Abstract
Objective:
To examine if clustering of cardiometabolic risk factors in pregnancy predicts type 2 diabetes and cardiovascular disease (CVD) risk at 10 years in women with gestational diabetes mellitus (GDM).
Study design and subjects:
A prospective case–control study in 150 GDM and 72 overweight women with normal glucose tolerance (NGT) measured cardiometabolic risk factors (body mass index (BMI), systolic blood pressure (SBP), fasting glucose, insulin, and triglycerides and high-density lipoprotein (HDL) cholesterol) at 28 weeks gestation and 6 months and 10 years after pregnancy. Cluster analysis of cardiometabolic risk factors in pregnancy was used to stratify GDM as ‘high' and ‘low risk' for diabetes and CVD risk at 10 years. The data in pregnancy were used to determine a simple method for assessing risk of future diabetes.
Results:
BMI in the 150 GDM at study entry was similar to NGT, but 35% of GDM fell into a ‘high-risk cluster' with elevated BMI, SBP, glucose, insulin and triglycerides and lower HDL levels. At 10 years, type 2 diabetes was sixfold higher in ‘high-risk' GDM (odds ratio (OR)=6.75, confidence interval (CI)=2.0, 22.7, P=0.002) compared with ‘low-risk' GDM and was not reported in NGT. The ‘high-risk' cluster predicted type 2 diabetes better than BMI>30 (OR=2.13, CI=0.71, 6.4, P=0.179) or fasting glucose >5.5 mmol l–1, (OR=4.56, CI=1.50, 13.85, P=0.007). We determined that GDM with any four of the cardiometabolic risk factors (BMI>30 kg m–2, fasting glucose>5.0 mmol l–1, insulin>7.8 mU l–1, triglycerides >2.4 mmol l–1, HDL<1.6 mmol l–1 or SBP>105 mm Hg) in pregnancy would be in a ‘high-risk' cluster.
Conclusions:
A metabolic syndrome-like cluster in pregnant GDM identifies risk for type 2 diabetes providing an opportunity to focus on rigorous lifestyle interventions after delivery to reduce the burden of disease attributed to this condition.
Keywords: lipids, insulin, blood pressure, glucose, body mass index, metabolic syndrome
Introduction
Obesity in the obstetric population is increasing to epidemic proportions worldwide1 and is likely to affect future incidence of gestational diabetes mellitus (GDM), subsequent type 2 diabetes and cardiovascular disease (CVD). Pregnancy offers an opportune time to assess CVD and diabetes risk at an early age. Women with GDM have a high rate of complications in pregnancy including stillbirth, macrosomia and pre-eclampsia2 and are prone to develop diabetes and vascular disease in the years following.3, 4, 5 GDM and their offspring have an increased risk of obesity and type 2 diabetes.6 Diabetes appears to negate the usual female cardiovascular advantage in women of reproductive age7 and has been associated with an increase in CVD risk in women compared with men.8, 9, 10
It has been suggested that pregnancy provides the necessary stress to unmask latent elements of the metabolic syndrome predisposing to an increase in long-term cardiovascular risk,11, 12 However, traditional CVD risk markers are not routinely measured in pregnancy because hormonal changes that occur in pregnancy influence the levels of many CVD markers. It is well known that pregnancy complications such as GDM and pre-eclampsia are associated with abnormalities that could reflect a predisposition to the metabolic syndrome and both of these complications are associated with an increased risk of coronary artery disease and stroke. Clustering of cardiovascular risk factors that comprise the metabolic syndrome (hypertension, obesity, impaired glucose tolerance and dyslipidemia) has been recognized since the 1920s.13 In this report, we describe a prospective case–control study in GDM and overweight women with normal glucose tolerance (NGT) that examined whether stratifying the GDM using cluster analysis of cardiometabolic risk markers measured during pregnancy predicted development of diabetes and CVD risk 10 years later.
Materials and methods
Patient population and study design
One hundred twenty-two pregnant women were recruited from outpatient clinics at King Edward Memorial Hospital, and Joondalup Health Campus in the metropolitan area of Perth, Western Australia between 1998 and 2001. All the women were referred for a 75-g oral glucose tolerance at 28 weeks gestation. One hundred fifty were classified as GDM using the prevailing criteria of the Australasian Diabetes in Pregnancy Society14 (fasting glucose⩾5.5 mmol l–1 and/or a 2-h glucose⩾8.0 mmol l–1, after a 75-g oral glucose tolerance test). The majority of the GDM (75%) presented with incident GDM. Seventy-two women were classified as having NGT. The GDM were further classified into ‘high-‘ and ‘low-risk' GDM using cluster analysis of traditional cardiovascular factors at 28 weeks gestation as described in the statistical methods.
All women were studied at 28 weeks gestation, and at 6 months and 10 years after their pregnancy in a prospective case–control study. The research nurse undertaking the study was blinded to the GDM cluster allocation when follow-up measurements were obtained. Women were excluded from the study if they had any known pre-existing chronic medical condition. The study was carried out in accordance with the Declaration of Helsinki. It was approved by the institutional ethics committees and informed written consent was obtained from all participants.
