Abstract
The nucleotide sequence of pKP1433 (55,417 bp), a blaKPC-2-carrying plasmid from Klebsiella pneumoniae sequence type 340, was determined. pKP1433 displayed extensive sequence and structural similarities with the IncN plasmids possessing the KPC-2-encoding Tn4401b isoform. However, the replication, partitioning, and stability of pKP1433 were determined by sequences related to diverse non-IncN plasmids.
TEXT
Most of the KPC-producing Klebsiella pneumoniae strains belong to sequence type 258 (ST258) or other STs of clonal complex 292 (1, 2). blaKPC commonly occurs as part of the Tn4401a isoform carried by similar IncFIIK plasmids (3, 4). However, an increasing diversity of clonal lineages and plasmids is being observed among KPC producers (5). We have previously reported on the sporadic occurrence in Greek hospitals of K. pneumoniae ST340 isolates containing a plasmid-borne blaKPC-2 gene associated with the Tn4401b isoform (5). We describe here the sequence of pKP1433, representing the KPC-2-encoding plasmids harbored by this distinct group of K. pneumoniae strains.
Plasmid pKP1433 could not be transferred by conjugation to Escherichia coli hosts (5) and was nontypeable by the PCR-based replicon typing (PBRT) method (6). Plasmid DNA was extracted from an E. coli DH5α transformant using the Qiagen large-construct kit (Qiagen, Hilden, Germany). Sequence analysis was performed by using the 454 genome sequencer FLX system on a standard DNA fragment library (Sistemas Genomicos, S.L., Valencia, Spain). The results were assembled into four contigs using 454 Newbler Assembler software (7). Gaps were filled by sequencing of PCR-produced fragments. The final contig assembly was carried out using Laser Gene software (DNAStar, Madison, WI). For sequence analysis and annotation, the BLAST algorithm (www.ncbi.nlm.nih.gov/BLAST), insertion sequence (IS) finder (www-is.biotoul.fr/), and open reading frame (ORF) finder (www.bioinformatics.org/sms/) were utilized.
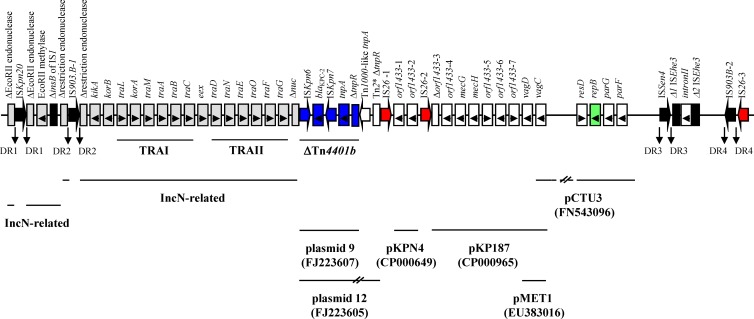
pKP1433 was 55,417 bp in size and included 59 coding sequences (48 complete and 11 truncated). The plasmid showed a complex structure, being mainly composed of an IncN-derived segment, as well as sequences of diverse origin, and was punctuated by mobile elements. A linear map of pKP1433 is shown in Figure 1.
Fig 1.
Linear depiction of pKP1433 (GenBank accession no. JX397875). ORFs are shown as rectangles containing arrowheads indicating direction of transcription. Intact insertion sequences (ISs) are represented by arrows, while truncated ISs appear as rectangles. The IncN-related sequences are shaded gray, and the non-IncN sequences are indicated as white rectangles. ΔTn4401b and repB are shown in blue and green, respectively. Red arrows indicate IS26 elements; the other ISs are shown in black. The lines below the map correspond to highly similar sequences from other plasmids. Target site duplications (TSDs) generated by transposition are marked as DR1, DR2, DR3, and DR4.
