Abstract
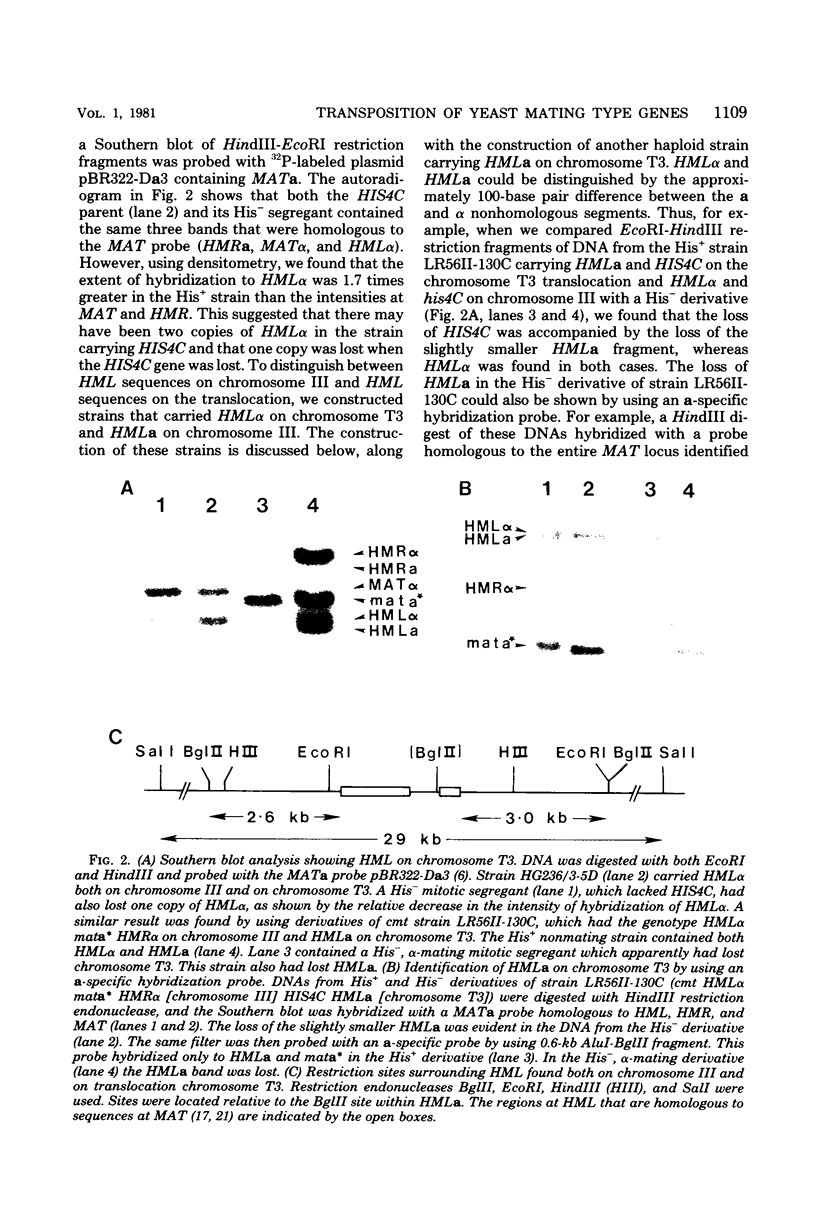
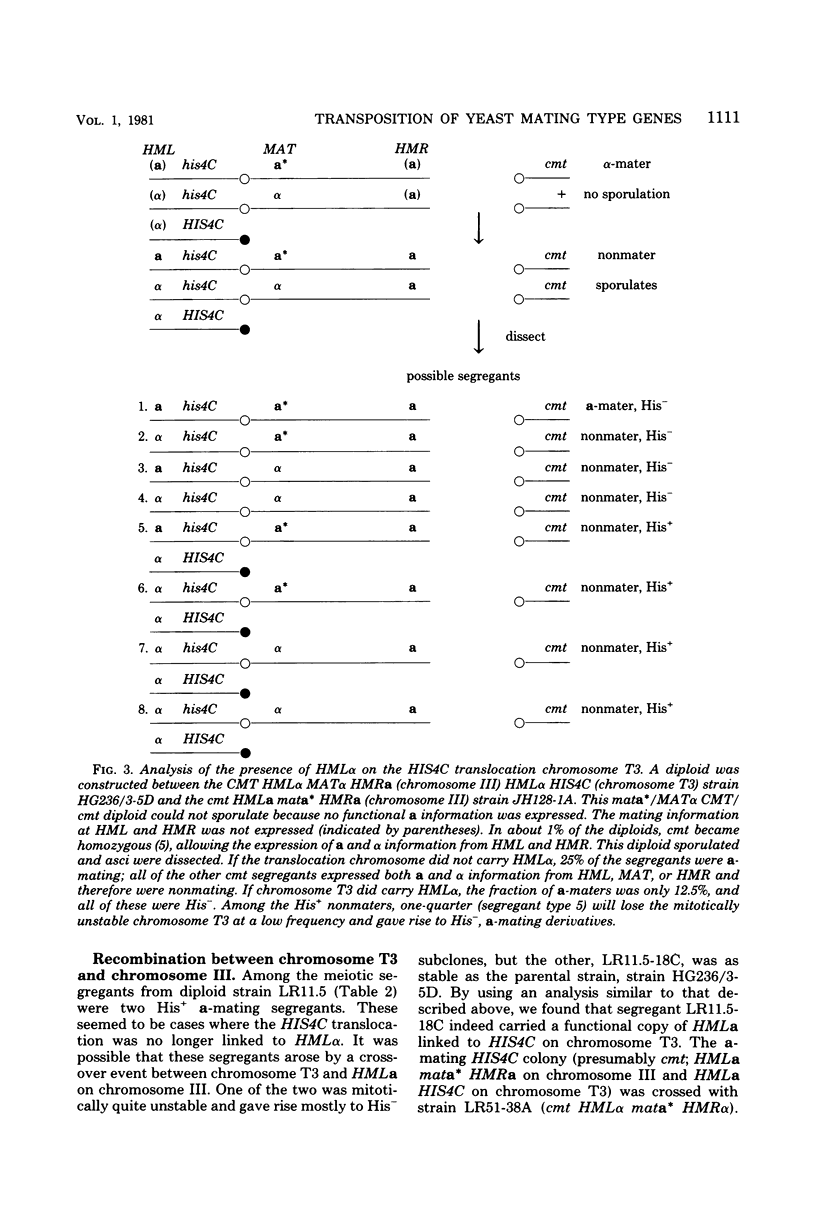
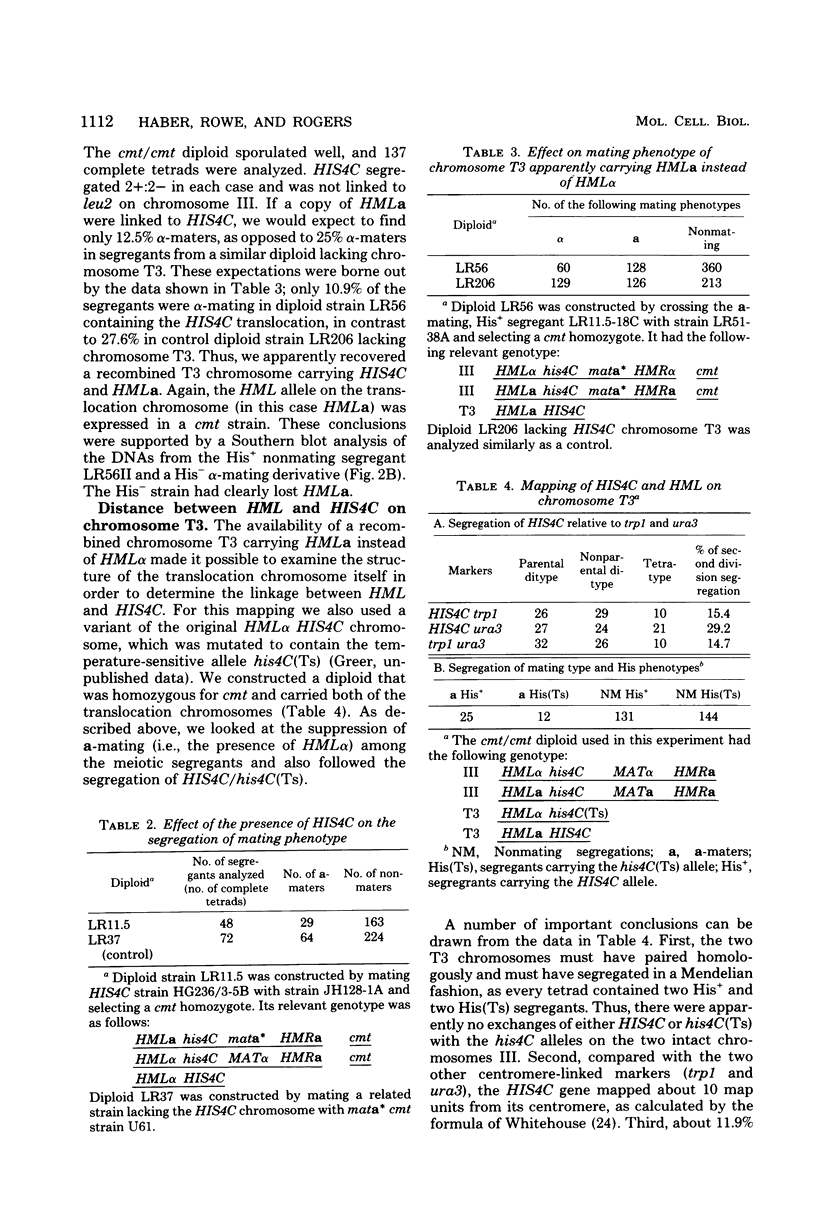
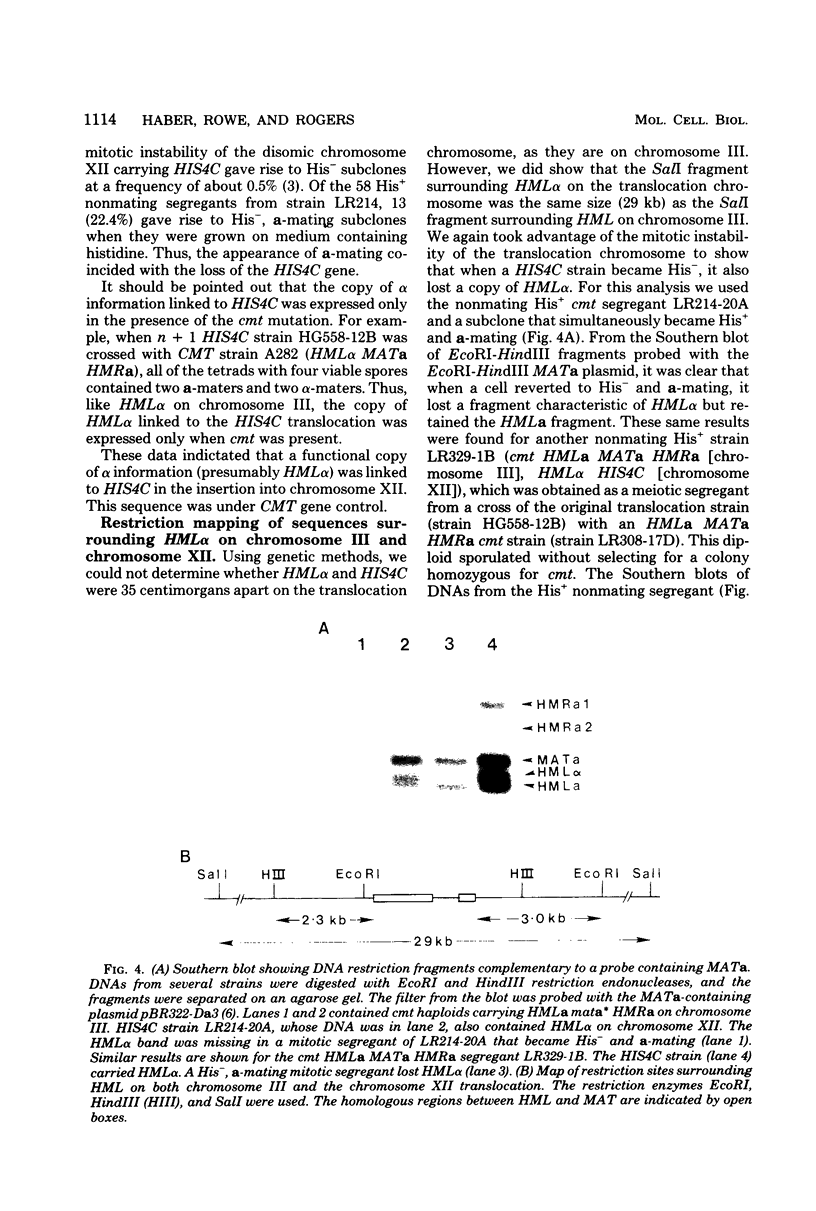
In the yeast Saccharomyces cerevisiae, the HIS4C gene lies on the left arm of chromosome III. We analyzed two chromosomal rearrangements that have HIS4C translocated either to chromosome XII or to a new translocation chromosome. Using the cmt mutation that allows expression of the normally silent copies of mating type genes, we found that both of these translocations also carried HML alpha, more than 30 map units distal to HIS4C which normally lies on chromosome III. In the case of the translocation chromosome (designated T3), we also found an exchange event between HML alpha on the translocation chromosome and HMLa on chromosome III. In diploids containing two T3 chromosomes (one carrying HML alpha and the carrying HMLa), we found that HML was 32 centimorgans from HIS4C, which was 10 centimorgans from an unknown centromere. In homothallic strains carrying HMLa MATa HMRa on chromosome III, switching from MATa to MAT alpha could occur by using the HML alpha on the translocation as the sole donor of alpha information. Transposition from HML alpha on chromosome T3 was about 20 to 40% as efficient as transposition from intact chromosome III. In contrast, transposition from the HML alpha inserted into chromosome XII was reduced about 100-fold. This reduced efficiency did not