the other fluoroquinolones) should be critically analyzed in perioperative pneumonic infections caused by Klebsiella species and especially by P. aeruginosa.
A recent study (22) postulated that the dosage per time unit of norepinephrine (noradrenaline) showed an inverse correlation (r = −5.2) with tissue penetration of antibiotics. It stands to reason that patients operated on with CPB need higher vasopressor support intraoperatively than do patients operated on with OPCAB (P = 0.01; Table 1). However, in our porcine study LVX was administered immediately after the end of surgery, when noradrenaline was similar in both groups (P = 0.073; Table 1); hence, perfusion was not impaired due to vasopressor support.
The alleged protein binding of total LVX concentration is 30%. Protein binding of LVX in plasma obtained in our study was slightly higher (32.8% for the CPB group and 37% for the OPCAB group). However, the amount of total plasma protein in pigs is lower than that in humans (23, 24), potentially resulting in higher unbound LVX concentrations in pigs.
Limiting factors.
This study is certainly underpowered. However, each pig served as its own control.
Student's t test and the Mann-Whitney U test showed a significant difference in regional penetration of LVX in upper versus lower lung areas, whereas ANOVA showed no differences for AUCs.
Although this effect was not measured, we suggest that the lateral positioning of the pigs caused gravity-induced atelectasis (ventilation-perfusion mismatch) in lower lung areas. Chest radiography was performed in all pigs. Computed tomography is the method of choice for the quantification of atelectasis, but it was not available in our biomedical research setting. In addition, even multislice computed tomography is unable to detect the extremely slim microdialysis probe. However, our data showed clearly that CPB affects regional antibiotic penetration into lung tissue.
Conclusion.
Our results demonstrate that the use of extracorporeal circulation (CPB) affects regional antibiotic penetration into lung tissue and that it has a greater effect than does perioperative positioning. Our results show the necessity of reevaluating common clinical antibiotic dosing schemes in ICU patients after cardiac surgery with CPB.
ACKNOWLEDGMENTS
This study was performed in the Department of Cardiothoracic and Vascular Anesthesia & Critical Care Medicine, Medical University of Vienna, Vienna General Hospital, Vienna, Austria. Support was provided solely by institutional and departmental sources.
We declare that we have no potential conflicts of interest.
Footnotes
Published ahead of print 15 April 2013
REFERENCES
- 1. Light RB. 1999. Pulmonary pathophysiology of pneumococcal pneumonia. Semin. Respir. Infect. 14:218–226 [PubMed] [Google Scholar]
- 2. Bouza E, Hortal J, Munoz P, Pascau J, Pérez MJ, Hiesmayr M. 2006. Postoperative infections after major heart surgery and prevention of ventilator-associated pneumonia: a one-day European prevalence study (ESGNI-008). J. Hosp. Infect. 64:224–230 [DOI] [PubMed] [Google Scholar]
- 3. Allou N, Kermarrec N, Muller C, Thabut G, Philip I, Lucet JC, Montravers P. 2010. Risk factors and prognosis of post-operative pneumonia due to Pseudomonas aeruginosa following cardiac surgery. J. Antimicrob. Chemother. 65:806–807 [DOI] [PubMed] [Google Scholar]
- 4. Riera M, Ibáñez J, Herrero J, Ignacio Sáez De Ibarra J, Enríquez F, Campillo C, Bonnín O. 2010. Respiratory tract infections after cardiac surgery: impact on hospital morbidity and mortality. J. Cardiovasc. Surg. 51:907–914 [PubMed] [Google Scholar]
- 5. Boyce JM, White RL, Spruill EY, Wall M. 1985. Cost-effective application of the Centers for Disease Control Guideline for Prevention of Nosocomial Pneumonia. Am. J. Infect. Control 13:228–232 [DOI] [PubMed] [Google Scholar]
- 6. Tschernko EM, Bambazek A, Wisser W, Partik B, Jantsch U, Kubin K, Ehrlich M, Klimscha W, Grimm M, Keznickl FP. 2002. Intrapulmonary shunt after cardiopulmonary bypass: the use of vital capacity maneuvers versus off-pump coronary artery bypass grafting. J. Thorac. Cardiovasc. Surg. 124:732–738 [DOI] [PubMed] [Google Scholar]