A sequence of 13,836 bp (nucleotides [nt] 1 to 13836) shared common features with the backbone of the IncN prototype plasmids R46 (GenBank accession no. AY046276) and pKM101 (8), including kikA, korB, and two tra arrays (TRAI and TRAII; 10.1 kb). Genes comprising TRAIII were not found, explaining the inability of pKP1433 to transfer via conjugation. Also, pKP1433 carried sequences (nt 13837 to 26798 and 51415 to 55417) that have been found in the KPC-encoding IncN plasmids 9, 12, and pBK31551 from K. pneumoniae strains isolated in the United States (9, 10). Adjacent to TRAII and within the coding sequence of the nuc gene, there was a truncated form of the KPC-2-encoding transposon Tn4401b (ΔTn4401b; 9,395 bp) that was 100% identical to that carried by plasmid 9 (9). ΔTn4401b consisted of ISKpn6, blaKPC-2, ISKpn7, tnpA, and part of tnpR (ΔtnpR). The latter gene had been truncated by the insertion of a Tn1000/Tn2* hybrid transposon comprising a Tn1000-like tnpA and part of Tn2* tnpR. This segment was 97% identical to that found in plasmid 12, although the associated ars operon was not present in pKP1433. Genes encoding a restriction endonuclease, an EcoRII methylase, and an EcoRII endonuclease were located downstream from kikA, as in the aforementioned KPC-encoding plasmids and other multiresistant IncN plasmids, such as pNL194 (11), pKOX105 (12), and pKP96 (13). An intact group II intron (2,270 bp; nt 45033 to 47302) was identified upstream from the EcoRII endonuclease. It included an intron RNA that may promote self-splicing and intron mobility, as well as an ORF encoding a multifunctional protein with reverse transcriptase and RNA maturase activities required for site-specific DNA insertion (14–16). A similar region (90% identity) has also been described in plasmid 9, although it is disrupted by the insertion of a copy of Tn4401b (9).
pKP1433 lacked the replication region and stability operon that are characteristic of the IncN plasmids. However, in the 24,616-bp segment adjoining the boundaries of the presumably IncN-derived part (nt 26799 to 51414), sequences most probably implicated in essential plasmid functions were identified. A repB-like sequence of 693 bp exhibiting 79, 73, and 71% identity with the repB genes of pCTU3 from Cronobacter turicensis strain z3032 (GenBank accession no. FN543096) (17), p14-120 from Salmonella sp. strain 14 (GenBank accession no. JQ418538), and pPAT9B05 from Pantoea sp. strain At-9b (GenBank accession no. CP002438), respectively, was located at positions 39393 to 40085. None of the latter plasmids could be assigned to any of the known incompatibility groups, thus explaining the failure of the PBRT method to classify pKP1433. The putative repB product of pKP1433 showed high amino acid sequence similarity (from 91 to 96%) with the replication initiation proteins of the aforementioned plasmids. The similarities with other replicases were significantly lower, the higher scores being observed with RepHI1A (77%) (18) and RepFIB (65%) (19). Upstream from repB, a single-strand initiation sequence motif (ssiF) was present (nt 40421 to 40476). A resD-like gene, belonging to the family of the xerCD site-specific recombinases, was located at positions 37992 to 38768. Its putative product (resolvase) is probably involved in the multimer resolution of pKP1433, thus contributing to plasmid maintenance (20). Of note, similar resD-like genes have also been found downstream from the repB genes of pCTU3 and p14-120.
A sequence resembling the parFG operon of plasmid pMET1 from K. pneumoniae FC1 (77% identity) (21) was carried by pKP1433 (nt 40619 to 41525), as well as pCTU3, at a similar position (i.e., upstream from repB). The ParF and ParG proteins are involved in the partitioning of plasmid replicates (22). Upstream from the parFG operon, a putative operator and a centromere-like site (parH) comprising five and eight degenerated 5′-ACTC-3′ tetramer motifs, respectively (arranged either in direct [n = 8] or inverted [n = 5] orientation), were identified between nt 41526 and nt 41756. Thus, parFG may be functional, contributing to pKP1433 maintenance.
