Abstract
INTRODUCTION:
Individuals with obesity and type 2 diabetes (T2D) are typically insulin resistant, exhibiting impaired skeletal muscle glucose uptake. Animal and cell culture experiments have shown that site-specific phosphorylation of the Rab-GTPase-activating proteins AS160 and TBC1D1 is critical for GLUT4 translocation facilitating glucose uptake, but their regulation in human skeletal muscle is not well understood.
METHODS:
Here, lean, obese and T2D subjects underwent a euglycemic-hyperinsulinemic clamp, and vastus lateralis muscle biopsies were obtained before, and at 30 and 180 min post insulin infusion.
RESULTS:
Obese and T2D subjects had higher body mass indexes and fasting insulin concentrations, and T2D subjects showed insulin resistance. Consistent with the clamp findings, T2D subjects had impaired insulin-stimulated phosphorylation of AS160 Thr642, a site previously shown to be important in glucose uptake in rodents. Interestingly, insulin-stimulated phosphorylation of TBC1D1 Thr590, a site shown to be regulated by insulin in rodents, was only increased in T2D subjects, although the functional significance of this difference is unknown.
CONCLUSION:
These data show that insulin differentially regulates AS160 and TBC1D1 phosphorylation in human skeletal muscle. Impaired insulin-stimulated glucose uptake in T2D subjects is accompanied by dysregulation of AS160 and TBC1D1 phosphorylation in skeletal muscle, suggesting that these proteins may regulate glucose uptake in humans.
Keywords: obesity, insulin, diabetes, TBC1D1, AS160
Introduction
In individuals with obesity and type 2 diabetes (T2D), insulin-stimulated glucose uptake into skeletal muscle is decreased, resulting in impaired glucose metabolism. Glucose uptake in muscle is regulated by GLUT4 translocation to the plasma membrane, and both the translocation process and insulin signal transduction, leading to GLUT4 translocation, are defective in patients with obesity and T2D.1 It is now well-established that insulin binding to its receptor, insulin receptor substrate proteins and phospoinositide 3 kinase proteins leads to phosphorylation and activation of Akt, with the Akt2-isoform being predominantly involved in glucose transport.1 In rodent skeletal muscle, Akt phosphorylates Akt substrate of 160 kDa (AS160/TBC1D4)2 and TBC1D1 (tre-2/USP6, BUB2, cdc16 domain family member 1).3 AS160 and TBC1D1 are highly homologous Rab-GTPase-activating proteins that promote hydrolysis of guanosine-5'-triphosphate to guanosine diphosphate on GLUT4-containing vesicles. In the guanosine diphosphate-bound form, GLUT4-containing vesicles are inhibited from translocating to the cell surface. Site-specific AS160 and/or TBC1D1 phosphorylation by Akt or other kinases is postulated to modulate Rab-GTPase-activating protein activity, releasing GLUT4 vesicles, and regulation of these sites is critical for controlling glucose uptake.4 In rodent skeletal muscle, insulin phosphorylates AS160 on multiple phospho-Akt-substrate (PAS) motifs,2, 3, 5 and mutation of four regulatory AS160 PAS-sites impairs insulin-stimulated skeletal muscle glucose uptake.5 In human skeletal muscle, T2D decreases AS160 PAS phosphorylation,6 but it increases with weight loss7 and exercise induces site-specific AS160 phosphorylation.8 A critical Akt consensus site on AS160 is Thr642, a 14-3-3 protein-binding site that regulates GLUT4 trafficking and insulin sensitivity in rodent skeletal muscle.9 The 5′ AMP-activated protein kinase consensus site AS160 Ser711 is highly regulated by insulin and by 5′ AMP-activated protein kinase. However, the physiologic relevance of insulin-induced AS160 Ser711 phosphorylation and whether insulin resistant subjects have alterations in AS160 Ser711 phosphorylation remains unknown.10 Here, we evaluated whether obese and T2D subjects exhibit impaired insulin-stimulated phosphorylation at these two sites in skeletal muscle.