appear to be caused by an alteration in the sequences immediately surrounding HML alpha in the translocation. The translocated HML alpha sequence was located in the same size (29-kilobase) SalI fragment as was found in chromosome III, and the same EcoRI, HindIII, and BglII restriction sites were also found. Furthermore, HML alpha was still under the control of the CMT gene, which maintains HML as a silent copy of mating type information. These results suggested that the position of the HML alpha sequence plays an important role in the efficiency of mating type switching.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Davidow L. S., Haber J. E. Relation between the efficiency of homothallic switching of yeast mating type genes and the distribution of cell types. Mol Cell Biol. 1981 Dec;1(12):1120–1124. doi: 10.1128/mcb.1.12.1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greer H., Fink G. R. Unstable transpositions of his4 in yeast. Proc Natl Acad Sci U S A. 1979 Aug;76(8):4006–4010. doi: 10.1073/pnas.76.8.4006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber J. E., Garvik B. A new gene affecting the efficiency of mating-type interconversions in homothallic strains of Saccharomyces cerevisiae. Genetics. 1977 Sep;87(1):33–50. doi: 10.1093/genetics/87.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber J. E., George J. P. A mutation that permits the expression of normally silent copies of mating-type information in Saccharomyces cerevisiae. Genetics. 1979 Sep;93(1):13–35. doi: 10.1093/genetics/93.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber J. E., Rogers D. T., McCusker J. H. Homothallic conversions of yeast mating-type genes occur by intrachromosomal recombination. Cell. 1980 Nov;22(1 Pt 1):277–289. doi: 10.1016/0092-8674(80)90175-0. [DOI] [PubMed] [Google Scholar]
- Haber J. E., Savage W. T., Raposa S. M., Weiffenbach B., Rowe L. B. Mutations preventing transpositions of yeast mating type alleles. Proc Natl Acad Sci U S A. 1980 May;77(5):2824–2828. doi: 10.1073/pnas.77.5.2824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harashima S., Oshima Y. Mapping of the homothallic genes, HM alpha and HMa, in Saccharomyces yeasts. Genetics. 1976 Nov;84(3):437–451. doi: 10.1093/genetics/84.3.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hicks J. B., Herskowitz I. Interconversion of Yeast Mating Types I. Direct Observations of the Action of the Homothallism (HO) Gene. Genetics. 1976 Jun;83(2):245–258. doi: 10.1093/genetics/83.2.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hicks J., Strathern J. N., Klar A. J. Transposable mating type genes in Saccharomyces cerevisiae. Nature. 1979 Nov 29;282(5738):478–473. doi: 10.1038/282478a0. [DOI] [PubMed] [Google Scholar]
- Kassir Y., Simchen G. Regulation of mating and meiosis in yeast by the mating-type region. Genetics. 1976 Feb;82(2):187–206. doi: 10.1093/genetics/82.2.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klar A. J., Fogel S. The Action of Homothallism Genes in Saccharomyces Diploids during Vegetative Growth and the Equivalence of hma and HMalpha Loci Functions. Genetics. 1977 Mar;85(3):407–416. doi: 10.1093/genetics/85.3.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klar A. J., McIndoo J., Hicks J. B., Strathern J. N. Precise mapping of the homothallism genes HML and HMR in Saccharomyces cerevisiae. Genetics. 1980 Oct;96(2):315–320. doi: 10.1093/genetics/96.2.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klar A. J., McIndoo J., Strathern J. N., Hicks J. B. Evidence for a physical interaction between the transposed and the substituted sequences during mating type gene transposition in yeast. Cell. 1980 Nov;22(1 Pt 1):291–298. doi: 10.1016/0092-8674(80)90176-2. [DOI] [PubMed] [Google Scholar]
- Mascioli D. W., Haber J. E. A CIS-Acting Mutation within the MATa Locus of SACCHAROMYCES CEREVISIAE That Prevents Efficient Homothallic Mating-Type Switching. Genetics. 1980 Feb;94(2):341–360. doi: 10.1093/genetics/94.2.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasmyth K. A., Tatchell K. The structure of transposable yeast mating type loci. Cell. 1980 Mar;19(3):753–764. doi: 10.1016/s0092-8674(80)80051-1. [DOI] [PubMed] [Google Scholar]
- Oshima T., Takano I. Mutants Showing Heterothallism from a Homothallic Strain of SACCHAROMYCES CEREVISIAE. Genetics. 1980 Apr;94(4):841–857. doi: 10.1093/genetics/94.4.841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petes T. D. Yeast ribosomal DNA genes are located on chromosome XII. Proc Natl Acad Sci U S A. 1979 Jan;76(1):410–414. doi: 10.1073/pnas.76.1.410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Strathern J. N., Herskowitz I. Asymmetry and directionality in production of new cell types during clonal growth: the switching pattern of homothallic yeast. Cell. 1979 Jun;17(2):371–381. doi: 10.1016/0092-8674(79)90163-6. [DOI] [PubMed] [Google Scholar]
- Strathern J. N., Spatola E., McGill C., Hicks J. B. Structure and organization of transposable mating type cassettes in Saccharomyces yeasts. Proc Natl Acad Sci U S A. 1980 May;77(5):2839–2843. doi: 10.1073/pnas.77.5.2839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takano I., Kusumi T., Oshima Y. An alpha mating-type allele insensitive to the mutagenic action of the homothallic gene system in Saccharomyces diastaticus. Mol Gen Genet. 1973 Oct 16;126(1):19–28. doi: 10.1007/BF00333478. [DOI] [PubMed] [Google Scholar]
- WHITEHOUSE H. L. K. Mapping chromosome centromeres by the analysis of unordered tetrads. Nature. 1950 Jun 3;165(4205):893–893. doi: 10.1038/165893a0. [DOI] [PubMed] [Google Scholar]
- de Bruijn F., Greer H. Physical evidence for a Saccharomyces cerevisiae transposable element which carries the his4C gene. Mol Cell Biol. 1981 Apr;1(4):381–386. doi: 10.1128/mcb.1.4.381. [DOI] [PMC free article] [PubMed] [Google Scholar]





