Abstract
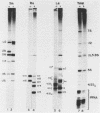
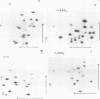
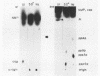
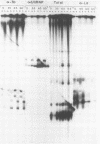
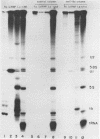
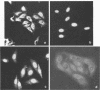
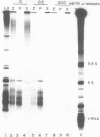
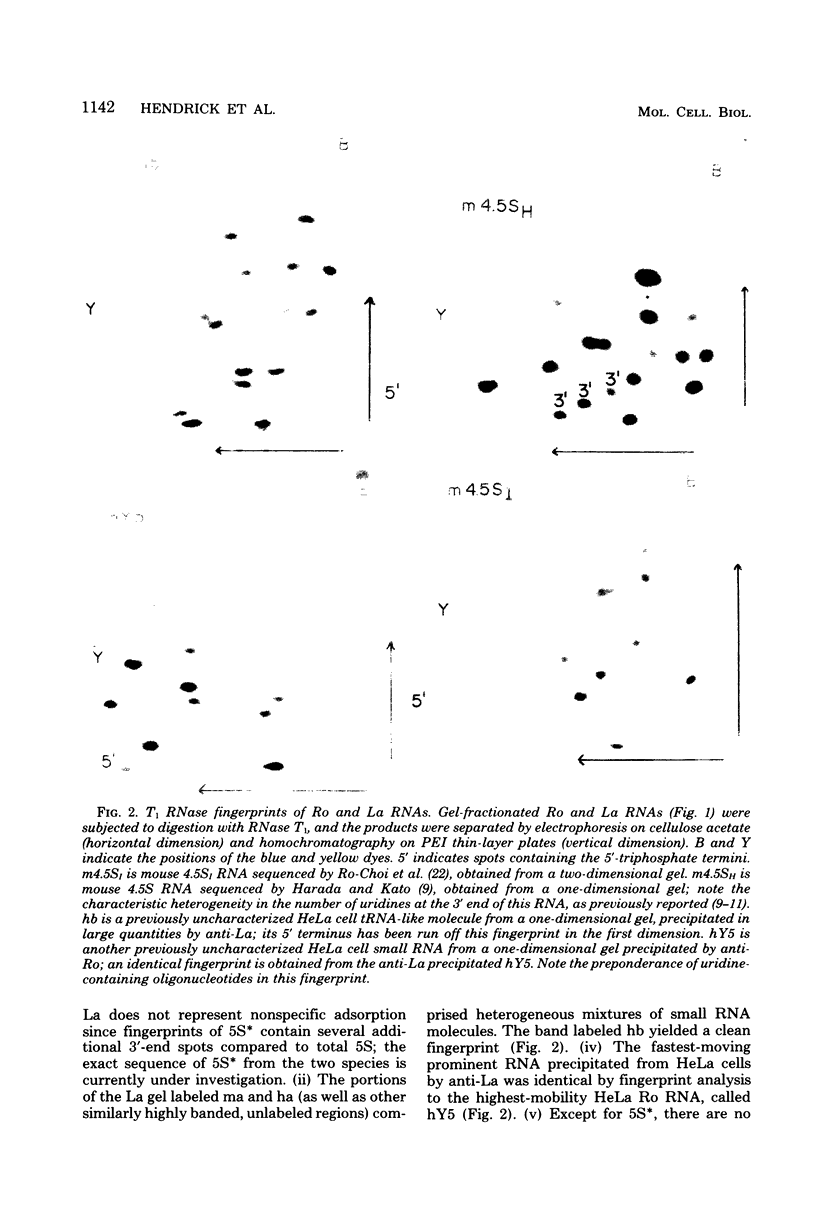
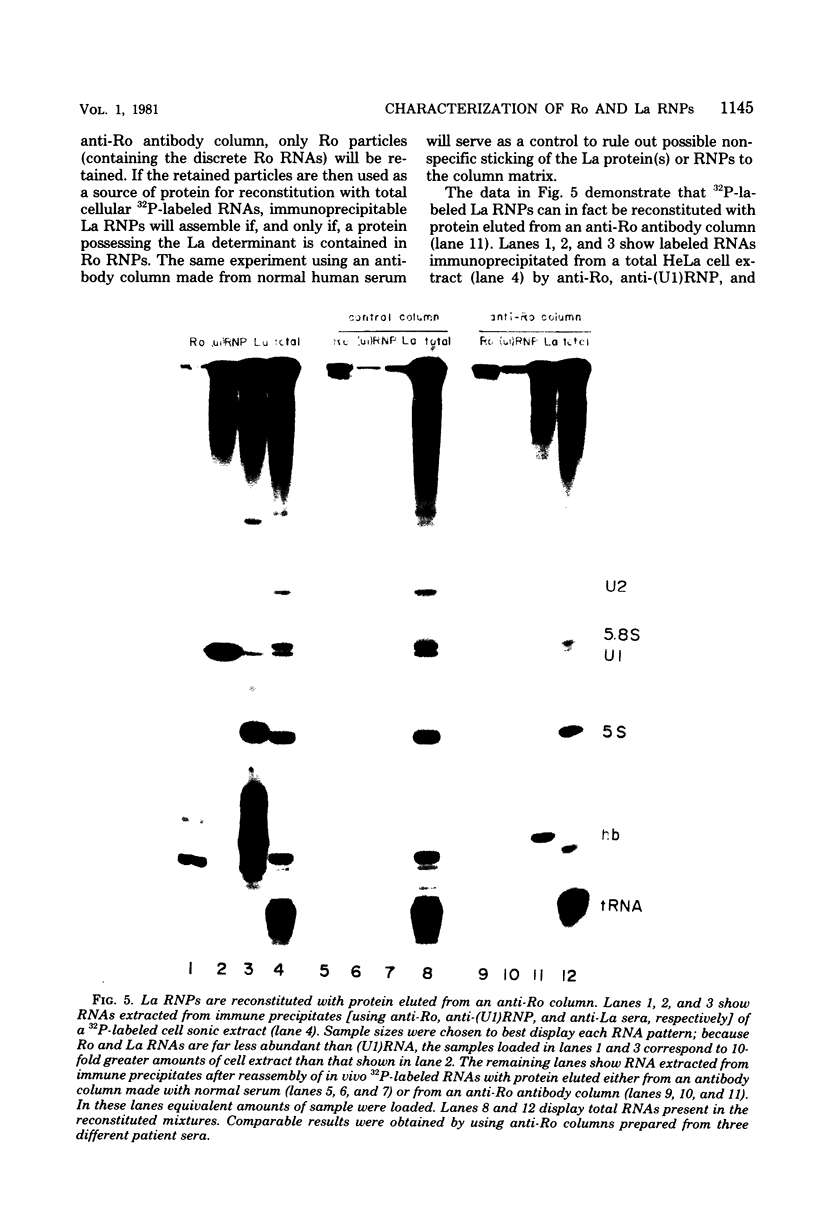
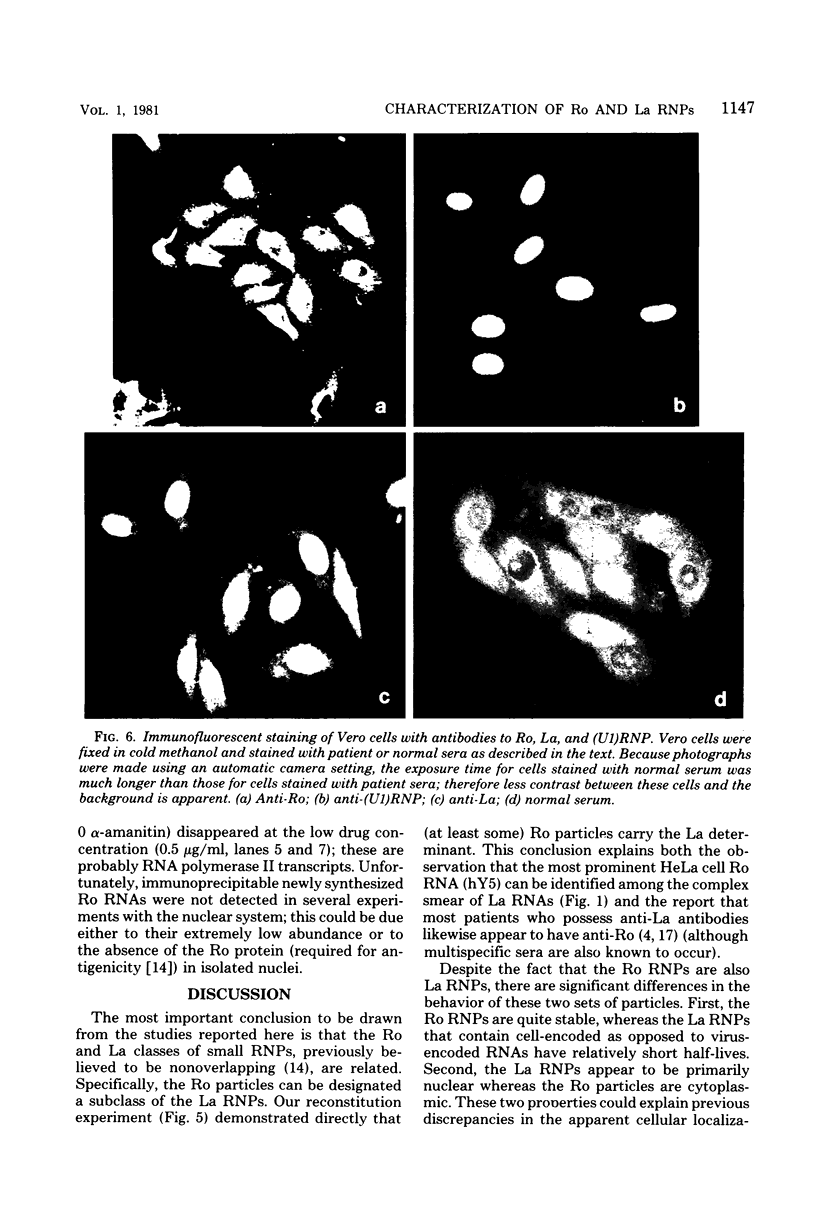
Small ribonucleic acid (RNA)-protein complexes precipitated by anti-Ro and anti-La antibodies from lupus patients have been examined with emphasis on their RNA components. In both ribonucleoprotein (RNP) classes, the numbers of different RNA molecules and their sequences vary between mouse and human cells. The complex mixtures of La RNAs include two previously sequenced 4.5S RNAs from mouse cells and 5S ribosomal RNA-like molecules from both mouse and human cells. All Ro and La RNAs possess 5-triphosphates. Some La RNAs have internal modifications typical of transfer RNAs. The Ro RNPs are quite stable and are localized by immunofluorescence in the cell cytoplasm, whereas the majority of the La RNPs turn over rapidly and reside in the nucleus. Despite these differences, reconstitution experiments show that the Ro particles carry the La as well as the Ro determinant. Studies using a nuclear transcription system demonstrate that most of the La RNAs are synthesized by RNA polymerase III. The possibility that the La protein(s) functions in the transcription or maturation of all RNA polymerase III transcripts is discussed.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akizuki M., Powers R., Holman H. R. A soluble acidic protein of the cell nucleus which reacts with serum from patients with systemic lupus erythermatosus and Sjögren's syndrome. J Clin Invest. 1977 Feb;59(2):264–272. doi: 10.1172/JCI108637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alspaugh M. A., Talal N., Tan E. M. Differentiation and characterization of autoantibodies and their antigens in Sjögren's syndrome. Arthritis Rheum. 1976 Mar-Apr;19(2):216–222. doi: 10.1002/art.1780190214. [DOI] [PubMed] [Google Scholar]
- Alspaugh M. A., Tan E. M. Antibodies to cellular antigens in Sjögren's syndrome. J Clin Invest. 1975 May;55(5):1067–1073. doi: 10.1172/JCI108007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alspaugh M., Maddison P. Resolution of the identity of certain antigen-antibody systems in systemic lupus erythematosus and Sjögren's syndrome: an interlaboratory collaboration. Arthritis Rheum. 1979 Jul;22(7):796–798. doi: 10.1002/art.1780220719. [DOI] [PubMed] [Google Scholar]
- Cashel M., Lazzarini R. A., Kalbacher B. An improved method for thin-layer chromatography of nucleotide mixtures containing 32P-labelled orthophosphate. J Chromatogr. 1969 Mar 11;40(1):103–109. doi: 10.1016/s0021-9673(01)96624-5. [DOI] [PubMed] [Google Scholar]
- Clark G., Reichlin M., Tomasi T. B., Jr Characterization of a soluble cytoplasmic antigen reactive with sera from patients with systemic lupus erythmatosus. J Immunol. 1969 Jan;102(1):117–122. [PubMed] [Google Scholar]
- Harada F., Kato N., Hoshino H. Series of 4.5S RNAs associated with poly(A)-containing RNAs of rodent cells. Nucleic Acids Res. 1979 Oct 25;7(4):909–917. doi: 10.1093/nar/7.4.909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harada F., Kato N. Nucleotide sequences of 4.5S RNAs associated with poly(A)-containing RNAs of mouse and hamster cells. Nucleic Acids Res. 1980 Mar 25;8(6):1273–1285. doi: 10.1093/nar/8.6.1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jelinek W., Leinwand L. Low molecular weight RNAs hydrogen-bonded to nuclear and cytoplasmic poly(A)-terminated RNA from cultured Chinese hamster ovary cells. Cell. 1978 Sep;15(1):205–214. doi: 10.1016/0092-8674(78)90095-8. [DOI] [PubMed] [Google Scholar]