- 7. Duggan M, Kavanagh BP. 2010. Best perioperative modifications of respiratory function. Pract. Res. Clin. Anaesthesiol. 24:145–155 [DOI] [PubMed] [Google Scholar]
- 8. Kerendi F, Halkos ME, Puskas JD, Lattouf OM, Guyton RA, Thourani VH. 2011. Impact of off-pump coronary artery bypass graft surgery on postoperative pulmonary complications in patients with chronic lung disease. Ann. Thorac. Surg. 91:8–15 [DOI] [PubMed] [Google Scholar]
- 9. Kirk KC, Aldridge RA, Sistino JJ. 2001. Coronary artery bypass grafting with and without cardiopulmonary bypass: a comparison analysis. J. Extra. Corpor. Technol. 33:86–90 [PubMed] [Google Scholar]
- 10. Hutschala D, Kinstner C, Skhirtladze K, Mayer-Helm BX, Zeitlinger M, Wisser W, Müller M, Tschernko E. 2008. The impact of perioperative atelectasis on antibiotic penetration into lung tissue: an in vivo microdialysis study. Intensive Care Med. 34:1827–1834 [DOI] [PubMed] [Google Scholar]
- 11. Vellinga NA, Ince C, Boerma EC. 2010. Microvascular dysfunction in the surgical patient. Curr. Opin. Crit. Care 16:377–383 [DOI] [PubMed] [Google Scholar]
- 12. Joukhadar C, Derenhdorf H, Müller M. 2001. Microdialysis, a novel tool for clinical studies of anti-infective agents. Eur. J. Clin. Pharmacol. 57:211–219 [DOI] [PubMed] [Google Scholar]
- 13. Dhanani J, Roberts JA, Chew M, Lipman J, Boots RJ, Paterson DL, Fraser JF. 2010. Antimicrobial chemotherapy and lung microdialysis: a review. Int. J. Antimicrob. Agents 36:491–500 [DOI] [PubMed] [Google Scholar]
- 14. National Research Council 2011. Guide for the care and use of laboratory animals, 8th ed National Academies Press, Washington, DC [Google Scholar]
- 15. Neckel U, Joukhadar C, Frossard M, Jäger W, Müller M, Mayer BX. 2000. Simultaneous determination of levofloxacin and ciprofloxacin in microdialysates and plasma by high-performance liquid chromatography. Anal. Chim. Acta 463:199–206 [Google Scholar]
- 16. Georgopoulos A, Buxbaum A, Straschil U, Graninger W. 1998. Austrian national survey of prevalence of antimicrobial resistance among clinical isolates of Streptococcus pneumoniae 1994-1996. Scand. J. Infect. Dis. 30:345–349 [DOI] [PubMed] [Google Scholar]
- 17. Westerdahl E, Lindmark B, Eriksson T, Friberg O, Hedenstierna G, Tenling A. 2005. Deep-breathing exercises reduce atelectasis and improve pulmonary function after coronary artery bypass surgery. Chest 128:3482–3488 [DOI] [PubMed] [Google Scholar]
- 18. Svircevic V, Nierich AP, Moons KG, Brandon Bravo Bruinsma GJ, Kalkman CJ, van Dijk D. 2009. Fast-track anesthesia and cardiac surgery: a retrospective cohort study of 7989 patients. Anesth. Analg. 108:727–733 [DOI] [PubMed] [Google Scholar]
- 19. Silbert BS, Myles PS. 2009. Is fast-track cardiac anesthesia now the global standard of care? Anesth. Analg. 108:689–691 [DOI] [PubMed] [Google Scholar]
- 20. Minkovich L, Djaiani G, Katznelson R, Day F, Fedorko L, Tan J, Carroll J, Cheng D, Karski J. 2007. Effects of alveolar recruitment on arterial oxygenation in patients after cardiac surgery: a prospective, randomized, controlled clinical trial. J. Cardiothorac. Vasc. Anesth. 21:375–378 [DOI] [PubMed] [Google Scholar]
- 21. Brunner M, Pernerstorfer T, Mayer BX, Eichler HG, Müller M. 2000. Surgery and intensive care procedures affect the target site distribution of piperacillin. Crit. Care Med. 28:1754–1759 [DOI] [PubMed] [Google Scholar]
- 22. Zeitlinger BS, Zeitlinger M, Leitner I, Müller M, Joukhadar C. 2007. Clinical scoring system for the prediction of target site penetration of antimicrobials in patients with sepsis. Clin. Pharmacokinet. 46:75–83 [DOI] [PubMed] [Google Scholar]
- 23. Ryan DM. 2003. Pharmacokinetics of antibiotics in natural and experimental superficial compartments in animals and humans. J. Antimicrob. Chemother. 31(Suppl D):1–16 [DOI] [PubMed] [Google Scholar]
- 24. Hannon JP, Bossone CA, Wade CE. 1990. Normal physiological values for conscious pigs used in biomedical research. Lab. Anim. Sci. 40:293–298 [PubMed] [Google Scholar]