TBC1D1 was first identified in adipocytes in culture,11 but is highly expressed in skeletal muscle with minimal expression in adipose tissue.3 Insulin increases TBC1D1 PAS phosphorylation in skeletal muscle,3, 12, 13 but, unlike AS160, TBC1D1 can regulate insulin-stimulated glucose transport through a PAS-independent mechanism. Exercise increases human TBC1D1 phosphorylation,14 and mutation of TBC1D1 on R125W, which regulates rodent skeletal muscle glucose transport, has been linked to female obesity.15 The Akt-targeted motif TBC1D1 Thr590, a main contributor to TBC1D1 PAS phosphorylation,3 is highly phosphorylated after insulin stimulation in rodent skeletal muscle and cell culture.3, 11, 13 We hypothesized that insulin stimulates TBC1D1 Thr590 in human skeletal muscle and examined whether obesity and T2D alter TBC1D1 Thr590 phosphorylation. In this study, we determined the effects of insulin stimulation on AS160 Thr642, AS160 Ser711 and TC1D1 Thr590 phosphorylation in lean, obese and T2D subjects.
Materials and Methods
Subjects
Obese patients with T2D (n=10), obese normal-glucose-tolerant subjects (n=9) and lean normal-glucose-tolerant subjects (n=8) were studied. Normal glucose tolerant and diabetic status (per American Diabetes Association criteria) was established with an oral glucose tolerance test and the Matsuda index of insulin sensitivity was calculated as described.16 Sulfonylureas were discontinued in the two T2D subjects 2 days before the clamp procedure to avoid hypoglycemia. Seven subjects with T2D were diet-controlled. The study was approved by the Institutional Review Board of the University of Texas Health Science Center, and the Joslin Diabetes Center, and subjects gave their informed written consent before participating in the study.
Measurement of peripheral insulin sensitivity and muscle biopsies
After an overnight fast, subjects underwent a 180-min euglycemic-hyperinsulinemic (160 mU m−2 min−1) clamp. Insulin-stimulated glucose metabolism (M) was determined based on the mean glucose infusion rate during the last 30 min of the clamp.17 Vastus lateralis muscle biopsies were obtained at baseline, 30 min and 180 min into the clamp.
Tissue processing, immunoblotting and plasma analyses
Frozen muscle biopsy samples were processed as previously described.3, 17 Antibodies against Akt, phospho-Akt Thr308, PAS, TBC1D1, AS160 Thr642, AS160 Ser711 and TBC1D1 Thr590 were from Cell Signaling Technologies (Danvers, MA, USA).10, 13, 18 The anti-AS160 antibodies were from Millipore (Billerica, MA, USA) and Abcam (Cambridge, MA, USA).10 Plasma insulin, plasma glucose and hemoglobin A1c (HbA1c) were measured as described previously.17
Statistical analysis
Data are presented as means±s.e.m. Increases in phosphorylation are expressed relative to lean subjects at baseline. Subjects' baseline characteristics were compared by one-way analysis of variance. Group and time course data were analyzed by two-way analysis of variance with repeated measures. Multiple comparisons were performed using the Holm-Sidak post-hoc test. The level of significance was set at P<0.05.
Results and discussion
Subject characterization
Compared with lean subjects, T2D subjects had a higher body mass index, fasting plasma glucose and HbA1c, and increased insulin resistance as determined by Homeostasis Model of Assessment - Insulin Resistance (HOMA-IR) and fasting insulin (Supplementary Table 1). Obese subjects had higher body mass index and showed a trend toward a lower Matsuda index (P=0.1) compared with lean subjects, suggesting peripheral insulin resistance (Supplementary Table 1), but fasting insulin levels (P=0.3) and M-value (P=0.4) were not significantly different compared with lean subjects.
Insulin stimulation increases Akt Thr308 and PAS phosphorylation
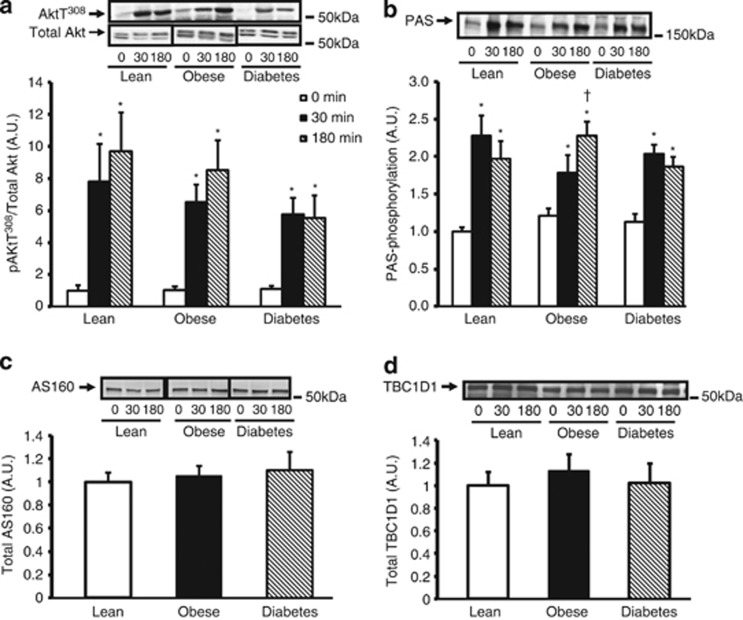
Akt is the primary upstream kinase regulating AS160 and TBC1D1 phosphorylation.3, 5 We measured phosphorylation of Akt on Thr308, a key phosphorylation site that regulates Akt activity, and found that after 180 min of insulin stimulation, Akt Thr308 phosphorylation was significantly reduced in T2D subjects compared with lean subjects (Figure 1a). These data are consistent with a previous study6 and indicate that the T2D subjects had impaired proximal insulin signaling. To assess overall AS160 and TBC1D1 phosphorylation on Akt substrate motifs, we used the PAS antibody.3 Insulin stimulation increased PAS phosphorylation approximately twofold in all groups, indicating no significant difference in overall PAS phosphorylation among subjects (Figure 1b).
Figure 1.
Insulin regulation of phospho-Akt Thr308 and PAS phosphorylation and AS160 and TB1D1 protein content. a, b, c and d: Vastus lateralis muscle biopsies were taken during the clamp procedure at baseline (0), 30 min and 180 min. Muscle samples were processed and immunoblotted with Akt, anti-phospho-Akt Thr308 antibody (a) and anti-PAS antibody (b). For the total-Akt blot, the upper band was identified as Akt. AS160 (c) and TBC1D1 (d) protein expression was assessed by immunoblotting. After quantification there were no significant differences in protein expression within groups during clamp, therefore, data from the three time points were pooled. *P<0.05 vs basal time point within the same group, †P<0.05 vs T2D subjects at similar time point. Data are means ± s.e.; n=8–10 per group.
As T2D subjects have impaired insulin-stimulated GLUT4 translocation and glucose uptake, but have normal GLUT4 levels in skeletal muscle,19 we also measured whether T2D subjects have altered AS160 and TBC1D1 protein content. An increase in AS160 and/or TBC1D1 protein content (in the basal state) could potentially contain Rab proteins in their inactive, guanosine diphosphate-bound form, preventing translocation of GLUT4. AS160 and TBC1D1 total protein expression was not different among subjects at baseline or during the clamp and, therefore, could not account for variation in insulin-stimulated glucose uptake among groups (Figures 1c and d). These data led us to determine if impaired glucose uptake in T2D could be associated with alterations in site-specific AS160 and TBC1D1 phosphorylation.
Insulin effects on AS160 phosphorylation
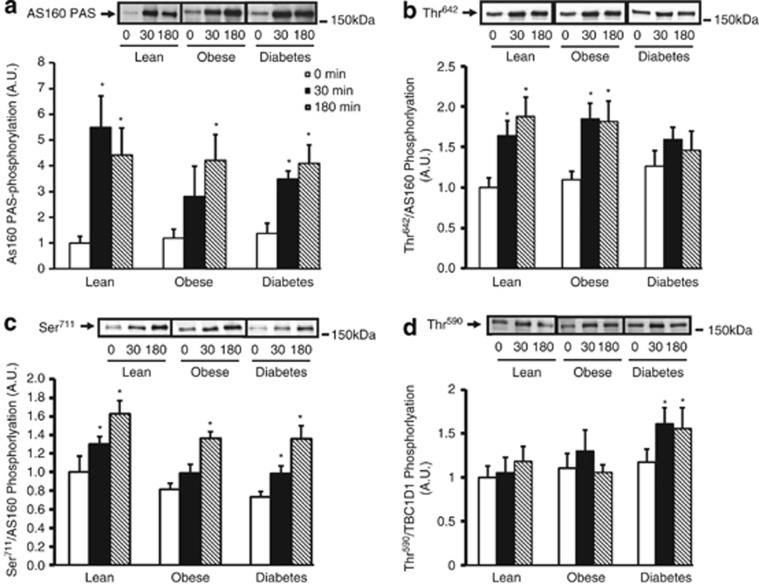
To determine AS160-specific PAS phosphorylation, we immunoprecipitated using an AS160 antibody.10 Baseline AS160 PAS phosphorylation was similar among groups. Although obese subjects had lower rates of phosphorylation compared with lean subjects after 30 min of the clamp, by 180 min, all groups had a fourfold increase in AS160 PAS phosphorylation (Figure 2a). These findings demonstrate that insulin-stimulated AS160 PAS phosphorylation is not different in obese and T2D subjects; rather, it is possible that phosphorylaton on other phospho-sites on AS160 and/or TBC1D1 could be impaired.
Figure 2.
Insulin regulates AS160 and TBC1D1 site-specific phosphorylation. a, b, c and d: AS160 (250 μg of protein lysate) was immunoprecipitated from basal and insulin-stimulated muscle samples and precipitates were immunoblotted with anti-PAS antibody (a). Basal and insulin-stimulated muscle lysates were immunoblotted with anti-phospho-AS160 Thr642 antibody (b), anti-phospho-AS160 Ser711 antibody (c) and anti-phospho-TBC1D1 Thr590 antibody (d). Results of phosphorylation levels (Figures 2b–d) were normalized to the amount of AS160 or TBC1D1 protein content for each individual sample. Data are means ± s.e.; n=8–10 per group. *P<0.05 vs basal time point within the same group.
We measured the effects of insulin on the Akt consensus site AS160 Thr642. Insulin stimulation increased phosphorylation at 30 and 180 min in lean and obese subjects; however, this effect was significantly impaired in subjects with T2D (Figure 2b). In mice, mutation of the equivalent phospho-site AS160 Thr649 inhibited insulin-stimulated glucose transport.9 Taken together, these data suggest that AS160 Thr642 could be a key phosphorylation site in human insulin-stimulated glucose uptake and a crucial phospho-site in impaired glucose uptake in T2D subjects.
Insulin similarly increased AS160 Ser711 phosphorylation in all the subjects at 180 min (Figure 2c). In mice, insulin stimulates AS160 Ser711 phosphorylation in a wortmannin-sensitive manner but independent of Akt2, implicating regulation by one or more upstream kinases.10 These data suggest that insulin-stimulated AS160 Ser711 phosphorylation is regulated independently of Akt Thr308 phosphorylation in human skeletal muscle. Furthermore, our finding that AS160 Ser711 phosphorylation is unaltered in obesity and T2D, along with previous work showing that mutation of AS160 on Ser711 does not disrupt insulin-stimulated glucose uptake in mouse skeletal muscle,10 suggest that this site is unlikely to contribute to insulin-stimulated glucose uptake in human skeletal muscle.
Insulin effects on TBC1D1 phosphorylation
TBC1D1 Thr590 is a predicted Akt consensus site that is phosphorylated by insulin in rodent skeletal muscle.13 In the current study, baseline TBC1D1 Thr590 phosphorylation was similar among all the subjects, and insulin stimulation did not increase TBC1D1 Thr590 phosphorylation in lean or obese subjects during the clamp. In contrast, insulin stimulation increased Thr590 phosphorylation in T2D subjects at 30 and 180 min (Figure 2d). These human findings differ from other species possibly because of expression of different splice variants of TBC1D1 in human skeletal muscle.14 Although studies in mouse skeletal muscle and adipocytes have demonstrated Akt2-dependent insulin-stimulated TBC1D1 Thr590 phosphorylation,11, 13 this phospho-site does not appear to be essential for regulation of glucose uptake, possibly given its lack of 14-3-3 binding, thought to be critical for glucose uptake.20 Also, TBC1D1 Thr590 phosphorylation could have inhibitory effects, given that phosphorylation of this site was only increased in T2D subjects.
In conclusion, this study is the first to show that insulin-stimulated skeletal muscle AS160 Thr642 phosphorylation is impaired in T2D subjects, thereby possibly leading to impaired glucose uptake. AS160 and TBC1D1 site-specific phosphorylation is differentially regulated in human skeletal muscle. Impaired insulin stimulation of AS160 Thr642 phosphorylation in T2D could be a new pharmacological target for improving impaired skeletal muscle glucose uptake in obesity and T2D.
Acknowledgments
This research was supported by the National Institutes of Health Grant AR42238 (to LJG). RJWM was supported by an American Diabetes Association mentor-based fellowship (to LJG), the VSBfonds Beurs, the Scholten-Cordes Foundation and the Haak Bastiaanse-Kuneman Foundation. MAC was supported by the NRSA grant F32DK083183. JTT was supported by a postdoctoral fellowship from The Danish Agency for Science, Technology and Innovation, Denmark. NM was supported by grants from the National Institutes of Health (K23-AG030979, RO1-DK80157 and RO1-DK089229) and the American Diabetes Association. Special thanks to Cell Signaling Technology (Danvers, MA, USA) for pan-TBC1D1 and TBC1D1 phospho-specific antibodies. This study was supported by the National Institutes of Health Grants AR42238 (to LJG) and K23-AG030979, RO1-DK80157, RO1-DK089229 and the American Diabetes Association (to NM).
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies this paper on the Nutrition & Diabetes website (http://www.nature.com/nutd)
Supplementary Material
References
- Bogan JS. Regulation of glucose transporter translocation in health and diabetes. Ann Rev Biochem. 2012;81:507–532. doi: 10.1146/annurev-biochem-060109-094246. [DOI] [PubMed] [Google Scholar]
- Bruss MD, Arias EB, Lienhard GE, Cartee GD. Increased phosphorylation of Akt substrate of 160 kDa (AS160) in rat skeletal muscle in response to insulin or contractile activity. Diabetes. 2005;54:41–50. doi: 10.2337/diabetes.54.1.41. [DOI] [PubMed] [Google Scholar]
- Taylor EB, An D, Kramer HF, Yu H, Fujii NL, Roeckl KS, et al. Discovery of TBC1D1 as an insulin-, AICAR-, and contraction-stimulated signaling nexus in mouse skeletal muscle. J Biol Chem. 2008;283:9787–9796. doi: 10.1074/jbc.M708839200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sano H, Kane S, Sano E, Miinea CP, Asara JM, Lane WS, et al. Insulin-stimulated phosphorylation of a Rab GTPase-activating protein regulates GLUT4 translocation. J Biol Chem. 2003;278:14599–14602. doi: 10.1074/jbc.C300063200. [DOI] [PubMed] [Google Scholar]
- Kramer HF, Witczak CA, Taylor EB, Fujii N, Hirshman MF, Goodyear LJ. AS160 regulates insulin- and contraction-stimulated glucose uptake in mouse skeletal muscle. J Biol Chem. 2006;281:31478–31485. doi: 10.1074/jbc.M605461200. [DOI] [PubMed] [Google Scholar]
- Karlsson HK, Zierath JR, Kane S, Krook A, Lienhard GE, Wallberg-Henriksson H. Insulin-stimulated phosphorylation of the Akt substrate AS160 is impaired in skeletal muscle of type 2 diabetic subjects. Diabetes. 2005;54:1692–1697. doi: 10.2337/diabetes.54.6.1692. [DOI] [PubMed] [Google Scholar]
- Jazet IM, Schaart G, Gastaldelli A, Ferrannini E, Hesselink MK, Schrauwen P, et al. Loss of 50% of excess weight using a very low energy diet improves insulin-stimulated glucose disposal and skeletal muscle insulin signalling in obese insulin-treated type 2 diabetic patients. Diabetologia. 2008;51:309–319. doi: 10.1007/s00125-007-0862-2. [DOI] [PubMed] [Google Scholar]
- Treebak JT, Frosig C, Pehmoller C, Chen S, Maarbjerg SJ, Brandt N, et al. Potential role of TBC1D4 in enhanced post-exercise insulin action in human skeletal muscle. Diabetologia. 2009;52:891–900. doi: 10.1007/s00125-009-1294-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S, Wasserman DH, MacKintosh C, Sakamoto K. Mice with AS160/TBC1D4-Thr649Ala knockin mutation are glucose intolerant with reduced insulin sensitivity and altered GLUT4 trafficking. Cell Metab. 2011;13:68–79. doi: 10.1016/j.cmet.2010.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treebak JT, Taylor EB, Witczak CA, An D, Toyoda T, Koh HJ, et al. Identification of a novel phosphorylation site on TBC1D4 regulated by AMP-activated protein kinase in skeletal muscle. Am J Physiol Cell Physiol. 2010;298:C377–C385. doi: 10.1152/ajpcell.00297.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roach WG, Chavez JA, Miinea CP, Lienhard GE. Substrate specificity and effect on GLUT4 translocation of the Rab GTPase-activating protein Tbc1d1. Biochem J. 2007;403:353–358. doi: 10.1042/BJ20061798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- An D, Toyoda T, Taylor EB, Yu H, Fujii N, Hirshman MF, et al. TBC1D1 regulates insulin- and contraction-induced glucose transport in mouse skeletal muscle. Diabetes. 2010;59:1358–1365. doi: 10.2337/db09-1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vichaiwong K, Purohit S, An D, Toyoda T, Jessen N, Hirshman MF, et al. Contraction regulates site-specific phosphorylation of TBC1D1 in skeletal muscle. Biochem J. 2010;431:311–320. doi: 10.1042/BJ20101100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jessen N, An D, Lihn AS, Nygren J, Hirshman MF, Thorell A, et al. Exercise increases TBC1D1 phosphorylation in human skeletal muscle. Am J Physiol Endocrinol Metab. 2011;301:E164–E171. doi: 10.1152/ajpendo.00042.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyre D, Farge M, Lecoeur C, Proenca C, Durand E, Allegaert F, et al. R125W coding variant in TBC1D1 confers risk for familial obesity and contributes to linkage on chromosome 4p14 in the French population. Hum Mol Genet. 2008;17:1798–1802. doi: 10.1093/hmg/ddn070. [DOI] [PubMed] [Google Scholar]
- Matsuda M, DeFronzo RA. Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diab Care. 1999;22:1462–1470. doi: 10.2337/diacare.22.9.1462. [DOI] [PubMed] [Google Scholar]
- Tantiwong P, Shanmugasundaram K, Monroy A, Ghosh S, Li M, DeFronzo RA, et al. NF-kappaB activity in muscle from obese and type 2 diabetic subjects under basal and exercise-stimulated conditions. Am J Physiol Endocrinol Metab. 2010;299:E794–E801. doi: 10.1152/ajpendo.00776.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vendelbo MH, Clasen BF, Treebak JT, Moller L, Krusenstjerna-Hafstrom T, Madsen M, et al. Insulin resistance after a 72-h fast is associated with impaired AS160 phosphorylation and accumulation of lipid and glycogen in human skeletal muscle. Am J Physiol Endocrinol Metab. 2012;302:E190–E200. doi: 10.1152/ajpendo.00207.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen O, Bak JF, Andersen PH, Lund S, Moller DE, Flier JS, et al. Evidence against altered expression of GLUT1 or GLUT4 in skeletal muscle of patients with obesity or NIDDM. Diabetes. 1990;39:865–870. doi: 10.2337/diab.39.7.865. [DOI] [PubMed] [Google Scholar]
- Pehmoller C, Treebak JT, Birk JB, Chen S, Mackintosh C, Hardie DG, et al. Genetic disruption of AMPK signaling abolishes both contraction- and insulin-stimulated TBC1D1 phosphorylation and 14-3-3 binding in mouse skeletal muscle. Am J Physiol Endocrinol Metab. 2009;297:E665–E675. doi: 10.1152/ajpendo.00115.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.