- Lerner E. A., Lerner M. R., Janeway C. A., Jr, Steitz J. A. Monoclonal antibodies to nucleic acid-containing cellular constituents: probes for molecular biology and autoimmune disease. Proc Natl Acad Sci U S A. 1981 May;78(5):2737–2741. doi: 10.1073/pnas.78.5.2737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerner M. R., Andrews N. C., Miller G., Steitz J. A. Two small RNAs encoded by Epstein-Barr virus and complexed with protein are precipitated by antibodies from patients with systemic lupus erythematosus. Proc Natl Acad Sci U S A. 1981 Feb;78(2):805–809. doi: 10.1073/pnas.78.2.805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerner M. R., Boyle J. A., Hardin J. A., Steitz J. A. Two novel classes of small ribonucleoproteins detected by antibodies associated with lupus erythematosus. Science. 1981 Jan 23;211(4480):400–402. doi: 10.1126/science.6164096. [DOI] [PubMed] [Google Scholar]
- Lerner M. R., Boyle J. A., Mount S. M., Wolin S. L., Steitz J. A. Are snRNPs involved in splicing? Nature. 1980 Jan 10;283(5743):220–224. doi: 10.1038/283220a0. [DOI] [PubMed] [Google Scholar]
- Lerner M. R., Steitz J. A. Antibodies to small nuclear RNAs complexed with proteins are produced by patients with systemic lupus erythematosus. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5495–5499. doi: 10.1073/pnas.76.11.5495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattioli M., Reichlin M. Heterogeneity of RNA protein antigens reactive with sera of patients with systemic lupus erythematosus. Description of a cytoplasmic nonribosomal antigen. Arthritis Rheum. 1974 Jul-Aug;17(4):421–429. doi: 10.1002/art.1780170413. [DOI] [PubMed] [Google Scholar]
- Nishimura S. Minor components in transfer RNA: their characterization, location, and function. Prog Nucleic Acid Res Mol Biol. 1972;12:49–85. [PubMed] [Google Scholar]
- Osborn M., Weber K. The display of microtubules in transformed cells. Cell. 1977 Nov;12(3):561–571. doi: 10.1016/0092-8674(77)90257-4. [DOI] [PubMed] [Google Scholar]
- Provost T. T. Subsets in systemic lupus erythematosus. J Invest Dermatol. 1979 Mar;72(3):110–113. doi: 10.1111/1523-1747.ep12530348. [DOI] [PubMed] [Google Scholar]
- Ro-Choi T. S., Redy R., Henning D., Takano T., Taylor C. W., Busch H. Nucleotide sequence of 4.5 S ribonucleic acid of Novikoff hepatoma cell nuclei. J Biol Chem. 1972 May 25;247(10):3205–3222. [PubMed] [Google Scholar]
- Rosa M. D., Gottlieb E., Lerner M. R., Steitz J. A. Striking similarities are exhibited by two small Epstein-Barr virus-encoded ribonucleic acids and the adenovirus-associated ribonucleic acids VAI and VAII. Mol Cell Biol. 1981 Sep;1(9):785–796. doi: 10.1128/mcb.1.9.785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross A., Brimacombe R. Experimental determination of interacting sequences in ribosomal RNA. Nature. 1979 Sep 27;281(5729):271–276. doi: 10.1038/281271a0. [DOI] [PubMed] [Google Scholar]
- Weinmann R., Brendler T. G., Raskas H. J., Roeder R. G. Low molecular weight viral RNAs transcribed by RNA polymerase III during adenovirus 2 infection. Cell. 1976 Apr;7(4):557–566. doi: 10.1016/0092-8674(76)90206-3. [DOI] [PubMed] [Google Scholar]
- Wise J. A., Weiner A. M. The small nuclear RNAs of the cellular slime mold Dictyostelium discoideum. Isolation and characterization. J Biol Chem. 1981 Jan 25;256(2):956–963. [PubMed] [Google Scholar]
- Yang V. W., Binger M. H., Flint S. J. Transcription of adenoviral genetic information in isolated nuclei. Characterization of viral RNA sequences synthesized in vitro. J Biol Chem. 1980 Mar 10;255(5):2097–2108. [PubMed] [Google Scholar]