Abstract
We describe here a simple, fast, and reliable bioassay method for therapeutic drug monitoring of voriconazole. Fifty-eight clinical and external quality control samples were evaluated with this microbiological assay, and results were compared with those obtained with a previously validated chromatographic method. A good correlation between both assays was observed. This particular microbiological method was demonstrated to be simple and offers enough precision and accuracy to perform voriconazole therapeutic drug monitoring in laboratories without specialized equipment.
TEXT
Voriconazole is used for the treatment of invasive fungal infections. It is currently considered the first-line agent for the treatment of invasive aspergillosis (1) and a valid alternative for treating invasive candidiasis (2).
It is available as both an oral and intravenous formulation and is characterized by a saturable hepatic metabolism influenced by genetic background and specific clinical conditions. Most published data have shown substantial inter- and intraindividual variabilities of trough concentrations, and recent published data have suggested that its efficacy and tolerance could be increased with therapeutic drug monitoring (TDM), a suitable strategy to maximize the cost-effectiveness of this treatment (3–5). Several retrospective studies and also two prospective studies recently published support the usefulness of TDM with voriconazole treatment (3–7). A target blood concentration of voriconazole ranging between 1.0 and 6.0 μg/ml (therapeutic range) has been demonstrated to have great utility for patient management (8–10). To evaluate antifungal concentrations, several quantification methods, most based on high-performance liquid chromatography (HPLC) (11–15) and microbiological assays (bioassay), have been described in the literature (16–19). Bioassays offer simplicity and lower costs and are therefore a valid alternative to chromatographic methods in clinical laboratories without specialized equipment. Nevertheless, some disadvantages have been described, such as lack of standardization and lower precision and accuracy than chromatographic methods, and also limited utility in cases of antifungal combined therapy.
The aim of this study was to develop a simple, fast, nonexpensive, and reliable bioassay for voriconazole quantification. A cross-validation between this assay and a previously validated chromatographic method was also performed (20) in order to address the effectiveness of the microbiological method for treatment follow-up.
The biological activity of voriconazole in serum samples was measured in a diffusion assay. Preparation of the media, reagents for the assay, and the test organism (Candida kefyr ATCC 28838; voriconazole MIC, 0.015 μg/ml) were previously described (21), including the following minor modifications. The turbidity of the test organism suspension was adjusted to 1.0 McFarland (optical density at 530 nm between 0.23 and 0.27; Thermospectronic, Genesis TM20). In addition, 30 wells (5.4-mm diameter) were bored aseptically with the help of a sterile cork borer in a well-spaced pattern. Voriconazole was kindly supplied by Pfizer (Pfizer S.A., Madrid, Spain). Twenty-five-microliter aliquots of clinical samples were pipetted in triplicate into individual wells. This amount of plasma was allowed to diffuse through the agar at room temperature for 1 h, and the plates were then incubated for 20 h at 37°C.
Human commercial serum (Sigma-Aldrich, Madrid, Spain) was used as the matrix for calibration standards (CS) and quality control samples (QC). Two separate stock solutions of voriconazole were used to prepare CS and QC.
A validation procedure was performed in different experiments including CS and QC. The concentrations evaluated are shown in Table 1.
Table 1.