The non-IncN-derived segment of pKP1433 also included a 6,715-bp sequence (nt 29676 to 36390) exhibiting 97% identity with a contiguous region described in pKP187 from K. pneumoniae strain 342 (23) that is related to the virulence plasmid pLVPK of this species (24). This sequence (not found in the plasmids sharing common replication and partition regions with pKP1433) contained nine ORFs (Δorf3, orf4, mceG, mceH, orf5, orf6, orf7, vagC, and vagD). The products of mceG and mceH may form a type I secretion system (25). The VagC and VagD proteins encoded by the vagCD operon (nt 35636 to 36279) are believed to be involved in the coordination of plasmid replication with cell division (26).
pKP1433 was punctuated by a variety of mobile elements (n = 8) (Fig. 1). Two of them, an IS903.B-like element (IS903.B-1) and a novel insertion sequence of the IS3 family (designated ISKpn20), were inserted in the restriction endonuclease and the EcoRII endonuclease of the IncN backbone. Target site duplications of 9 bp (GCCATGCGC; direct repeat 2 [DR2]) and 4 bp (GAGG; DR1) at the boundaries of the latter ISs indicated insertions by transposition. The non-IncN region included three copies of IS26, a second copy of IS903.B (IS903.B-2), an ISSen4, and an ISEhe3 disrupted by intron II. Transposition of IS903.B-2 and ISSen4 within pKP1433 was indicated by the presence of target site duplications of 9 bp (GTTCTCCGG; DR4) and of 3 bp (AAG; DR3). Of note, the non-IncN region was bracketed by two IS26 copies in reverse orientation (Fig. 1).
The sequencing data may indicate that the progenitor of pKP1433 was an IncN plasmid carrying the KPC-encoding Tn4401b. A plausible hypothesis is that, at a certain step of the evolution of pKP1433, the IncN formed with another plasmid a fusion multireplicon structure that, after resolution, evolved further through rearrangements facilitated by insertion sequences. Nevertheless, en block acquisition of an IncN-derived large segment containing Tn4401b by a plasmid carrying the repB gene cannot be excluded. Whichever is the case, the IS26 elements bordering the two distinct segments constituting pKP1433 most probably played an important role in shaping this novel plasmid. The findings of this study underscore the diversification potential of plasmids carrying important resistance determinants such as blaKPC-2.
Nucleotide sequence accession number.
The complete nucleotide sequence of plasmid pKP1433 has been assigned GenBank accession no. JX397875.
ACKNOWLEDGMENT
This work was supported by funding from the European Commission (TROCAR contract HEALTH-F3-2008-223031).
Footnotes
Published ahead of print 29 April 2013
REFERENCES
- 1. Tzouvelekis LS, Markogiannakis A, Psichogiou M, Tassios PT, Daikos GL. 2012. Carbapenemases in Klebsiella pneumoniae and other Enterobacteriaceae: an evolving crisis of global dimensions. Clin. Microbiol. Rev. 25:682–707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Woodford N, Turton JF, Livermore DM. 2011. Multiresistant gram-negative bacteria: the role of high-risk clones in the dissemination of antibiotic resistance. FEMS Microbiol. Rev. 35:736–755 [DOI] [PubMed] [Google Scholar]
- 3. Cuzon G, Naas T, Truong H, Villegas MV, Wisell KT, Carmeli Y, Gales AC, Venezia SN, Quinn JP, Nordmann P. 2010. Worldwide diversity of Klebsiella pneumoniae that produce β-lactamase blaKPC-2 gene. Emerg. Infect. Dis. 16:1349–1356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Leavitt A, Chmelnitsky I, Carmeli Y, Navon-Venezia S. 2010. Complete nucleotide sequence of KPC-3-encoding plasmid pKpQIL in the epidemic Klebsiella pneumoniae sequence type 258. Antimicrob. Agents Chemother. 54:4493–4496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Giakkoupi P, Papagiannitsis CC, Miriagou V, Pappa O, Polemis M, Tryfinopoulou K, Tzouvelekis LS, Vatopoulos AC. 2011. An update of the evolving epidemic of blaKPC-2-carrying Klebsiella pneumoniae in Greece (2009-10). J. Antimicrob. Chemother. 66:1510–1513 [DOI] [PubMed] [Google Scholar]
- 6. Carattoli A, Bertini A, Villa L, Falbo V, Hopkins KL, Threlfall EJ. 2005. Identification of plasmids by PCR-based replicon typing. J. Microbiol. Methods 63:219–228 [DOI] [PubMed] [Google Scholar]
- 7. Margulies M, Egholm M, Altman WE, Attiya S, Bader JS, Bemben LA, Berka J, Braveman MS, Chen YJ, Chen Z, Dewell SB, Du L, Fierro JM, Gomes XV, Godwin BC, He W, Helgesen S, Ho CH, Irzyk GP, Jando SC, Alenquer ML, Jarvie TP, Jirage KB, Kim JB, Knight JR, Lanza JR, Leamon JH, Lefkowitz SM, Lei M, Li J, Lohman KL, Lu H, Makhijani VB, McDade KE, McKenna MP, Myers EW, Nickerson E, Nobile JR, Plant R, Puc BP, Ronan MT, Roth GT, Sakris GJ, Simons JF, Simpson JW, Srinivasan M, Tartaro KR, Tomasx A, Vogt KA, Volkmer GA, Wang SH, Wang Y, Weiner MP, Yu P, Begley RF, Rothberg JM. 2005. Genome sequencing in microfabricated high-density picolitre reactors. Nature 437:376–380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Winans SC, Walker GC. 1985. Conjugal transfer system of the IncN plasmid pKM101. J. Bacteriol. 161:402–410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gootz TD, Lescoe MK, Dib-Hajj F, Dougherty BA, He W, Della-Latta P, Huard RC. 2009. Genetic organization of transposase regions surrounding blaKPC carbapenemase genes on plasmids from Klebsiella strains isolated in a New York City hospital. Antimicrob. Agents Chemother. 53:1998–2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chen L, Chavda KD, Fraimow HS, Mediavilla JR, Melano RG, Jacobs MR, Bonomo RA, Kreiswirth BN. 2013. Complete nucleotide sequences of blaKPC-4- and blaKPC-5-harboring IncN and IncX plasmids from Klebsiella pneumoniae strains isolated in New Jersey. Antimicrob. Agents Chemother. 57:269–276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Miriagou V, Papagiannitsis CC, Kotsakis SD, Loli A, Tzelepi E, Legakis NJ, Tzouvelekis LS. 2010. Sequence of pNL194, a 79.3-kilobase IncN plasmid carrying the blaVIM-1 metallo-β-lactamase gene in Klebsiella pneumoniae. Antimicrob. Agents Chemother. 54:4497–4502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Carattoli A, Aschbacher R, March A, Larcher C, Livermore DM, Woodford N. 2010. Complete nucleotide sequence of the IncN plasmid pKOX105 encoding VIM-1, QnrS1 and SHV-12 proteins in Enterobacteriaceae from Bolzano, Italy compared with IncN plasmids encoding KPC enzymes in the U. S. A. J. Antimicrob. Chemother. 65:2070–2075 [DOI] [PubMed] [Google Scholar]
- 13. Shen P, Jiang Y, Zhou Z, Zhang J, Yu Y, Li L. 2008. Complete nucleotide sequence of pKP96, a 67 850 bp multiresistance plasmid encoding qnrA1, aac(6′)-Ib-cr and blaCTX-M-24 from Klebsiella pneumoniae. J. Antimicrob. Chemother. 62:1252–1256 [DOI] [PubMed] [Google Scholar]
- 14. Dai L, Zimmerly S. 2002. The dispersal of five group II introns among natural populations of Escherichia coli. RNA 8:1294–1307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Rajakumar K, Sasakawa C, Adler B. 1997. Use of a novel approach, termed island probing, identifies the Shigella flexneri she pathogenicity island which encodes a homolog of the immunoglobulin A protease-like family of proteins. Infect. Immun. 65:4606–4614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Tobe T, Hayashi T, Han CG, Schoolnik GK, Ohtsubo E, Sasakawa C. 1999. Complete DNA sequence and structural analysis of the enteropathogenic Escherichia coli adherence factor plasmid. Infect. Immun. 67:5455–5462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Stephan R, Lehner A, Tischler P, Rattei T. 2011. Complete genome sequence of Cronobacter turicensis LMG 23827, a food-borne pathogen causing deaths in neonates. J. Bacteriol. 193:309–310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sherburne CK, Lawley TD, Gilmour MW, Blattner FR, Burland V, Grotbeck E, Rose DJ, Taylor DE. 2000. The complete DNA sequence and analysis of R27, a large IncHI plasmid from Salmonella typhi that is temperature sensitive for transfer. Nucleic Acids Res. 28:2177–2186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Spiers AJ, Bhana N, Bergquist PL. 1993. Regulatory interactions between RepA, an essential replication protein, and the DNA repeats of RepFIB from plasmid P307. J. Bacteriol. 175:4016–4024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Drieux L, Decré D, Frangeul L, Arlet G, Jarlier V, Sougakoff W. 2013. Complete nucleotide sequence of the large conjugative pTC2 multireplicon plasmid encoding the VIM-1 metallo-β-lactamase. J. Antimicrob. Chemother. 68:97–100 [DOI] [PubMed] [Google Scholar]
- 21. Soler Bistué AJ, Birshan D, Tomaras AP, Dandekar M, Tran T, Newmark J, Bui D, Gupta N, Hernandez K, Sarno R, Zorreguieta A, Actis LA, Tolnasky ME. 2008. Klebsiella pneumoniae multiresistance plasmid pMET1: similarity with the Yersinia pestis plasmid pCRY and integrative conjugative elements. PLoS One 3:e1800. 10.1371/journal.pone.0001800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Barillà D, Hayes F. 2003. Architecture of the ParF*ParG protein complex involved in prokaryotic DNA segregation. Mol. Microbiol. 49:487–499 [DOI] [PubMed] [Google Scholar]
- 23. Fouts DE, Tyler HL, DeBoy RT, Daugherty S, Ren Q, Badger JH, Durkin AS, Huot H, Shrivatava S, Kothari S, Dodson RJ, Mohamoud Y, Khouri H, Roesch LF, Krogfelt KA, Struve C, Triplett EW, Methé BA. 2008. Complete genome sequence of the N2-fixing broad host endophyte Klebsiella pneumonia 342 and virulence predictions verified in mice. PLoS Genet. 4:e1000141. 10.1371/journal.pgen.1000141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Chen YT, Chang HY, Lai YC, Pan CC, Tsai SF, Peng HL. 2004. Sequencing and analysis of the large virulence plasmid pLVPK of Klebsiella pneumoniae CG43. Gene 337:189–198 [DOI] [PubMed] [Google Scholar]
- 25. Lagos R, Baeza M, Corsini G, Hetz C, Strahsburger E, Castillo JA, Vergara C, Monasterio O. 2001. Structure, organization and characterization of the gene cluster involved in the production of microcin E492, a channel-forming bacteriocin. Mol. Microbiol. 42:229–243 [DOI] [PubMed] [Google Scholar]
- 26. Pullinger GD, Lax AJ. 1992. A Salmonella dublin virulence plasmid locus that affects bacterial growth under nutrient-limited conditions. Mol. Microbiol. 6:1631–1643 [DOI] [PubMed] [Google Scholar]