The women were studied at 28 weeks gestation, before any dietary intervention or hypoglycemic therapy commenced. They had standard clinical monitoring in the outpatient clinic until delivery and were studied again at 6 months postpartum and then 10 years after their pregnancy. A comprehensive study of cardiovascular risk factors was carried out at the three time points and included fasting glucose, insulin, triglycerides, high-density lipoprotein (HDL) cholesterol and high sensitivity C-reactive protein (Hs-CRP) that were measured in the Department of Pathology and Laboratory Medicine at Royal Perth Hospital. A research nurse measured weight and height, and blood pressure (BP) using a Dinamap 1846SX oscillometic monitor (Criticon Inc., Tampa, FL, USA). Average systolic and diastolic BPs were calculated from five measurements at 1-min intervals after 5-min seated rest.
Questionnaires were administered regarding obstetric and medical history, parity and pre-pregnancy body weight, marital status, education level, ethnic background and family history of hypertension and diabetes. After delivery, details of infant birth weight and postnatal complications were obtained from hospital records.
At the 10-year follow-up, diagnosed diabetes and hypertension were ascertained from medical records and measurement of fasting glucose (⩾7 mmol l–1) and BP >140/90 mm Hg measured using a Dinamap 1846SX. Inquiry was made about additional pregnancies and pregnancy complications in the 10 years. An assessment of current level of physical activity and alcohol and cigarette consumption and medication was obtained from questionnaire.
Statistical analysis
Cluster analysis in GDM
Definitions of the metabolic syndrome rely on cut-points for quantitative variables where each variable is linearly related to CVD risk. The traditional cut-points for components of the metabolic syndrome are not suitable for use in pregnant women because the hormonal changes associated with pregnancy affect lipid levels, insulin resistance and BP. To overcome the problem of defining the metabolic syndrome in pregnancy, we used k-means cluster analysis to define two groups with higher or lower values that constitute the metabolic syndrome in non-pregnant adults. Cluster analysis is a statistical method that uses algorithms, which minimize within-group variation and maximize between-group variation of the clustering variables.15 Clustering variables were body mass index (BMI), systolic BP (SBP), fasting glucose, insulin, HDL cholesterol and triglycerides measured at 28 weeks of pregnancy before commencing dietary intervention or hypoglycemic therapy. Two clusters were identified. We refer to the group with the higher values as the ‘high-risk cluster' group.
Differences in SBP, and BMI and fasting glucose, insulin, triglycerides, HDL cholesterol and Hs-CRP in the ‘high' and ‘low-risk' clusters and NGT at study entry and 6 months and 10 years were assessed using one-way analysis of variance and least square differences to adjust for multiple comparisons. Log transformation was undertaken when necessary to normalize the data.
The study was designed to have 80% power at P<0.05 to detect a difference of 0.3 mmol l–1 in glucose; 0.35 mml l–1 in triglycerides; 0.14 mmol l–1 in HDL cholesterol; a 2.6 unit difference in BMI and 4 mm Hg difference in SBP between the clusters and NGT.
Differences in discriminate variables were assessed using χ2 analysis. Logistic regression models with a robust standard error term were used to predict type 2 diabetes 10 years after pregnancy, with adjustment for differences in age, family history of diabetes, level of education, pregnancy weight gain, insulin treatment during pregnancy and smoking. The robust standard errors were used to accommodate the nested data with clustering of observation times within individuals. Bayesian Information Criterion (BIC) was used to compare different models, where a smaller BIC represents a better model fit.16
The potential for bias resulting from loss to follow-up was investigated using a Heckman selection model.17
Determination of cardiometabolic risk factor cut-points for clinical use in GDM
Recursive partitioning developed by Breiman et al.18 was used to define suitable cut-points for each of the clustering variables in pregnancy. To avoid the problem of multi-collinearity that is inevitable in the analysis of clusters we ran two recursive partitioning analyses, one with insulin, glucose and BMI and the other with SBP, triglycerides and HDLs. We used the resulting cut-points to determine the sensitivity and specificity for cluster membership depending on whether 6, 5 or 4 of the cluster variables exceeded the cut-point for patient classification.
Results
A total of 150 GDM and 72 overweight NGT were studied during pregnancy. Follow-up measurements were obtained in 90% of GDM and 86% of NGT at 6 months post-partum and 75% of GDM and 70% of NGT at 10 years after pregnancy. BMI in GDM at the study entry was similar to NGT, 30.2±5.0 and 30.2±7.5 kg m–2, respectively.