Within-day and between-day variabilities of CS and QCa
| Type of variability and Cnom (μg/ml) | CS |
QC |
||||
|---|---|---|---|---|---|---|
| CCS (μg/ml) (mean ± SD) | Precision (CV %) | Accuracy (RE %) | CQC (μg/ml) (mean ± SD) | Precision (CV%) | Accuracy (RE %) | |
| Within-day variabilityb | ||||||
| 0.25 | 0.25 ± 0.005 | 2.22 | 1.19 | 0.25 ± 0.013 | 5.45 | 0.26 |
| 0.5 | 0.48 ± 0.014 | 3.03 | −2.66 | 0.55 ± 0.043 | 7.75 | 11.75 |
| 1 | 0.93 ± 0.049 | 5.31 | −6.07 | 1.01 ± 0.088 | 8.72 | 1.52 |
| 2 | 2.08 ± 0.111 | 5.36 | 4.16 | |||
| 3 | 3.03 ± 0.055 | 1.82 | 1.06 | 3.24 ± 0.083 | 2.57 | 8.32 |
| 4 | 4.08 ± 0.256 | 6.27 | 2.23 | |||
| 6 | 6.02 ± 0.106 | 1.77 | 0.43 | 6.12 ± 0.21 | 3.58 | 6.18 |
| 8 | 7.89 ± 0.827 | 10.50 | −1.50 | |||
| Between-day variabilityc | ||||||
| 0.25 | 0.25 ± 0.015 | 5.88 | 3.34 | 0.24 ± 0.023 | 9.67 | −2.47 |
| 0.5 | 0.48 ± 0.032 | 6.70 | −2.37 | 0.50 ± 0.060 | 12.05 | 0.832 |
| 1 | 0.94 ± 0.053 | 5.65 | −5.23 | 1.00 ± 0.105 | 10.49 | 0.149 |
| 2 | 2.11 ± 0.257 | 7.45 | 5.54 | |||
| 3 | 3.07 ± 0.150 | 4.90 | 2.49 | 3.32 ± 0.269 | 8.10 | 10.96 |
| 4 | 4.23 ± 0.312 | 7.39 | 5.78 | |||
| 6 | 5.98 ± 0.355 | 5.94 | −0.20 | 6.38 ± 0.703 | 11.02 | 6.37 |
| 8 | 7.73 ± 0.849 | 10.98 | −3.32 | |||
Cnom, nominal or theoretical concentration; CCS, experimental concentration of the calibration standards; CQC, experimental concentration of the quality controls.
n = 3 for CS and n = 6 for QC for within-day variability calculations.
n = 9 for CS and n = 24 for QC for between-day variability calculations.
The assay was repeated over 4 days for QC, over 2 days for CS, and for 1 day for clinical samples. Zones of inhibition were measured using a metric caliper micrometer (Fowler, Ultra-cal II; Sylvac, Bévilard, Switzerland) by two different analysts (blinded measures), taking the average of both measurements as the final result.
The bioassay results were tested for linearity, precision, and accuracy. The criteria for acceptability of data included accuracy (relative error [% RE]) within a 15% deviation from the nominal values and precision within 15% of the coefficient of variation (% CV), except for the lower limit of quantification (LLOQ), for which data should not exceed 20% of the CV (22, 23). The standard curve was logarithmic in the range of 0.25 to 8 μg/ml. The LLOQ was 0.25 μg/ml, which was slightly better than that from other recently published assays (0.5 to 3.00 μg/ml) (16, 19). Between- and within-day values for accuracy and precision of the CS and the QC samples are shown in Table 1. Precision and accuracy were within the limits established by international guidelines, with coefficients of variation lower than 12%, a value comparable with that for other published bioassays (17–19). Bioassay variability has been reported to be associated with the natural antimicrobial serum activity or biases in the measurements of the inhibition zones (24, 25). Although natural antifungal serum activity is difficult to determine, no zones of inhibition were obtained from any of the blank commercial serum samples included in each sample set performed. In addition, the dynamic range defined for this bioassay (0.25 to 8.00 μg/ml) included the therapeutic range considered for clinical decisions (from 1.0 to 6.0 μg/ml).
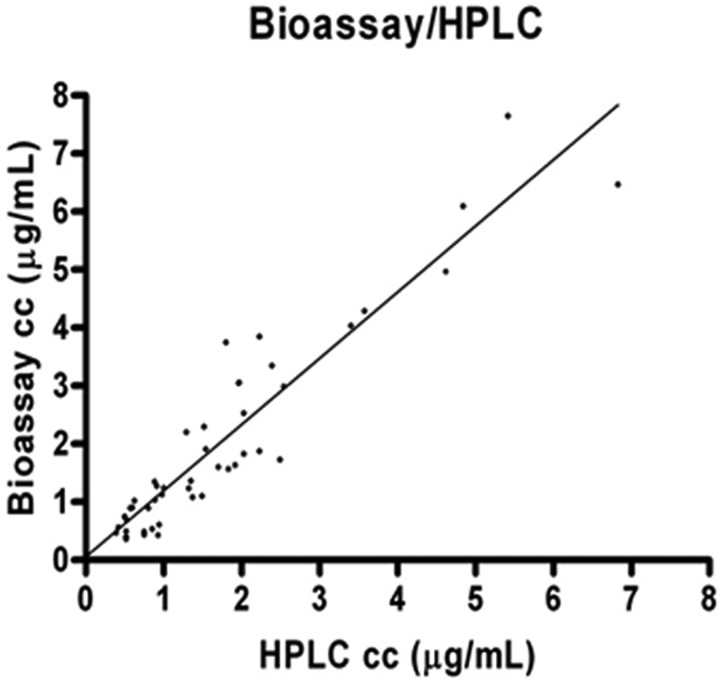
A cross-validation was also performed, using a total of 58 human serum trough samples. Samples belong to 11 patients (46 samples) and 12 external QC samples received from an international interlaboratory proficiency testing program for antifungal drug quantifications. Agreement between measurements was evaluated by using the intraclass correlation coefficient (ICC; Paws 18.0; Paws Statistics, Madrid, Spain). A P value of <0.01 was considered statistically significant. Results were also compared by using least-squares linear regression standard techniques (Prism 4; GraphPad, La Jolla, CA). For these calculations, the chromatographic method was considered the reference method. The correlation between chromatographic and bioassay methodologies in clinical samples was statistically significant, as indicated by the ICC value (0.965; range, 0.941 to 0.979; P < 0.01) and the linear regression analysis [bioassay: 1.1347(HPLC result) + 0.0543; R2 = 0.899; coefficient of correlation, 0.9481; n = 58 runs] (Fig. 1). Several practical advantages for this bioassay could be enumerated compared to others previously described. One of them was the number of samples that could be evaluated (10 samples in triplicate). This fact reduced costs, working time, and biases in the measuring of the inhibition zones. Another benefit to add is the small volume of serum required to perform this bioassay (75 μl), which indicates it is a good alternative when the amount of serum is not adequate (e.g., pediatric patients). One more practical advantage is the flexibility of the assay. Concerning the test organism, several strains have been used (17–19), but a standard microorganism has not been defined for performing this methodology, and theoretically any strain of Candida showing a voriconazole susceptibility profile might be suitable as the test organism if it provided reliable and symmetric zones of growth inhibition.
Fig 1.
Scatter plot of voriconazole serum concentrations (in μg/ml) as measured by HPLC and bioassay. Linear regression results: bioassay result = 1.1347(HPLC result) + 0.053; R2 = 0.899; coefficient of correlation, 0.948; n = 58 samples.
In contrast, bioassay-based techniques show some limitations. This method quantifies the antifungal activity but fails to identify the drug or its active metabolites, and inhibition zones can be modified by concomitant antifungal drugs. Combined antifungal therapy should be taken into account, and a bioassay is not adequate for use for TDM in those patients.
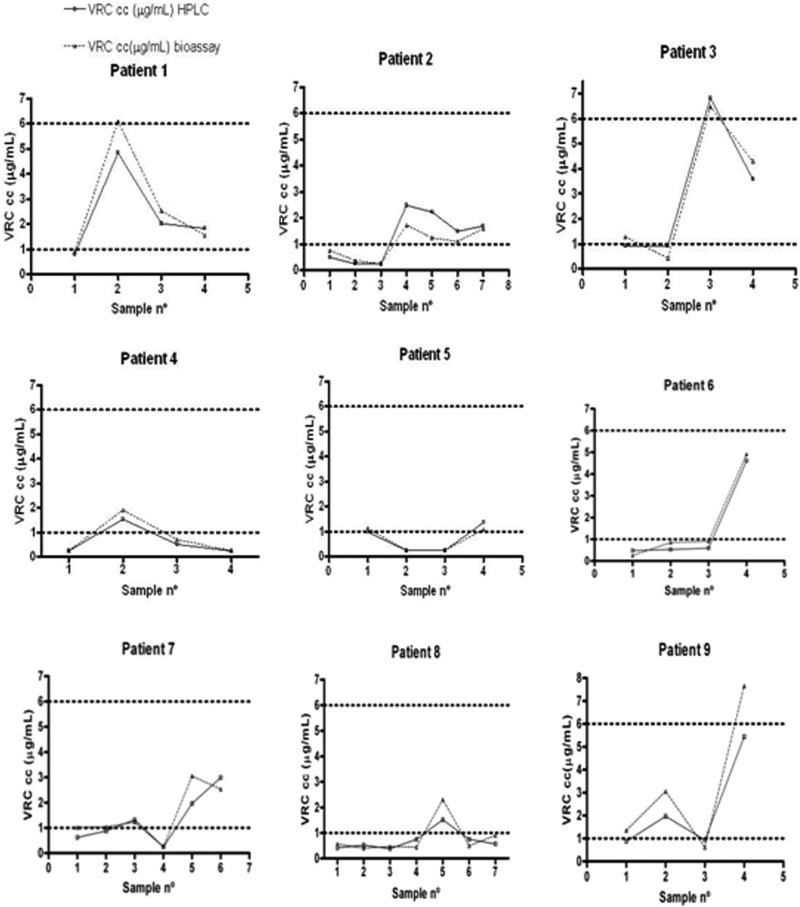
Plots representing sequential serum drug concentrations measured by HPLC and bioassay were created for each patient with two or more samples (GraphPad Prism 4), keeping in consideration the target therapeutic range of 1 to 6 μg/ml (8–10) (Fig. 2). The graphical representation was intended to allow easy detection of discordant results that might affect clinical decisions, knowing that values out of the therapeutic range would imply a dose change. Concordance between individual data was checked, scoring as agreement (AgM) those cases in which both measurements were within the therapeutic range and differences were less than 20%; the classification minor error (MiE) was assigned when both of the measurements were in the same region of the therapeutic range but differences were more than 20%; a major error (MaE) classification was assigned when measurements were in different regions of the therapeutic range and the differences were less than 20%; a very major error (VmE) classification was assigned when results were in different regions of the therapeutic range and differences were more than 20%. The overall percentages of the AgM and MiE were close to 90.00% (41.37% and 48.72%, respectively). Six results were coded as MaE or VmE (Table 2), and values reported from the bioassay were slightly higher (HPLC concentration average, 1.388 μg/ml, versus bioassay concentration average, 1.636 μg/ml). In our cohort of patients, good and reliable monitoring of voriconazole levels could be performed by bioassay. The main drawback in the evaluation of the clinical samples was the limited access to clinical records. In general, a more exhaustive follow-up should be recommended in those cases for which bioassay results are close to the upper and lower limits of the therapeutic range (1.00 and 6.00 μg/ml).
Fig 2.
Plots of voriconazole (VRC) measurements for the nine patients included in the validation of the voriconazole bioassay.
Table 2.
Sample results coded as MaE and VmE
| Patient no. | Sample no. | Concn (μg/ml) based on: |
% difference between values | |
|---|---|---|---|---|
| HPLC | Bioassay | |||
| 5 | 1 | 0.98 | 1.13 | 13.27 |
| 9 | 1 | 0.88 | 1.35 | 34.81 |
| 9 | 4 | 5.41 | 7.65 | 29.28 |
| 3 | 1 | 0.91 | 1.28 | 28.90 |
| 7 | 1 | 0.62 | 1.02 | 39.21 |
| 7 | 2 | 0.88 | 1.03 | 14.56 |
According to the high concordance values found, this bioassay is a reliable and nonexpensive tool to perform TDM of voriconazole, especially for clinical laboratories where chromatography facilities are not available.
ACKNOWLEDGMENTS
We thank Pfizer for supply of the voriconazole powder.
This study has been partially financed by Fondo de Investigaciones Sanitarias (PI09/0624). E. Cendejas-Bueno received a predoctoral fund from Instituto de Salud Carlos III (AFTDOC 11/02, Spain).
In the past 5 years, M.C.E. has received grant support from Astellas Pharma, bioMérieux, Gilead Sciences, Merck Sharp and Dohme, Pfizer, Schering Plough, and Soria Melguizo SA. He has been an advisor/consultant to the Panamerican Health Organization, Gilead Sciences, Merck Sharp and Dohme, Pfizer, and Schering Plough. He has been paid for talks on behalf of Gilead Sciences, Merck Sharp and Dohme, Pfizer, and Schering Plough. The other authors have no conflicts to declare.
Footnotes
Published ahead of print 6 May 2013
REFERENCES
- 1. Herbrecht R, Denning DW, Patterson TF, Bennett JE, Greene RE, Oestmann JW, Kern WV, Marr KA, Ribaud P, Lortholary O, Sylvester R, Rubin RH, Wingard JR, Stark P, Durand C, Caillot D, Thiel E, Chandrasekar PH, Hodges MR, Schlamm HT, Troke PF, de Pauw B. 2002. Voriconazole versus amphotericin B for primary therapy of invasive aspergillosis. N. Engl. J. Med. 347:408–415 [DOI] [PubMed] [Google Scholar]
- 2. Rex JH, Walsh TJ, Sobel JD, Filler SG, Pappas PG, Dismukes WE, Edwards JE. 2000. Practice guidelines for the treatment of candidiasis. Infectious Diseases Society of America. Clin. Infect. Dis. 30:662–678 [DOI] [PubMed] [Google Scholar]
- 3. Pascual A, Calandra T, Bolay S, Buclin T, Bille J, Marchetti O. 2008. Voriconazole therapeutic drug monitoring in patients with invasive mycoses improves efficacy and safety outcomes. Clin. Infect. Dis. 46:201–211 [DOI] [PubMed] [Google Scholar]
- 4. Trifilio S, Ortiz R, Pennick G, Verma A, Pi J, Stosor V, Zembower T, Mehta J. 2005. Voriconazole therapeutic drug monitoring in allogeneic hematopoietic stem cell transplant recipients. Bone Marrow Transplant. 35:509–513 [DOI] [PubMed] [Google Scholar]
- 5. Trifilio SM, Yarnold PR, Scheetz MH, Pi J, Pennick G, Mehta J. 2009. Serial plasma voriconazole concentrations after allogeneic hematopoietic stem cell transplantation. Antimicrob. Agents Chemother. 53:1793–1796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Park WB, Kim NH, Kim KH, Lee SH, Nam WS, Yoon SH, Song KH, Choe PG, Kim NJ, Jang IJ, Oh MD, Yu KS. 2012. The effect of therapeutic drug monitoring on safety and efficacy of voriconazole in invasive fungal infections: a randomized controlled trial. Clin. Infect. Dis. 55:1080–1087 [DOI] [PubMed] [Google Scholar]
- 7. Pascual A, Csajka C, Buclin T, Bolay S, Bille J, Calandra T, Marchetti O. 2012. Challenging recommended oral and intravenous voriconazole doses for improved efficacy and safety: population pharmacokinetics-based analysis of adult patients with invasive fungal infections. Clin. Infect. Dis. 55:381–390 [DOI] [PubMed] [Google Scholar]
- 8. Andes D, Pascual A, Marchetti O. 2009. Antifungal therapeutic drug monitoring: established and emerging indications. Antimicrob. Agents Chemother. 53:24–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bruggemann RJ, Donnelly JP, Aarnoutse RE, Warris A, Blijlevens NM, Mouton JW, Verweij PE, Burger DM. 2008. Therapeutic drug monitoring of voriconazole. Ther. Drug Monit. 30:403–411 [DOI] [PubMed] [Google Scholar]
- 10. Lewis RE. 2007. Pharmacodynamic implications for use of antifungal agents. Curr. Opin. Pharmacol. 7:491–497 [DOI] [PubMed] [Google Scholar]
- 11. Chhun S, Rey E, Tran A, Lortholary O, Pons G, Jullien V. 2007. Simultaneous quantification of voriconazole and posaconazole in human plasma by high-performance liquid chromatography with ultra-violet detection. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 852:223–228 [DOI] [PubMed] [Google Scholar]
- 12. Gage R, Stopher DA. 1998. A rapid HPLC assay for voriconazole in human plasma. J. Pharm. Biomed. Anal. 17:1449–1453 [DOI] [PubMed] [Google Scholar]
- 13. Gordien JB, Pigneux A, Vigouroux S, Tabrizi R, Accoceberry I, Bernadou JM, Rouault A, Saux MC, Breilh D. 2009. Simultaneous determination of five systemic azoles in plasma by high-performance liquid chromatography with ultraviolet detection. J. Pharm. Biomed. Anal. 50:932–938 [DOI] [PubMed] [Google Scholar]
- 14. Kahle K, Langmann P, Schirmer D, Lenker U, Keller D, Helle A, Klinker H, Heinz WJ. 2009. Simultaneous determination of voriconazole and posaconazole concentrations in human plasma by high-performance liquid chromatography. Antimicrob. Agents Chemother. 53:3140–3142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Langman LJ, Boakye-Agyeman F. 2007. Measurement of voriconazole in serum and plasma. Clin. Biochem. 40:1378–1385 [DOI] [PubMed] [Google Scholar]
- 16. Adams AI, Steppe M, Froehlich PE, Bergold AM. 2006. Comparison of microbiological and UV-spectrophotometric assays for determination of voriconazole in tablets. J. AOAC Int. 89:960–965 [PubMed] [Google Scholar]
- 17. Pascual A, Nieth V, Calandra T, Bille J, Bolay S, Decosterd LA, Buclin T, Majcherczyk PA, Sanglard D, Marchetti O. 2007. Variability of voriconazole plasma levels measured by new high-performance liquid chromatography and bioassay methods. Antimicrob. Agents Chemother. 51:137–143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Perea S, Pennick GJ, Modak A, Fothergill AW, Sutton DA, Sheehan DJ, Rinaldi MG. 2000. Comparison of high-performance liquid chromatographic and microbiological methods for determination of voriconazole levels in plasma. Antimicrob. Agents Chemother. 44:1209–1213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Steinmann J, Huelsewede J, Buer J, Rath PM. 2011. Comparison and evaluation of a novel bioassay and high-performance liquid chromatography for the clinical measurement of serum voriconazole concentrations. Mycoses 54:e421–e428 [DOI] [PubMed] [Google Scholar]
- 20. Cendejas-Bueno E, Rodriguez-Tudela JL, Cuenca-Estrella M, Gomez-Lopez A. 2013. Development and validation of a fast HPLC/photodiode array detection method for the measurement of voriconazole in human serum samples. A reference laboratory experience. Enferm. Infecc. Microbiol. Clin. 31:23–28 [DOI] [PubMed] [Google Scholar]
- 21. Cendejas-Bueno E, Forastiero A, Rodriguez-Tudela JL, Cuenca-Estrella M, Gomez-Lopez A. 2012. HPLC/UV or bioassay: two valid methods for posaconazole quantification in human serum samples. Clin. Microbiol. Infect. 18:1229–1235 [DOI] [PubMed] [Google Scholar]
- 22. EMEA 2010. Guideline on validation of bioanalytical method validation. European Medicines Agency, London, England [Google Scholar]
- 23. FDA 2001. Guidance for industry: bioanalytical method validation. FDA, Rockville, MD [Google Scholar]
- 24. Duvvuru S, Brummer E, Morelli R, Stevens DA. 1998. Isolation of a human serum protein that inhibits the growth of Cryptococcus neoformans. Mycopathologia 144:1–7 [DOI] [PubMed] [Google Scholar]
- 25. Zhou ZH, Zhang Y, Hu YF, Wahl LM, Cisar JO, Notkins AL. 2007. The broad antibacterial activity of the natural antibody repertoire is due to polyreactive antibodies. Cell Host Microbe 1:51–61 [DOI] [PMC free article] [PubMed] [Google Scholar]