Abstract
Objective:
To investigate whether the effects on weight loss and cardiometabolic risk factor reduction of two technology-mediated lifestyle interventions for 15 months in a primary care-based translation trial sustained at 24 months (that is, 9 months after the end of intervention).
Design:
This study analyzed data from an extended follow-up of participants in the original ‘E-LITE' (Evaluation of Lifestyle Interventions to Treat Elevated Cardiometabolic Risk in Primary Care)-randomized controlled trial, which demonstrated the effectiveness of two adapted Diabetes Prevention Program (DPP) lifestyle interventions compared with usual primary care.
Subjects:
E-LITE randomized 241 overweight or obese participants with pre-diabetes and/or metabolic syndrome to receive usual care alone (n=81) or usual care plus a coach-led (n=79) or self-directed intervention (n=81). The interventions provided coach-led group behavioral weight-loss treatment or a take-home, self-directed DVD using the same 12-week curriculum, followed by 12 additional months of technology-mediated coach contact and self-monitoring support. Participants received no further intervention after month 15. A blinded assessor conducted 24-month visits by following the measurement protocols of the original trial. Measurements include weight and cardiometabolic risk factors (waist circumference, fasting plasma glucose, resting blood pressure, triglycerides, high- and low-density lipoprotein cholesterol, total cholesterol and triglyceride to high-density lipoprotein cholesterol ratio).
Results:
At month 24, mean±s.e. changes in body mass index (trial primary outcome) and weight (kg) from baseline were –1.9±0.3 (P=0.001) and –5.4±0.9 (P<0.001) in the coach-led intervention, and –1.6±0.3 (P=0.03) and –4.5±0.9 (P=0.001) in the self-directed intervention, compared with –0.9±0.3 and 2.4±0.9 in the usual care group. In addition, both interventions led to a greater percentage of participants maintaining ⩾7% weight loss and sustained improvements in waist circumference and fasting plasma glucose levels than usual care.
Conclusion:
This study shows sustained benefits of the two primary care-based, technology-mediated DPP lifestyle interventions. The findings warrant replication in long-term studies involving diverse populations.
Keywords: BMI, weight loss, diabetes prevention program, lifestyle intervention, primary care
Introduction
We previously published findings from E-LITE (Evaluation of Lifestyle Interventions to Treat Elevated Cardiometabolic Risk in Primary Care), a three-arm randomized trial, demonstrating the effectiveness of two adapted Diabetes Prevention Program (DPP) lifestyle interventions for weight loss and cardiometabolic risk factor reduction among overweight or obese participants with pre-diabetes and/or metabolic syndrome over 15 months of follow-up compared with usual care.1 This study extends those findings to include outcomes at 24 months—9 months after trial completion. We hypothesized that participants of both E-LITE interventions would sustain greater weight loss and improvements in cardiometabolic risk factors (for example, waist circumference and fasting plasma glucose levels) at 24 months than controls.
Materials and methods
Overweight or obese adults with pre-diabetes and/or metabolic syndrome were recruited within a primary care clinic and randomized to receive usual care alone (n=81) or usual care plus a coach-led (n=79) or self-directed (n=81) behavioral weight-loss intervention. The interventions delivered the DPP-based Group Lifestyle Balance core curriculum2, 3 for 12 weeks (intensive treatment phase) through in-clinic small groups or take-home DVD, supplemented with technology-mediated coach contact and self-monitoring that lasted for 12 more months (maintenance phase). Participants received no further intervention after month 15. Heart 360 is a publically available tool; participants could have continued using this tool if they chose to do so, but coach contact ceased after month 15. Baseline mean (s.d.) age was 53 (11) years, mean body mass index (BMI) was 32.0 (5.4) kg m−2, 47% were female and 78% were non-Hispanic white. Outcome assessors blinded to treatment assignment obtained written informed consent for an extended follow-up until 24 months (through additional funding) from 179 of 194 participants who attended the 15-month visits. Of these, 158 completed the 24-month assessment, and for an additional 13 participants, weights were obtained from electronic medical records (n=11) or by self-report (n=2). A blinded assessor conducted 24-month visits by following the measurement protocols of the original trial.1, 4
Between-group differences at 24 months were evaluated for BMI (trial primary outcome), weight and cardiometabolic risk factors (waist circumference, fasting plasma glucose, blood pressure, triglycerides, high- and low-density lipoprotein cholesterol, total cholesterol and triglyceride to high-density lipoprotein cholesterol ratio). Tests of group by time interactions were performed by intention-to-treat with repeated-measures mixed-effects linear (for continuous outcomes) or logistic models (for categorical outcomes). As in the main study,1 these models used the same fixed and random effects, with missing data handled directly through maximum likelihood estimation via mixed modeling. Primary analyses used all available data (study measured, medical record and self-report); sensitivity analyses were also performed that included only participants with study-measured values. All analyses were conducted using SAS, version 9.2 (SAS Institute Inc., Cary, NC, USA).
Results
Extended follow-up participants (171 or 71% of the 241 trial participants) were similar to the original sample on baseline characteristics and were comparably distributed among study groups (64%, self-directed group; 75%, coach-led group; and 74%, usual care group; P=0.26).
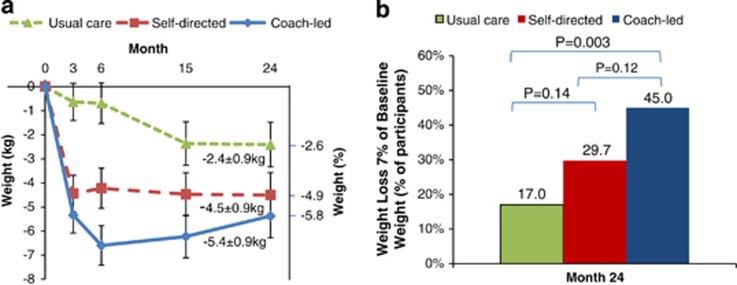
At month 24, the mean±s.e. change in BMI from baseline was –1.9±0.3 in the coach-led intervention (P=0.001 vs usual care), –1.6±0.3 in the self-directed intervention (P=0.03 vs usual care) and –0.9±0.3 in the usual care group. The mean±s.e. change in weight from baseline was –5.4±0.9 kg in the coach-led intervention, –4.5±0.9 kg in the self-directed intervention and –2.4±0.9 kg in the usual care group, corresponding to a weight change of –5.8%, –4.9% and –2.6%, respectively (Figure 1a). The percentage of participants who achieved the 7% DPP-based weight-loss goal at 24 months was 45% in the coach-led intervention (P=0.003) and 30% in the self-directed intervention (P=0.14) vs 17% in the usual care group (Figure 1b). Results remained unchanged in sensitivity analyses using study-measured weights only.
Figure 1.
Estimated mean (±s.e.) weight change (a) and categorical weight loss (b) in the intention-to-treat population.
Compared with usual care, significant improvements were maintained at 24 months for waist circumference and fasting plasma glucose level in the coach-led (P<0.001 for both) and self-directed interventions (P<0.001 and P=0.04, respectively), triglyceride to high-density lipoprotein ratio in the coach-led intervention (P=0.03) and total cholesterol level in the self-directed intervention (P=0.04).
Discussion
This extended study shows that the previously demonstrated effects of the two primary care-based, technology-enhanced DPP lifestyle interventions on weight loss and cardiometabolic risk factors persisted through 24 months after treatment initiation (21 months after completion of the intensive treatment phase and 9 months after completion of the maintenance phase). The literature on weight-loss therapies reveals that short-term weight loss is often more achievable than long-term maintenance.5, 6 In behavioral interventions, participants typically achieve maximal weight loss around 6 months after beginning treatment, but many regain 30–35% of their lost weight in the year following treatment. Continued coach contact and self-monitoring are among a small number of strategies that can effectively prevent or reduce weight regain.5, 6 However, their cost to implement (for example, staffing requirements, participant burden) poses significant access and adherence barriers. Technologies (for example, Internet, e-mail and mobile devices) may offer practical, affordable and scalable alternatives to traditional face-to-face only interventions. Evidence on the effectiveness of technology interventions for weight loss is limited and for weight-loss maintenance is even more limited.7 The current study usefully expands this literature, and the interventions have good reach and adoption potential.8 It is important to note, however, that 29% of the trial participants were lost to the extended follow-up through 24 months, and that the participants had narrow demographics (as described previously1). The findings warrant replication in long-term studies involving diverse populations.
Acknowledgments
We wish to thank the participants and their families who made this study possible. We also would like to acknowledge the Diabetes Prevention Support Center (DPSC) of the University of Pittsburgh for training and support in the Group Lifestyle Balance program; the E-LITE coach-led and self-directed interventions were derived from this material.
The original E-LITE trial was supported by grant R34DK080878 from the National Institute of Diabetes and Digestive and Kidney Diseases, and a Scientist Development Grant award (0830362N) from the American Heart Association. The extension was supported by grant R21HS019550 from the Agency for Healthcare Research and Quality. The Palo Alto Medical Foundation Research Institute also provided internal funding. Dr Lavori acknowledges support by the Clinical and Translational Science Award 1UL1 RR025744 for the Stanford Center for Clinical and Translational Education and Research (Spectrum) from the National Center for Research Resources. The content is solely the responsibility of the authors and does not necessarily represent the official views of any of the funding agencies. No sponsor or funding source had a role in the design or conduct of the study; collection, management, analysis or interpretation of the data; or preparation, review or approval of the manuscript.
The authors declare no conflict of interest.
References
- Ma J, Yank V, Xiao L, Lavori PW, Wilson SR, Rosas LG, et al. Translating the Diabetes Prevention Program Lifestyle Intervention for weight loss into primary care: a randomized trial. JAMA Intern Med. 2013;173:113–121. doi: 10.1001/2013.jamainternmed.987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer MK, Kriska AM, Venditti EM, Miller RG, Brooks MM, Burke LE, et al. Translating the Diabetes Prevention Program: a comprehensive model for prevention training and program delivery. Am J Prev Med. 2009;37:505–511. doi: 10.1016/j.amepre.2009.07.020. [DOI] [PubMed] [Google Scholar]
- Kramer MK, Kriska AM, Venditti EM, Semler LN, Miller RG, McDonald T, et al. A novel approach to diabetes prevention: evaluation of the Group Lifestyle Balance program delivered via DVD. Diabetes Res Clin Pract. 2010;90:e60–e63. doi: 10.1016/j.diabres.2010.08.013. [DOI] [PubMed] [Google Scholar]
- Ma J, King AC, Wilson SR, Xiao L, Stafford RS. Evaluation of lifestyle interventions to treat elevated cardiometabolic risk in primary care (E-LITE): a randomized controlled trial. BMC Fam Pract. 2009;10:71. doi: 10.1186/1471-2296-10-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeffery RW, Drewnowski A, Epstein LH, Stunkard AJ, Wilson GT, Wing RR, et al. Long-term maintenance of weight loss: current status. Health Psychol. 2000;19 (1 Suppl:5–16. doi: 10.1037/0278-6133.19.suppl1.5. [DOI] [PubMed] [Google Scholar]
- Turk MW, Yang K, Hravnak M, