Abstract
The plasma and intrapulmonary pharmacokinetics (PK) of intravenous (i.v.) GSK2251052, a novel boron-containing antimicrobial, were evaluated in healthy adult subjects. Thirty subjects underwent bronchoscopy and timed bronchoalveolar lavage (BAL) either following a single dose (cohort 1) or after 5 twice-daily doses (cohort 2) of 1,500 mg GSK2251052 i.v. Serial PK and safety assessments were obtained throughout the study. Bronchoscopy was performed on a single occasion in each subject at 2, 6, or 12 h after start of infusion. Noncompartmental analysis was performed to calculate PK parameters. Thirty subjects completed the study. The mean clearance (CL), volume of distribution at steady state (Vss), and half-life (t1/2) values were 22 liters/h, 231 liters, and 10.7 h, respectively. Approximately 30% of the dose was excreted unchanged in urine. The GSK2251052 concentrations in epithelial lining fluid (ELF) and alveolar macrophages (AM) were approximately 50% and 500 to 600%, respectively, compared to the concentration in plasma. the GSK2251052 exposures in ELF and AM were comparable following single- and repeat-dose administration. The most frequently reported drug-related adverse event (AE) was mild to moderate infusion site reactions (7 subjects) that occurred primarily in the repeat-dose cohort. No serious drug-related AEs or clinically significant trends in laboratory values, vital signs, or electrocardiograms were observed. GSK2251052 given as a 1,500-mg infusion was generally tolerated following single- or repeat-dose administration. GSK2251052 distributes into both the ELF and AM of healthy volunteers, which supports further study in patients with pneumonia.
INTRODUCTION
GSK2251052 is a novel boron-containing inhibitor of bacterial enzyme leucyl-tRNA synthetase (LeuRS) with in vitro activity against Gram-negative pathogens, including multidrug-resistant strains of Enterobacteriaceae, and was previously in development for the treatment of serious Gram-negative bacterial infections. LeuRS is an essential enzyme that catalyzes the coupling of the amino acid leucine onto its corresponding tRNALeu, which are used by the ribosome for protein synthesis. Inhibition of LeuRS prevents protein synthesis and stops the growth of the bacteria. Through the unique chemical-binding properties of the boron atom, GSK2251052 binds to the editing active site of bacterial LeuRS and forms a boron adduct with the 3′ terminus of tRNALeu that locks the tRNALeu to LeuRS in an unproductive state (1).
GSK2251052 has in vitro activity against common Gram-negative pathogens, such as Escherichia coli, Klebsiella pneumoniae, Pseudomonas aeruginosa, Acinetobacter baumannii, and Enterobacter species. GSK2251052 has a MIC90 value of 1 μg/ml against recent clinical isolates, including resistant strains of E. coli, Klebsiella spp., Enterobacter spp., Citrobacter spp., Proteus spp., Morganella morganii, and Serratia marcescens (2). The microbiological activity of GSK2251052 was not affected by clinically relevant resistance mechanisms, including efflux pumps (3), extended-spectrum β-lactamases (ESBLs), KPC, and AmpC (1, 2).
Following single and repeat intravenous (i.v.) dosing, GSK2251052 exhibited dose-proportional increases in area under the plasma concentration-time curve (AUC) and maximum plasma concentrations (Cmax). On average, the systemic clearance was approximately 18 liters/h, while the elimination half-life was approximately 11 h (4). Intravenous regimens of 750 mg or 1,500 mg are expected to achieve target plasma exposures associated with efficacy in animal models (5–7).
Data from preclinical lung infection models indicate that GSK2251052 achieves sufficient pulmonary concentrations to demonstrate efficacy against K. pneumoniae and P. aeruginosa, major pathogens implicated in hospital- and ventilator-associated pneumonia (6–8). The purpose of this study was to evaluate the plasma and intrapulmonary pharmacokinetics (PK) of GSK2251052 in healthy volunteers to assess its potential utility in patients with pneumonia.
(These data were presented in part at the 22nd Annual European Congress of Clinical Microbiology and Infectious Diseases Meeting, London, United Kingdom, 31 March to 3 April 2012.)
MATERIALS AND METHODS
Study design and subjects.
This was a prospective, open-label, nonrandomized, two-part study that evaluated the plasma and intrapulmonary concentrations of intravenous GSK2251052 administered after single and repeat doses in healthy adults. Key eligibility criteria included healthy male or female subjects aged 18 to 55 years, body mass index (BMI) of 18.5 to 30 kg/m2 inclusive, and no clinically significant abnormalities in vital signs or electrocardiographs (ECGs) or in chemistry, hematology, or liver function tests. Key exclusion criteria included women of child-bearing potential, pregnant or lactating females, presence of contraindications to bronchoalveolar lavage (BAL), including hypercapnia of >50 mm Hg, refractory hypoxemia, reactive airway disease or asthma, unstable angina or acute myocardial infarction in the previous 6 months, heart failure, severe hemostatic alterations, use of medications that could not be interrupted during the study, positive HIV or hepatitis B or C virus status, and recent alcohol or smoking history. Adverse events (AEs) were graded based on the FDA “Toxicity Grading Scale for Healthy Adult and Adolescent Volunteers Enrolled in Preventive Vaccine Clinical Trials” (9). All subjects gave written informed consent. The protocol was approved by the Western Institutional Review Board.
Fifteen subjects received a single 1,500-mg dose on day 1 and were assigned to 1 of 3 subgroups (5 per group) for bronchoscopy at either 2, 6, or 12 h after the start of infusion. In the repeat-dose cohort, another 15 subjects received 1,500 mg GSK2251052 every 12 h for 5 doses over 3 days and were similarly assigned to 1 of the 3 bronchoscopy subgroups (5 per group). GSK2251052 was administered as a 1-h intravenous infusion in all subjects. Each subject participated in the study for approximately 6 or 7 weeks, which included a 30-day screening period, a 1- or 3-day treatment period, and a 2-week follow-up. The study was conducted at Davita Clinical Research, Minneapolis, MN.
Bronchoscopy/bronchoalveolar lavage.
One standardized bronchoscopy with BAL was conducted by an experienced pulmonologist in each subject at one of three protocol-specified time points, 2, 6, or 12 h after start of infusion. Prior to bronchoscopy, subjects were premedicated with the following: nebulized 4% lidocaine with atropine, 2% topical lidocaine, 1% lidocaine (if needed), codeine, midazolam, and fentanyl. A fiber optic bronchoscope (Olympus P-20 and P-20D; Olympus America, Inc., Melville, NY, USA) was inserted into a subsegment of the right middle lobe. Four 50-ml aliquots of sterile 0.9% normal saline solution were instilled into the middle lobe of the right lung, and each specimen was immediately aspirated. The volume of the first aspirated sample (BAL1) was collected separately, measured, recorded, and placed on ice. The volumes of samples 2, 3, and 4 were combined (BAL2), measured, and recorded and immediately placed on ice. A minimum 4-ml aliquot was removed from both BAL1 and BAL2, refrigerated, and sent for cell count and differential. The remaining volumes of BAL1 and BAL2 were immediately centrifuged at 400 × g for 5 min, to separate alveolar macrophages (AM) (pellet) and epithelial lining fluid (ELF) (supernatant). A minimum 2-ml aliquot of supernatant was removed for determining urea concentrations in BAL fluid for both BAL1 and BAL2 and was refrigerated until shipped to MEDTOX Laboratories (St. Paul, MN) for urea analysis. The remaining BAL1 and BAL2 supernatant and associated cell pellets (4 samples total) were transferred into storage vials and frozen at −70°C until analyzed for GSK2251052 concentrations in alveolar macrophages (pellet) and epithelial lining fluid (supernatant). Plasma urea concentrations were also determined at the same time as bronchoscopy to provide a dilution ratio for the BAL samples.
Pharmacokinetic samples.
Blood samples, for plasma, were obtained for GSK2251052 concentration analysis at the following sampling times: predose (within 30 min of dosing), during the infusion at exactly 0.5 and 1 h (end of infusion), and 1.25, 1.5, 2, 4, 5, 6, 8, 10, 12, 24, 36, and 48 h after the start of the infusion on day 1 (single-dose cohort) or on day 3 after the 5th dose (repeat-dose cohort). Blood draws at 2, 6, and 12 h coincided with the BAL procedure or, if necessary, within 15 min of the BAL. Blood samples (approximately 2 ml) were collected into anticoagulant K2 EDTA tubes and immediately chilled on crushed ice. Plasma was separated by centrifugation at approximately 1,700 × g and 4°C for approximately 10 min and transferred to polypropylene specimen containers. Plasma samples were frozen and stored at approximately −80°C prior to analysis. Pooled urine was collected at the following times: 0 to 6 h, 6 to 12 h, 12 to 18 h, 18 to 24 h, 24 to 36 h, and 36 to 48 h after the start of the infusion. Urine samples were collected into amber polypropylene containers cooled with crushed ice or refrigeration. At the end of each collection period, following the recording of volume and/or weight, an aliquot (approximately 7.5 ml) of the urine sample was transferred to a plastic container and stored at −80°C prior to analysis.
Bioanalytical procedures for determination of plasma, urine, and pulmonary GSK2251052 concentrations.
Bioanalytical methods were developed, validated, and used for the determination of plasma, urine, and pulmonary GSK2251052 concentrations by CPR Pharma Services (Thebarton, Australia). The plasma concentrations of GSK2251052 were measured using a method based on protein precipitation followed by liquid chromatography-tandem mass spectrometry (LC-MS/MS) analysis. Briefly, a 100-μl aliquot of plasma was added to 300 μl of methanol containing trifluoroacetic acid (TFA; 0.1% vol/vol) and [2H213C]-GSK2251052 in polypropylene tubes and mixed. The samples were centrifuged for 5 min, and the supernatants then carefully transferred to clean glass tubes and dried under warm nitrogen. The samples were reconstituted in 200 μl of methanol-water (5:95, vol/vol) containing 0.1% trifluoroacetic acid before transfer to polypropylene (0.3-ml) vials or 96-well plates prior to analysis. Next, 20-μl sample aliquots were injected onto an LC-MS/MS system consisting of a Shimadzu high-performance liquid chromatography (HPLC) system with a Waters Xbridge column (3.5 μm) and an MDS Sciex 4000 API-4000 mass spectrometer (Applied Biosystems/MDS Sciex, Canada). Mobile phase A consisted of methanol-water (5:95, vol/vol) containing 0.1% trifluoroacetic acid, and mobile phase B consisted of methanol-water (95:5, vol/vol) containing 0.1% trifluoroacetic acid. The column was eluted at a flow rate of 0.3 ml/min with a gradient starting at 0% B and increasing linearly to 30% B from 0 to 3 min. MS/MS analyses were performed using a TurboIonSpray interface operated in the positive mode and a probe temperature of 400°C. The analyte and its internal standard were measured by multiple-reaction monitoring of the following [M+H]+ transitions: GSK2251052, m/z 238 → 202, and [2H213C]-GSK2251052, m/z 241 → 205. Data collection and integration were performed using Analyst software (version 1.5; Applied Biosystems/MDS Sciex, Canada). Quantification was based on analyte/internal standard peak area ratios and calculated using a weighted 1/x2 linear regression model. The operating range of the assay was 1 to 2,000 ng/ml.
The LC-MS/MS method used to determine the concentrations of GSK2251052 in urine was very similar to that described for plasma. The method used a dilution procedure where aliquots of urine (30 μl) were added to an internal standard solution of [2H213C]-GSK2251052 in 1,000 μl of methanol containing trifluoroacetic acid (0.1%, vol/vol). The samples were analyzed following the injection of a 2-μl aliquot onto the LC-MS/MS system described above for the plasma method. The operating range of the urine assay was 100 to 200,000 ng/ml. Plasma and urine samples were analyzed in a total of 7 and 5 analytical runs, respectively. The mean inter-run precision and accuracy values were within 3 and 4%, respectively, for plasma and 3% for urine.
The determination of GSK2251052 concentrations in human epithelial lining fluid utilized a dilution procedure followed by LC-MS/MS analysis and detection. Interference-free “blank” ELF was prepared from control human BAL fluid by centrifugation for 5 min at 300 × g (Eppendorf 5810). The supernatant, or ELF, was transferred to a polypropylene container and stored at −80°C until use. The residual pellet, containing alveolar macrophages, was resuspended in phosphate-buffered saline (PBS) and stored at −80°C until use. The analytical methodology used was similar to that previously described for urine and plasma. Aliquots of the ELF samples (50 μl) were added to an internal standard solution (50 μl) in water, and injections (up to 10 μl) were made onto the HPLC system. The column was eluted at a flow rate of 0.3 ml/min with a gradient starting at 0% B and increasing linearly to 25% B from 0 to 9 min. The operating range of the assay was 1 to 2,000 ng/ml. The ELF and AM samples were each analyzed in one analytical run.
The AM concentrations of GSK2251052 were measured by using a validated method based on protein precipitation followed by LC-MS/MS analysis. Interference-free blank alveolar macrophage fluid was prepared from control human BAL fluid as described above. The PBS-suspended AM sample (30 μl) was placed into a 1.5 ml-microcentrifuge tube, followed by 20 μl of methanol containing 0.1% TFA and the internal standard (50 μl in water). The sample was mixed to a vortex and centrifuged at 13,000 rpm for 10 min in an Eppendorf 5244 centrifuge, and 10 μl of the supernatant was injected onto the LC-MS/MS system. The HPLC methods were as described above for ELF analysis. The operating range of the assay was 1 to 2,000 ng/ml. The mean inter-run precision and accuracy were within 2 and 3% for ELF and 5 and 3% for AM.
Urea in serum and ELF.
The analysis of urea nitrogen in serum and BAL fluid was performed via spectrophotometric assay on a Beckman Coulter AU480 chemistry analyzer using a Beckman Coulter urea nitrogen reagent (kit number OSR6134) by MedTox Laboratories. The standard curve for serum was prepared as recommended by the manufacturer and ranged from 1.00 to 100 mg/dl. For BAL fluid samples, a modification in the manufacturer's procedure was made, and standard curves were prepared in normal saline over a range of urea nitrogen concentrations from 0.050 to 8.000 mg/dl. For both assays, the standard curves were linear and the relative accuracies ranged from 96.2 to 101.6% in serum and from 102 to 103.8% in BAL fluid. Calibrators were prepared in physiological saline solution, and quality control samples were prepared in human BAL fluid. The analyzer measured urea nitrogen based on the urea hydrolysis. Briefly, urea is hydrolyzed enzymatically by urease to yield ammonia and carbon dioxide. The ammonia and α-oxoglutarate are converted to glutamate in a reaction catalyzed by l-glutamate dehydrogenase (GLDH). Simultaneously, a molar equivalent of reduced NADH is oxidized. Two molecules of NADH are oxidized for each molecule of urea hydrolyzed. The rate of change in absorbance at 340 nm due to the disappearance of NADH is directly proportional to the urea nitrogen concentration in the sample. This assay measured urea nitrogen concentrations. The urea nitrogen measurements were converted into urea by multiplying the results by a factor of 2.14.
Calculation of recovered ELF volume and GSK2251052 concentrations in ELF and AM.
The calculation of ELF volume and GSK2251052 concentrations in ELF and AM was performed with BAL2 fluid (10, 11). The relative amount of ELF recovered within the BAL fluid was calculated according to the urea dilution method in reference 12. The concentration of GSK2251052 in ELF was estimated from the concentration of GSK2251052 in BAL aspirate supernatant and the ratio of the urea concentration in BAL aspirate supernatant to that in serum. Thus, the concentration of GSK2251052 in ELF was determined as follows: 052ELF = 052BAL × (UREAserum/UREABAL), where 052BAL is the concentration of GSK2251052 measured in the BAL aspirate supernatant, UREAserum is the concentration of urea in serum, and UREABAL is the concentration of urea in the BAL aspirate supernatant. The concentration of GSK2251052 in AM was determined as follows: 052AM = (052BAL-P)/(WBCBAL × VBAL-S × average cell volume × % cell count), where 052BAL-P is the amount of GSK2251052 in BAL fluid pellet in the 5 ml-cell suspension, VBAL-S is the volume of BAL fluid recovered from the BAL procedure, WBCBAL is the white blood cell concentration in BAL fluid prior to centrifugation (cells/mm3), and average cell volume is based on a mean value of 2.48 μl/106 cells (12). A differential cell count of the BAL fluid was performed to determine percent cell count (% cell count), including both macrophages and monocytes.
Pharmacokinetics and statistical analyses.
Plasma pharmacokinetic parameters for each subject were estimated by noncompartmental analysis using WinNonlin version 5.2 (Pharsight Corporation, Cary, NC). The maximal drug concentration in plasma (Cmax), time needed to reach the maximum concentration (Tmax), area under the concentration-time curve extrapolated to infinity [AUC0-∞], area under the concentration-time curve over the dosing interval [AUC0–12], elimination rate constant (λz), elimination half-life (t1/2), systemic clearance (CL), renal CL (CLR), urinary recovery of GSK2251052 (Ae), percentage of GSK2251052 excreted in urine (fe), and volume of distribution at steady-state (Vss) were determined. GSK2251052 pharmacokinetic parameters were summarized as geometric mean values with between-subject variability estimates (percent coefficient of variation [%CV]), except for Tmax, which was summarized as the median and range.
The Cmax and AUC0–12 values for ELF and AM were determined from the composite profile using the mean values at 2, 6, and 12 h postdose on days 1 and 3. AM/plasma and ELF/plasma ratios were determined based on the AUC0–12 and Cmax values and at each sampling time by dividing the concentrations of GSK2251052 in the AM or ELF by those in the plasma, respectively, at each sampling time. GSK2251502 is only approximately 10% protein bound (unpublished data); therefore, total plasma concentrations were used in all calculations.
RESULTS
Subjects.
Thirty subjects (15 in the single-dose cohort and another 15 in the repeat-dose cohort) enrolled in and completed the study. The mean ages (±standard deviation [±SD]) of the subjects were 30.8 ± 9.3 and 34.6 ± 10.9 years and the mean BMIs (±SD) were 26.6 ± 2.2 and 24.2 ± 2.9 kg/m2 in cohort 1 (single dose) and cohort 2 (repeat dose), respectively. The majority of subjects were male (n = 28, 93%) and of Caucasian heritage (n = 25, 83%).
GSK2251052 was generally tolerated, with no serious adverse events (AEs) or withdrawals. In the single-dose cohort, diarrhea, infusion site pain, and hypotension were reported by 1 subject each. All events were considered mild in severity, except for hypotension occurring after the BAL procedure, which was moderate and not considered study drug related. In the repeat-dose cohort, 10 subjects reported AEs, of which 5 subjects had multiple AEs. All AEs were reported to be mild and consisted of the following: infusion site reactions, influenza-like illness, chest pain, dizziness, headache, hiccups, oropharyngeal pain, rhinitis, contact dermatitis, and orthostatic hypotension. The most-common drug-related AE was infusion site reactions in 6 subjects, followed by chest pain, dizziness, and orthostatic hypotension in 2 subjects or fewer. The reason for one subject's chest pain appeared to be musculoskeletal pain; however, given no previous reports of chest pain with GSK2251052, the investigator could not rule out a relation to study drug. Infusion site reactions occurred in 7/30 subjects (23%) (1 in the single-dose cohort and 6 in the repeat-dose cohort) and consisted of phlebitis, irritation, pain, or infiltration of grade 1 or 2 in severity. All subjects completed treatment, and no subject prematurely withdrew from the investigational product or study. Subjects reported pain, burning, tingling, and warmth within 5 to 15 min from the start of infusion, usually with the second or third dose, and symptoms were progressive in nature. The symptoms resolved soon after dose completion without intervention in the majority of subjects. In two subjects, peripheral vein infiltration occurred in the forearm, requiring the placement of an alternate line. Additionally, one subject experienced grade 1 erythema in a 4- to 8-cm area surrounding the vein where there appeared to be infiltration with possible extravasation of drug substance. Another subject developed red streaking along the vein of grade 1 severity. No other subjects in the study reported injection site reactions.
Plasma pharmacokinetics.
Following single-dose administration of 1,500 mg GSK2251052 via intravenous infusion over 1 h, GSK2251052 was eliminated slowly, with a median t1/2 value of 10.7 h (Table 1). On average, CL was low, 23.1 liters/h on day 1 and 20.6 liters/h on day 3. The average CLR was 6.62 liters/h, and the fe of GSK2251052 after 48 h was approximately 27.8% of the total dose administered. The mean Vss (231 liters) exceeded the total body water. Following single-dose administration, the geometric mean (%CV) GSK2251052 AUC0-∞ and Cmax were 65.0 μg · h/ml (13.6%) and 21.6 μg/ml (20.4%), respectively (Table 1). Following repeat dose administration, AUC0-12 was 72.9 μg · h/ml (15.4%) and Cmax was 24.0 μg/ml (15.4%) (Table 1).
Table 1.
Plasma pharmacokinetic parameters of GSK2251052a
| Treatment cohort (n = 15), time of sampling | AUCb (μg · h/ml) | Cmax (μg/ml) | CL (liter/h) | Vss (liter) | t1/2c (h) | Tmaxc (h) | CLR (liter/h) | fe (%) |
|---|---|---|---|---|---|---|---|---|
| Single dose, day 1 | 65.0 (13.6) | 21.6 (20.4) | 23.1 (13.6) | 231 (20.7) | 10.7 (8.62–12.3) | 1.00 (0.50–1.05) | 6.62 (16.0) | 26.8 (11.5) |
| Repeat dose, day 3 | 72.9 (12.5) | 24.0 (15.4) | 20.6 (12.5) | 1.00 (0.53–1.00) |
Data are presented as geometric mean (%CV) unless otherwise indicated.
The AUC0–∞ value was determined for the single-dose cohort, and the AUC0–12 value for the repeat-dose cohort.
Data are presented as median (range).
ELF and AM.
The numbers (mean ± SD) of cells recovered in pooled BAL fluid (BAL2) from the single-dose cohort and repeat-dose cohort were (163 ± 66.1) × 106 and (182 ± 208) × 106 cells/liter, respectively. The mean (± SD) percentages of cells that were classified as monocytes and macrophages were 90.1% ± 11.1% and 88.4% ± 9.87% for the single- and repeat-dose cohort, respectively.
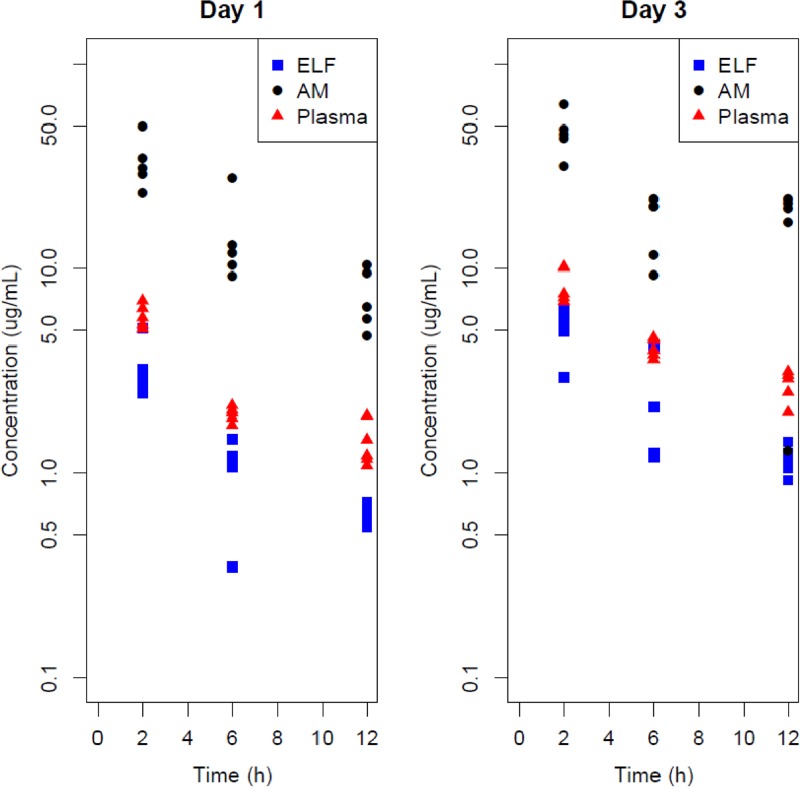
The concentrations of GSK2251052 in AM, ELF, and plasma and the AM/plasma and ELF/plasma ratios are displayed in Table 2 (composite PK parameters) and Table 3 (point-to-point comparison) and graphically in Figure 1. Following a single dose, the GSK2251052 concentrations in ELF were 53.8% based on composite AUC and, on average (%CV), 56.5% (32.7%), 54.7% (41.0%), and 47.4% (14.9%) at the 2-, 6-, and 12-h BAL sampling points, respectively, relative to the concentrations in plasma. The ELF concentrations of GSK2251052 after multiple twice-daily doses were 53.4% based on composite AUC and, on average (%CV), 60.2% (24.6%), 53.4% (57.3%), and 43.6% (16.8%) at 2, 6, and 12 h, respectively, compared to the concentrations in plasma.
Table 2.
Area under the concentration-time curve and maximum concentration for GSK2251052 in plasma, alveolar macrophages, and epithelial lining fluid
| Sample | Day | No. of subjects in subgroup | AUC0–12 (μg · h/ml)a |
Cmax (μg/ml) |
||
|---|---|---|---|---|---|---|
| Composite | Plasma ratio | Composite | Plasma ratio | |||
| Plasma | 1 | 5 | 30.0 | NAb | 5.89 | NA |
| 3 | 5 | 55.1 | 8.39 | |||
| AM | 1 | 5 | 186 | 6.21 | 33.3 | 5.65 |
| 3 | 5 | 275 | 4.98 | 45.9 | 5.47 | |
| ELF | 1 | 5 | 16.1 | 0.538 | 3.30 | 0.560 |
| 3 | 5 | 29.4 | 0.534 | 5.01 | 0.597 | |
AUC0–12 is based on concentrations at 2-, 6-, and 12-h sampling times.
NA, not applicable.
Table 3.
Concentrations of GSK2251052 in alveolar macrophages and epithelial lining fluid and corresponding plasma ratios for each study day and sampling time
| Treatment cohort (n =15), time of sampling | BAL sampling time (h)a | No. of subjects in subgroup | Concn in AM (μg/ml) | AM/plasma ratio | Concn in ELF (μg/ml) | ELF/plasma ratio |
|---|---|---|---|---|---|---|
| Single dose, day 1 | 2 | 5 | 33.3 (29.8)b | 5.73 (29.5) | 3.30 (31.5) | 0.565 (32.7) |
| 6 | 5 | 147 (5.14) | 7.26 (44.6) | 1.05 (40.2) | 0.547 (41.0) | |
| 12 | 5 | 7.340 (33.9) | 5.44 (30.5) | 0.635 (12.9) | 0.474 (14.9) | |
| Repeat dose, day 3 | 2 | 5 | 45.9 (24.6) | 5.49 (16.7) | 5.01 (25.1) | 0.602 (24.6) |
| 6 | 5 | 16.5 (34.5) | 4.05 (35.3) | 2.17 (55.8) | 0.534 (57.3) | |
| 12 | 5 | 16.0 (52.8) | 5.66 (53.4) | 1.16 (16.5) | 0.436 (16.8) |
In each treatment cohort, subjects were divided into 3 subgroups based on time of bronchoscopy (2, 6, or 12 h postdose).
Concentration and ratio data are presented as mean (%CV).
Fig 1.
GSK2251052 concentrations in plasma, epithelial lining fluid (ELF), and alveolar macrophages (AM) on day 1 and day 3.
In contrast, the GSK2251052 concentrations in AM relative to those in plasma were 621% based on composite AUC and, on average (%CV), 573% (29.5%), 726% (44.6%), and 544% (30.5%) at the 2-, 6-, and 12-h BAL sampling points, respectively, relative to the concentrations in plasma following a single dose and 498% based on composite AUC and, on average (%CV), 549% (16.7%), 405% (35.3%), and 566% (53.4%), at the 2-, 6-, and 12-h BAL sampling points, respectively, relative to the concentrations in plasma following multiple doses.
DISCUSSION
This is the first study to document the distribution of GSK2251052 in AM and ELF over the dosing interval in healthy volunteers. All subjects completed the study, and all had acceptable cell recovery from pulmonary fluids for determination of drug concentrations. The plasma pharmacokinetic data from this study were consistent with those observed in the first-in-human (FIH) study, further supporting these study findings (4).
It is widely believed that antibiotic concentrations in the pulmonary compartment correlate with efficacy for the treatment of pneumonia. Agents that distribute adequately into ELF are expected to be active against extracellular pathogens, while those that concentrate in AM are anticipated to be effective against intracellular pathogens. On average, the GSK2251052 concentrations in ELF were approximately half of those observed in plasma, while the AM concentrations were approximately 5-fold greater than those in plasma. There was no apparent delay in reaching the ELF and AM sites, indicating rapid pulmonary penetration after the first dose of GSK2251052. This is especially important in critically ill patients, in whom it is vital to rapidly achieve sufficient antibiotic concentrations at the site of infection. As expected based on the elimination t1/2 of GSK2251052, there was some accumulation observed in plasma, ELF, and AM with steady-state dosing. After both single- and repeat-dose administration, the drug concentrations declined in parallel in the ELF, AM, and plasma sites, as the ELF/plasma and AM/plasma ratios were maintained over the sampling interval.
The degree of ELF penetration by GSK2251052 compared to the concentration in plasma observed in humans (approximately 50%) is slightly higher than that observed in mice (approximately 34%) (6). Unbound drug concentrations were used in mice for the determination of ELF/fAUC (AUC for the free, unbound fraction), while total drug concentrations were used in the present study for ELF/AUC determination. In the neutropenic murine pneumonia infection model, the PK/PD (pharmacodynamic) index most associated with efficacy was the AUC/MIC ratio, with free-drug ratios of 7 to 10 in ELF and 10 to 30 in plasma demonstrating efficacy (6). Based on the composite AUC for ELF observed in this study (AUC0–12 of 29.4 μg · h/ml), achieving these target values (with BID dosing) should be possible for pathogens with MICs up to and including 4 μg/ml. GSK2251052 has a MIC90 of 1 μg/ml against recent clinical isolates, including resistant strains of Escherichia coli, Klebsiella pneumoniae, Enterobacter spp., Citrobacter spp., Proteus spp., Morganella morganii, and Serratia marcescens (2). A population pharmacokinetic plasma/lung model has been developed and was planned to be used to conduct Monte Carlo simulations to help select the dose regimen(s) that would be considered adequate to treat patients with pneumonia (13).
The overall safety profile of GSK2251052 administered i.v. in this study was similar to that seen in prior i.v. studies. Also as seen previously, mild decreases in reticulocytes, red cell counts, and hemoglobin were observed following multiple dosing; however, these changes were reversible upon drug discontinuation. The clinical relevance of these findings is unknown. Contrary to the FIH study, dosing in 7/15 repeat-dose subjects (and 1 single-dose subject) was associated with mild infusion site reactions during the infusion. Most subjects completed dosing in the original infusion site despite the reaction, while a few subjects resumed therapy after a new line was placed in the alternate arm. No subject withdrew from the study due to this adverse event. Since the dose and duration of infusion in the present study were not changed from the FIH study, these reactions are thought to be related to the higher final drug concentration of 15 mg/ml in 100 ml normal saline employed in this study. In future studies, GSK2251052 will be diluted in a higher infusion volume to mitigate the risk of infusion-related reactions.
In the present study, GSK2251052 penetration was approximately 50% in ELF and 500 to 600% in AM relative to the concentration in plasma in healthy volunteers. GSK2251052 was generally tolerated following a 1,500-mg infusion, except for injection site reactions reported in the repeat-dose cohort that appeared to be concentration dependent. Recently, the development of GSK2251052 was discontinued by GlaxoSmithKline due to the identification of microbiological findings of resistance in a small number of patients in a phase 2b trial for the treatment of complicated urinary tract infections (cUTI). Although therapeutic response (resolution of clinical signs and symptoms and clearance of baseline pathogen from the urine) was observed in some patients, a subpopulation of patients had rapid emergence of GSK2251052 resistance in bacterial isolates during therapy, prompting the discontinuation of the cUTI trial and subsequent termination of a concomitant complicated intra-abdominal infection phase 2 trial. The details of this finding will be the subject of a future manuscript.
Given the favorable pharmacodynamics of this boron-containing class of antimicrobials, the potential to successfully address the unmet need for treatment of multidrug-resistant Gram-negative infections worldwide remains hopeful (14).
Footnotes
Published ahead of print 6 May 2013
REFERENCES
- 1. Hernandez V, Akama T, Alley M, Baker S, Mao W, Rock F, Zhang YK, Zhang Y, Zhou Y, Crepin T, Cusack S, Palencia A, Nieman J, Anugula M, Baek M, Diaper C, Ha C, Kermane M, Lu X, Mohammad R, Savariraj K, Sharma R, Singh R, Subedi R, Plattner J. 2010. Discovery and mechanism of action of AN3365: a novel boron-containing antibacterial agent in clinical development for Gram-negative infections, abstr F1-1637. Abstr. 51st Intersci. Conf. Antimicrob. Agents Chemother American Society for Microbiology, Washington, DC [Google Scholar]
- 2. Mendes RE, Alley MRK, Sader HS, Biedenbach DJ, Jones RN. 2013. Potency and spectrum of activity of AN3365, a novel boron-containing protein synthesis inhibitor, tested against clinical isolates of Enterobacteriaceae and nonfermentative Gram-negative bacilli. Antimicrob. Agents Chemother. 57:2849–2857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mendez RE, Biedenbach DJ, Alley MRK, Sader HS, Jones RN. 2011. Potency and spectrum of activity of AN3365, a novel boron-containing protein synthesis inhibitor, tested against Enterobacteriaceae, abstr F1-1638. Abstr. 51st Intersci. Conf. Antimicrob. Agents Chemother American Society for Microbiology, Washington, DC: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zane LT, Shakib S, Milne R, Liu L, Baker S, Heerinckx FA. 2011. Safety, tolerability, and pharmacokinetics of a novel Gram-negative antimicrobial, GSK2251052, in healthy subjects. Clin. Microbiol. Infect. 17(Suppl s4):s434 [Google Scholar]
- 5. Bulik CC, Okusanya OO, Bhavnani SM, Lepak A, Forrest A, Ambrose PG, Hoover JL, Andes DR. 2012. Evaluation of the pharmacokinetics-pharmacodynamics of GSK2251052 against gram-negative bacilli in a murine-thigh infection model, abstr A-1270. Proc. 52nd Intersci. Conf. Antimicrob. Agents Chemother American Society for Microbiology, Washington, DC [Google Scholar]
- 6. Andes DR, Okusanya OO, Bulik CC, Lepak A, Bhavnani SM, Forrest A, Hoover JL, Ambrose PG. 2012. Evaluation of the pharmacokinetics-pharmacodynamics of GSK2251052 against gram-negative bacilli using data from a neutropenic murine-pneumonia infection model, abstr A-1271. Abstr. 52nd Intersci. Conf. Antimicrob. Agents Chemother American Society for Microbiology, Washington, DC [Google Scholar]
- 7. Hoover J, Mininger C, Rittenhouse S. 2012. GSK2251052, a novel LeuRS inhibitor, is effective against multi-drug resistant Pseudomonas aeruginosa in a mouse pneumonia model, abstr B-1308. Proc. 52nd Intersci. Conf. Antimicrob. Agents Chemother American Society for Microbiology, Washington, DC [Google Scholar]
- 8. Craven DE. 2000. Epidemiology of ventilator-associated pneumonia. Chest 117(4 suppl 2):186S–187S [DOI] [PubMed] [Google Scholar]
- 9. U.S. Department of Health and Human Services 2007. Guidance for industry: toxicity grading scale for healthy adult and adolescent volunteers enrolled in preventive vaccine clinical trials. U.S. FDA, Rockville, MD: http://www.fda.gov/downloads/BiologicsBloodVaccines/GuidanceComplianceRegulatoryInformation/Guidances/Vaccines/ucm091977.pdf [DOI] [PubMed] [Google Scholar]
- 10. Rodvold KA, Danziger LH, Gotfried MH. 2003. Steady-state plasma and bronchopulmonary concentrations of intravenous levofloxacin and azithromycin in healthy adults. Antimicrob. Agents Chemother. 47:2450–2457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Nix DE. 1998. Intrapulmonary concentrations of antimicrobial agents. Infect. Dis. Clin. North Am. 12:631–645 [DOI] [PubMed] [Google Scholar]
- 12. Rennard SI, Basset G, Lecossier D, O'Donnell KM, Pinkston P, Martin PG, Crystal RG. 1986. Estimation of volume of epithelial lining fluid recovered by lavage using urea as marker of dilution. J. Appl. Physiol. 60:532–538 [DOI] [PubMed] [Google Scholar]
- 13. Tenero D, Patel P, Dumont E, Tomayko J. 2012. Development of a plasma/lung population pharmacokinetic (PPK) model for GSK2251052. Clin. Pharmacol. Drug Dev. 1:216. 10.1177/2160763X12454673 [DOI] [Google Scholar]
- 14. Spellberg B, Guidos R, Gilbert D, Bradley J, Boucher HW, Scheld WM, Bartlett JG, Edwards J, Jr, Infectious Diseases Society of America 2008. The epidemic of antibiotic-resistant infections: a call to action for the medical community from the Infectious Diseases Society of America. Clin. Infect. Dis. 46:155–164 [DOI] [PubMed] [Google Scholar]