Identification of a ‘high-risk' metabolic cluster in GDM
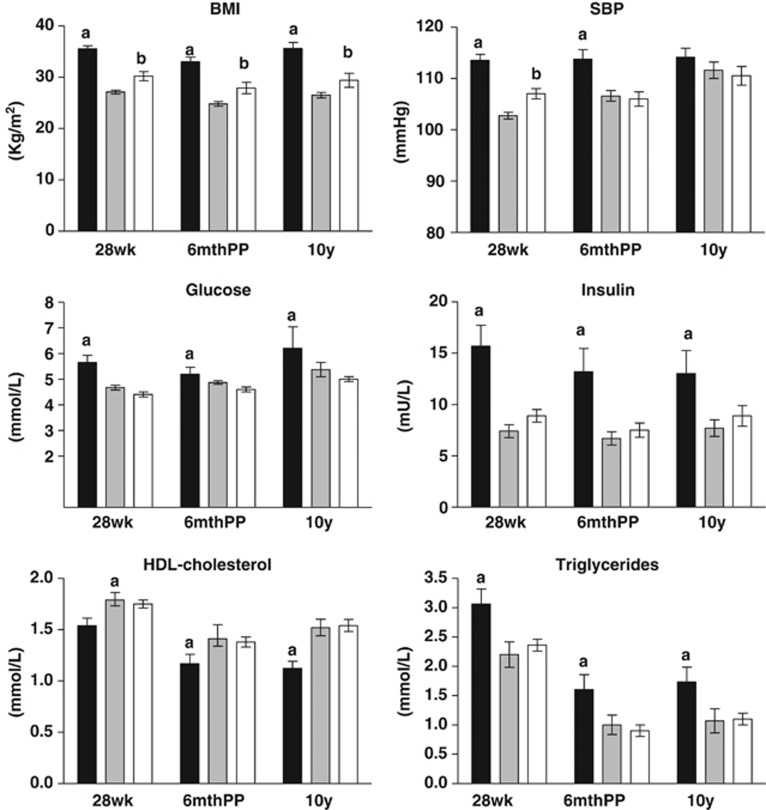
Cluster analysis using traditional cardiometabolic risk factors measured at 28 weeks gestation identified, a ‘high-risk' cluster representing 35% of GDM. The ‘high-risk' cluster had significantly elevated SBP, BMI, fasting blood glucose, triglycerides, insulin and lower HDL cholesterol. The degree of separation of the main features of the ‘high-' and ‘low-risk' clusters are shown in Table 1. The ‘high-risk' cluster had elevated Hs-CRP 9.7±1 mg l–1 compared with the ‘low-risk' cluster 5.5±0.4 mg l–1. At 28 weeks gestation, NGT were comparable to the ‘low-risk' cluster with respect to fasting glucose 4.46±0.04 mmol l–1; insulin 8.9±0.6 mU l–1; triglycerides 2.36±0.1 mmol l–1, HDL cholesterol 1.75±0.04 mmol l–1 and Hs-CRP 6.56±0.66 mg l–1. SBP and BMI in NGT were 107±1 mm Hg and 30.2±0.9 kg m–2, respectively, and lower than the ‘high-risk' cluster (P=0.001) but higher than the ‘low-risk' cluster (P=0.02, P=0.001, respectively; Figure 1).
Table 1. Comparison of the differences in cluster variables between the ‘high-' and ‘low'-risk cluster GDM and women with NGT at 28 weeks of pregnancy.
| Variable | ‘High-risk' GDM | ‘Low-risk' GDM | NGT |
|---|---|---|---|
| N | 53 | 97 | 72 |
| Fasting glucose (mmol l–1) | 5.6±0.9a | 4.7±0.5 | 4.6±0.4 |
| BMI (kg m–2) | 35.5±5.0 a | 27.3±4.1b | 30.2±7.6 |
| Fasting insulin (mU l–1) | 15.7±8.8a | 7.4±3.5 | 8.9±5.3 |
| SBP (mm Hg) | 112±9a | 103±7b | 107±10 |
| Fasting triglycerides (mmol l–1) | 3.0±1.0a | 2.2±0.7 | 2.4±0.8 |
| Fasting HDL cholesterol (mmol l–1) | 1.5±0.3a | 1.8±0.3 | 1.8±0.4 |
Abbreviations: ANOVA, analysis of variance; BMI, body mass index; GDM, gestational diabetes mellitus; HDL, high-density lipoprotein; NGT, normal glucose tolerance; SBP, systolic blood pressure.
Data shown are mean and s.d.
Differences between the each of the clusters and NGT were analyzed using one-way ANOVA.
P<0.01 compared with low-risk cluster and NGT.
P<0.05 compared with NGT.
Figure 1.
BMI, SBP, fasting glucose, insulin, triglycerides and HDL cholesterol in the ‘high-risk' (black bars) low-risk' clusters (grey bars), and NGT group (white bars) at 28 weeks gestation, 6 months and 10 years post-partum. Values are mean±s.e.m., aP<0.01 compared with ‘low-risk' cluster and NGT, bP<0.05 compared with ‘low-risk' cluster.
Demographics, obstetric history and family history of CVD risk factors at study entry
At entry to the study the ‘high-risk' cluster was younger than the ‘low-risk' cluster but similar to NGT. The distribution of ethnic backgrounds was similar to the ‘low-risk' cluster and NGT and was comprised mainly of women with a Caucasian background (Table 2). The ‘high-risk' cluster were less educated than the ‘low-risk' cluster and NGT, completing year 12 high school (P<0.001) and obtaining a tertiary qualification (P=0.025) less often (Table 2). Parity was similar between clusters and NGT (Table 2). Diagnosed GDM or pre-eclampsia, in a previous pregnancy was 20% and 19%, respectively, in the GDM clusters (Table 2) and was reported infrequently in NGT. Family history of hypertension was similar between the clusters and NGT (Table 2). Family history of diabetes was reported more often in the ‘high-risk' cluster (P=0.006; Table 2).
Table 2. Demographics, obstetric and family history of diabetes and hypertension and neonatal outcomes of the groups obtained at the index pregnancy.
| ‘High-risk' | ‘Low-risk' | NGT | |
|---|---|---|---|
| (n=53) | (n=97) | (n=72) | |
| Age (years) | 31.3±0.7 | 33.8±0.5a | 32.6±0.4 |
| Ethnic background | |||
| Caucasian | 67.9% | 78.4% | 84.9% |
| Asian/aboriginal/middle- eastern | 32.1% | 21.6% | 15.1% |
| Level of education | |||
| Completed year 12 | 39.6% | 71.1% a | 61% |
| Tertiary qualification | 17% | 37.1% b | 31% |
| Obstetric history | |||
| Parity | |||
| Nulliparous % | 15.1% | 20.6% | 20.6% |
| Multiparous % | 84.9% | 79.4% | 79.4% |
| Previous gestational diabetes % | 23.5% | 18.3% | 2.9% |
| Previous pre-eclampsia % | 27.9% | 15.4% | 0% |
| Family history | |||
| Hypertension % | 62.3% | 53.7% | 57.5% |
| Diabetes % | 75.5% | 52.6% a | 53.4% |
| Obstetric and neonatal outcomes | |||
| Pre-eclampsia % | 17% | 7.3% | 0% |
| Birth weight (g) | 3321±103 | 3329±49 | 3495±57 |
| Birth length (cm) | 49.7±0.5 | 49.6±0.3 | 49.6±0.3 |
| Head circumference (cm) | 34.6±0.4 | 34.7±0.3 | 35.0±0.2 |
| Gender of babies | |||
| % Boys | 56.9% | 48.4% | 44.4% |
| % Girls | 43.1% | 51.6% | 55.6% |
Abbreviations: ANOVA, analysis of variance; NGT, normal glucose tolerance.
Values are mean±s.e.m.
P<0.01 compared with ‘high-risk' cluster.
P<0.05 compared with ‘high-risk' cluster using one-way ANOVA for continuous variables and χ2 analysis for descriptive variables.
Neonatal outcomes and pregnancy complications in the index pregnancy
Birth weight, birth length and head circumference of the babies was similar in all groups (Table 2). The incidence of pre-eclampsia was 10% in GDM and not different between the clusters. Pre-eclampsia was not reported in NGT.
Cardiovascular risk profile 6 months after pregnancy
Using the clusters assigned in pregnancy, BMI, fasting glucose, SBP and triglycerides were significantly elevated in the ‘high-risk' cluster compared with the ‘low-risk' cluster and NGT. In contrast, HDL was significantly reduced in the ‘high-risk' cluster (Figure 1). The differences in triglycerides, HDL cholesterol, BMI, insulin, glucose and SBP between the clusters were still present after adjusting for differences in age and educational background. At 6 months post-partum, BMI in NGT was significantly lower than the ‘high-risk' cluster and higher than the ‘low-risk' cluster. SBP, glucose, triglycerides, HDL and insulin differed significantly from the ‘high-risk' cluster but not the ‘low-risk' cluster (Figure 1).
Follow-up at 10 years
At 10 years type 2 diabetes, was reported more frequently in the ‘high-risk' cluster (38.6%) compared with the ‘low-risk' cluster (6.2%, P=0.001) and was not reported in NGT (Table 3). Reporting of diagnosed hypertension and hypercholesterolemia was similar between the clusters (Table 3). Subsequent pregnancies in the 10 years, were reported in ∼50% of women. The ‘high-risk' cluster reported more births than the ‘low-risk' cluster P=0.03 (Table 3). Reporting of recurrence of GDM or hypertension in a subsequent pregnancy was similar between the GDM clusters. Reporting of these complications in NGT was infrequent (Table 3). Although measurements were not obtained from all women at 10 years, those not studied had a similar cluster distribution to the original cohort (30% high-risk cluster) and similar baseline BMI, lipids, glucose, insulin and SBP to their cluster (data not shown). The Heckman selection model produced an inverse Mills ratio of 0.52, P=0.263, which suggests that loss to follow-up is not related to development of diabetes.
Table 3. Diagnosed type 2 diabetes, hypertension and hypercholesterolemia at 10 years, obstetric history since the index pregnancy, and consumption of alcohol, tobacco, tea and coffee and level of exercise at 10 years in the ‘high' and ‘low'-risk cluster GDM and NGT.
| ‘High-risk' | ‘Low-risk' | NGT | |
|---|---|---|---|
| (n=40) | (n=72) | (n=48) | |
| Diagnosed conditions since index pregnancy | |||
| Type 2 diabetes | 38.6% | 6.2%a | 0% |
| Hypertension | 10.3% | 4.8% | 2.2% |
| Hypercholesterolemia | 26.9% | 12.9% | 8.3% |
| Obstetric history since index pregnancy | |||
| Subsequent pregnancies | |||
| Yes % | 62.5% | 47.0% | 60.4% |
| No % | 37.5% | 53.0% | 39.6% |
| No. of births | 1.56±0.15 | 1.19±0.09b | 1.59±0.18 |
| GDM in subsequent pregnancy | 41.7% | 53.6% | 10.3% |
| Hypertension in subsequent pregnancy | 8.3% | 10.3% | 6.9% |
| Self-reported beverage consumption and smoking at 10 years | |||
| Tea drinkers % | 67.5% | 70.8% | 63% |
| Coffee drinkers % | 55.0% | 76.9%b | 79% |
| Consumed alcohol in last week | 35% | 59.1%b | 54.2% |
| Quantity of alcohol consumed (g) | 19.8±15.4 | 54.5±12.0a | 37.2±6.5 |
| Smokers % | 25% | 16.7% | 14.6% |
| Self-reported exercise at 10 years | |||
| Once a month | 32.5% | 9.1%a | 8.3% |
| 1–2 times per week | 17.5% | 37.9% | 54.2% |
| 3–6 times per week | 32.5% | 31.8% | 16.7% |
| Daily | 17.5% | 21.2% | 20.8% |
Abbreviations: ANOVA, analysis of variance; GDM, gestational diabetes mellitus; NGT, normal glucose tolerance.
Values are mean±s.e.m.
P<0.01 or
P<0.05 compared with ‘high-risk' cluster using one-way ANOVA for continuous variables and χ2 analysis for descriptive variables.
The ‘high-risk' cluster reported reduced participation in exercise (walking, gardening and sport; Table 3). Reporting of tobacco and tea consumption was similar between the clusters but coffee drinkers were less prevalent in the ‘high-risk' cluster (P=0.019) and these women reported consumption of less alcohol (Table 3). In NGT, reporting of exercise was similar to the ‘low-risk cluster' (Table 3). Alcohol and tea consumption of NGT were not different to either cluster, but coffee, was consumed by 79% of NGT and was similar to the ‘low-risk' cluster' (Table 3).
Cardiovascular risk profile and CVD risk score 10 years after pregnancy
At 10 years, the pattern of CVD risk factors in both the clusters and NGT was similar those in pregnancy and at 6 months post-partum with the exception of SBP that was not different between the clusters (Figure 1). After adjusting for differences in age, educational level and the number of subsequent pregnancies the significant differences (P<0.001), were still found in triglycerides HDL cholesterol and BMI insulin and glucose. Hs-CRP remained elevated in the ‘high-risk' cluster at 10 years (7.0±1.3 mg l–1) compared with the ‘low-risk' cluster (3.1±0.6 mg l–1) and NGT (3.7±1.1 mg l–1, P<0.01).
Predictors of type 2 diabetes at 10 years in GDM
Logistic regression and the BIC were used to examine whether cut-points for BMI⩾30 kg m–2 or fasting glucose >5.5 mmol l–1 in pregnancy were better predictors of type 2 diabetes at 10 years than membership of the ‘high-risk' cluster. The ‘high-risk' cluster predicted the development of type 2 diabetes at 10 years (odds ratio (OR)=6.75, 95% confidence interval (CI)=2.0, 22.7, P=0.002, BIC=88.5). BMI⩾30 kg m–2 did not predict diabetes (OR=2.13, CI =0.71, 6.4, P=0.179, BIC=97.7). Fasting glucose >5.5 mmol l–1 at diagnosis also predicted type 2 diabetes (OR=4.56, CI=1.50, 13.85, P=0.007, BIC=92.4) but the larger BIC indicates the model containing the ‘high-risk' cluster is a better model, correctly predicting development of diabetes in 12 women as opposed to 8 women using fasting glucose >5.5 mmol l–1.
Smoking, weight change in pregnancy, insulin treatment in pregnancy and gestation at delivery were examined as confounders in the logistic regression analysis. Insulin treatment in pregnancy appeared to protect against development of diabetes at 10 years OR=0.124, CI=0.003, 0.461, P=0.002 but did not change the relationship between the high-risk cluster and diabetes at 10 years OR=6.88, CI=1.7, 28.7, P=0.008.
A simple method for determining high-risk GDM for clinicians
We recognized it would be clinically useful if a simple method could be used for future identification of GDM that fitted our definition of ‘high-risk' cluster membership. Using recursive partitioning of cardiometabolic risk factors measured in GDM at 28 weeks gestation, we defined cut-points for each of the risk factors. (Table 4). We determined that exceeding the defining level for any four of the cardiometabolic risk factors provided the greatest sensitivity 88.7% (CI=77–95%) and specificity 95.9% (CI=89.9–98.9%) for allocation of GDM to the high-risk cluster.
Table 4. Cardiometabolic risk factor defining levels for determining GDM at ‘high-risk' of future diabetes and cardiovascular disease.
|
Four or more of the six risk factors | |
|---|---|
| Risk factor | Defining level |
| Body mass index (kg m–2) | >30 |
| Fasting | |
| Glucose (mmol l–1) | >5.0 |
| Insulin (mU l–1) | >7.8 |
| Triglycerides (mmol l–1) | >2.4 |
| HDL cholesterol (mmol l–1) | <1.6 |
| Systolic blood pressure (mm Hg) | >105 |
Abbreviations: GDM, gestational diabetes mellitus; HDL, high-density lipoprotein.
Exceeding the cut-point for any 4 of these measurements will place GDM in the high-risk pregnancy cluster and at significant risk for development of type 2 diabetes and cardiovascular disease.
Discussion
In this prospective case–control study of overweight women with GDM, we identified 35% of women with GDM who had substantially higher BMI, BP, fasting glucose, insulin and triglycerides, and lower HDL. This ‘high-risk cluster paralleled several features of the metabolic syndrome in non-pregnant adults and at 10 years had increased rates of diabetes and a worse CVD risk profile than the ‘low-risk' GDM and overweight NGT. We have shown for the first time that a metabolic syndrome-like profile in GDM is a better predictor of incidence of diabetes at 10 years than high fasting glucose or elevated BMI. We have formulated a simple, sensitive, specific method based on cardiometabolic risk measurements obtained at diagnosis of GDM to aid clinicians in assessment of future risk of developing diabetes and CVD.
The ‘high-risk' cluster differed from low-risk' GDM in that they were slightly younger, less educated and reported family history of diabetes more often. Their metabolic syndrome-like CVD profile remained significantly different after adjusting for age, level of education and family history of diabetes. In keeping with their metabolic syndrome-like profile the ‘high-risk' cluster had higher Hs-CRP levels compared with the ‘low-risk' cluster and NGT during pregnancy. Pre-eclampsia has been shown to be associated with an increased CVD risk after pregnancy,4, 19 but in our study the incidence of pre-eclampsia in two clusters was not different. An elevated 2-h glucose in the glucose tolerance test distinguished the ‘low-risk' GDM cluster from NGT, who were otherwise similar in most respects.
At 6 months post-partum, after the hormonal influences of pregnancy had resolved the metabolic syndrome profile of the ‘high-risk' cluster was still evident. These differences persisted ten years after the index pregnancy, with the exception of SBP that no longer differed between the clusters. Hs-CRP remained significantly higher in the ‘high-risk' cluster at 10 years. This finding is in keeping with reports of increased low-grade inflammation in non-pregnant women with previous GDM20, 21 and women with the metabolic syndrome.22
In regression analysis, membership of the ‘high-risk' cluster that incorporated a number of cardiovascular abnormalities, was a better predictor of type 2 diabetes at 10 years than a high fasting glucose (>5.5 mmol l–1) measured at the time of diagnosis of GDM. Interestingly, obesity (BMI>30) at study entry did not predict the development of diabetes at 10 years, suggesting that this component of the ‘high-risk' cluster was not on its own the driver for development of diabetes. This observation is supported by the absence of reported diabetes in the overweight NGT whose BMI were consistently higher than the ‘low-risk' cluster GDM.
Previous studies have identified that women with a past history of GDM are more likely to have impaired glucose regulation and the metabolic syndrome 1–10 years after pregnancy.20, 22, 23, 24 Post-partum elevated CRP has been also been reported to coincide with these features. Reports that pre-pregnancy weight and insulin secretion predict glucose tolerance 5 years after GDM25 suggest that many of these features may be constitutional. A large retrospective population-based cohort study looking at glucose intolerance in pregnancy showed that women with GDM had a higher risk of a cardiovascular events over 12.3 years with an adjusted hazard ratio of 1.66 compared with women who had NGT in pregnancy.26 That study also showed that even mild glucose intolerance in pregnancy conferred an increased risk for CVD.26 We postulate that the increased risk for CVD in women with mild glucose intolerance may be due to abnormalities in other CVD risk factors such as lipids and insulin that were not measured in that study. Our findings suggest that a sub-set of women with GDM have a number of other metabolic abnormalities including a tendency to greater obesity, dyslipidemia, low-grade inflammation and elevated BP that together predispose to increased CVD risk and diabetes. This hypothesis is supported by our observation that overweight women who had a NGT test in pregnancy were similar to the ‘low-risk' cluster in terms of their lipid profile, insulin levels, markers of inflammation, family history and level of education.
In our study, 70% of the original cohort was re-studied at 10 years. Although incomplete follow-up could be seen as a weakness in our study, our analysis for bias because of the incomplete follow-up showed that there was no significant bias in our findings that could have occurred as a result of loss to follow-up.
Lifestyle measures recorded at 10 years indicated that the ‘high-risk' cluster exercised significantly less than the other groups with 32% of women reporting that they exercised only once a month. Alcohol consumers were similar between the clusters but alcohol consumption was lower in the ‘high-risk' cluster. Tea consumption was similar across the groups but coffee consumption in the ‘high-risk' cluster was reported less often than the ‘low-risk' cluster or NGT. These findings are interesting in view of the accumulating epidemiological evidence suggesting that physical activity,27 coffee drinking28, 29, 30 and alcohol consumption31, 32 protect against type 2 diabetes.
In conclusion, we have shown that the cardiometabolic risk profile as assessed by cluster analysis in pregnancies with GDM strongly correlates with the development of diabetes and persistence of CVD risk 10 years later. Our data suggest that in spite of the hormonal effects of pregnancy, measurement of traditional CVD risk factors in conjunction with a detailed family history in pregnant women with GDM may offer better opportunities to identify women with GDM who are at increased risk of subsequent diabetes and CVD. A strength of this study is that measurement of traditional CVD risk factors at diagnosis of GDM has enabled us to derive a simple formula that should significantly aid clinicians in identification of those women who should be targeted for intervention after pregnancy.
Acknowledgments
We thank Jackie Ritchie for her excellent assistance with recruitment and follow-up of the women studied. This study was funded by a project grant from the National Heart Foundation of Australia.
The authors declare no conflict of interest.
References
- Kanagalingam MG, Forouhi NG, Greer IA, Sattar N. Changes in booking body mass index over a decade: retrospective analysis from a Glasgow Maternity Hospital. Brit J Obstet Gynecol. 2005;112:1431–1433. doi: 10.1111/j.1471-0528.2005.00685.x. [DOI] [PubMed] [Google Scholar]
- Suhonen L, Teramo K. Hypertension and preeclampsia in women with gestational glucose-intolerance. Acta Obstet Gynecol Scand. 1993;72:269–272. doi: 10.3109/00016349309068036. [DOI] [PubMed] [Google Scholar]
- Lauenborg J, Hansen T, Jensen DM, Vestergaard H, Molsted-Pedersen L, Hornnes P, et al. Increasing incidence of diabetes after gestational diabetes: a long-term follow-up in a Danish population. Diabetes Care. 2004;27:1194–1199. doi: 10.2337/diacare.27.5.1194. [DOI] [PubMed] [Google Scholar]
- Bellamy L, Casas J-P, Hingorani AD, Williams D. Type 2 diabetes mellitus after gestational diabetes: a systematic review and meta-analysis. Lancet. 2009;373:1773–1779. doi: 10.1016/S0140-6736(09)60731-5. [DOI] [PubMed] [Google Scholar]
- Shah BR, Retnakaran R, Booth GL. Increased risk of cardiovascular disease in young women following gestational diabetes mellitus. Diabetes Care. 2008;31:1668–1669. doi: 10.2337/dc08-0706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dabelea D, Hanson RL, Lindsay RS, Pettitt DJ, Imperatore G, Gabir MM, et al. Intrauterine exposure to diabetes conveys risks for type 2 diabetes and obesity: a study of discordant sibships. Diabetes. 2000;49:2208–2211. doi: 10.2337/diabetes.49.12.2208. [DOI] [PubMed] [Google Scholar]
- Barrett-Connor E. Sex differences in coronary heart disease—why are women so superior? The 1995 Ancel Keys lecture. Circulation. 1997;95:252–264. doi: 10.1161/01.cir.95.1.252. [DOI] [PubMed] [Google Scholar]
- Barrett-Connor EL. Why is diabetes mellitus a stronger risk factor for fatal ischemic heart disease in women than in men? The Rancho Bernardo Study. JAMA. 1991;265:627–631. [PubMed] [Google Scholar]
- Lee WL, Cheung AM, Cape D, Zinman B. Impact of diabetes on coronary artery disease in women and men: a meta-analysis of prospective studies. Diabetes Care. 2000;23:962–968. doi: 10.2337/diacare.23.7.962. [DOI] [PubMed] [Google Scholar]
- Wannamethee SG, Papacosta O, Lawlor DA, Whincup PH, Lowe GD, Ebrahim S, et al. Do women exhibit greater differences in established and novel risk factors between diabetes and non-diabetes than men? The British Regional Heart Study and British Women's Heart Health Study. Diabetologia. 2012;55:80–87. doi: 10.1007/s00125-011-2284-4. [DOI] [PubMed] [Google Scholar]
- Kaaja RJ, Greer IA. Manifestations of chronic disease during pregnancy. JAMA. 2005;294:2751–2757. doi: 10.1001/jama.294.21.2751. [DOI] [PubMed] [Google Scholar]
- Barden AE, Beilin LJ, Ritchie L, Walters BN, Michael C. Does a predisposition to the metabolic syndrome sensitize women to develop pre-eclampsia. J Hypertens. 1999;17:1307–1315. doi: 10.1097/00004872-199917090-00011. [DOI] [PubMed] [Google Scholar]
- Eckel RH, Grundy SM, Zimmet PZ. The metabolic syndrome. Lancet. 2005;365:1415–1428. doi: 10.1016/S0140-6736(05)66378-7. [DOI] [PubMed] [Google Scholar]
- Hoffman L, Nolan C, Wilson JD, Oats JJ, Simmons D. Gestational diabetes mellitus—management guidelines. The Australasian Diabetes in Pregnancy Society. Med J Aust. 1998;169:93–97. doi: 10.5694/j.1326-5377.1998.tb140192.x. [DOI] [PubMed] [Google Scholar]
- Everitt B, Landau S, Leese M. Cluster Analysis. Edward Arnold Publishers Ltd.: London; 2001. [Google Scholar]
- Raftery AE. Choosing models for cross-classifications—comment. Am Sociol Rev. 1986;51:145–146. [Google Scholar]
- Heckman JJ. Sample selection bias as a specification error. Econometrica. 1979;47:153–161. [Google Scholar]
- Breiman L, Friedman JH, Olshen RA, Stone CJ. Classification and Regression Trees. Wadsworth: Belmont, CA, USA; 1983. [Google Scholar]
- Fraser A, Nelson SM, Macdonald-Wallis C, Cherry L, Butler E, Sattar N, et al. Associations of pregnancy complications with calculated cardiovascular disease risk and cardiovascular risk factors in middle age: the Avon Longitudinal Study of Parents and Children. Circulation. 2012;125:1367–1380. doi: 10.1161/CIRCULATIONAHA.111.044784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Cianni G, Lencioni C, Volpe L, Ghio A, Cuccuru I, Pellegrini G, et al. C-reactive protein and metabolic syndrome in women with previous gestational diabetes. Diab Metab Res Rev. 2007;23:135–140. doi: 10.1002/dmrr.661. [DOI] [PubMed] [Google Scholar]
- Di Benedetto A, Russo GT, Corrado F, Di Cesare E, Alessi E, Nicocia G, et al. Inflammatory markers in women with a recent history of gestational diabetes mellitus. J Endocrinol Invest. 2005;28:34–38. doi: 10.1007/BF03345527. [DOI] [PubMed] [Google Scholar]
- Wildman RP, Kaplan R, Manson JE, Rajkovic A, Connelly SA, Mackey RH, et al. Body size phenotypes and inflammation in the Women's Health Initiative Observational Study. Obesity. 2011;19:1482–1491. doi: 10.1038/oby.2010.332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madarasz E, Tamas G, Tabak AG, Kerenyi Z. Carbohydrate metabolism and cardiovascular risk factors 4 years after a pregnancy complicated by gestational diabetes. Diabetes Res Clin Pract. 2009;85:197–202. doi: 10.1016/j.diabres.2009.05.001. [DOI] [PubMed] [Google Scholar]
- Tam WH, Yang XL, Chan JCN, Ko GTC, Tong PCY, Ma RCW, et al. Progression to impaired glucose regulation, diabetes and metabolic syndrome in Chinese women with a past history of gestational diabetes. Diabetes-Metab Res Rev. 2007;23:485–489. doi: 10.1002/dmrr.741. [DOI] [PubMed] [Google Scholar]
- Metzger BE, Cho NH, Roston SM, Radvany R. Prepregnancy weight and antepartum insulin-secretion predict glucose-tolerance 5 years after gestational diabetes-mellitus. Diabetes Care. 1993;16:1598–1605. doi: 10.2337/diacare.16.12.1598. [DOI] [PubMed] [Google Scholar]
- Retnakaran R, Shah BR. Mild glucose intolerance in pregnancy and risk of cardiovascular disease: a population-based cohort study. Canadian Med Assoc J. 2009;181:371–376. doi: 10.1503/cmaj.090569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown WJ, Burton NW, Rowan PJ. Updating the evidence on physical activity and health in women. Am J Prevent Med. 2007;33:404–411. doi: 10.1016/j.amepre.2007.07.029. [DOI] [PubMed] [Google Scholar]
- Lin WY, Xaiver Pi-Sunyer F, Chen CC, Davidson LE, Liu CS, Li TC, et al. Coffee consumption is inversely associated with type 2 diabetes in Chinese. Eur J Clin Invest. 2011;41:659–666. doi: 10.1111/j.1365-2362.2010.02455.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Lee ET, Cowan LD, Fabsitz RR, Howard BV. Coffee consumption and the incidence of type 2 diabetes in men and women with normal glucose tolerance: the Strong Heart Study. Nutr Metab Cardiovasc Dis. 2011;21:418–423. doi: 10.1016/j.numecd.2009.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsson SC, Virtamo J, Wolk A. Coffee consumption and risk of stroke in women. Stroke. 2011;42:908–912. doi: 10.1161/STROKEAHA.110.603787. [DOI] [PubMed] [Google Scholar]
- Beilin LJ, Puddey IB. Alcohol and hypertension—an update. Hypertension. 2006;47:1035–1038. doi: 10.1161/01.HYP.0000218586.21932.3c. [DOI] [PubMed] [Google Scholar]
- Koppes LLJ, Dekker JM, Hendriks HFJ, Bouter LM, Heine RJ. Moderate alcohol consumption lowers the risk of type 2 diabetes—a meta-analysis of prospective observational studies. Diabetes Care. 2005;28:719–725. doi: 10.2337/diacare.28.3.719. [DOI] [PubMed] [Google Scholar]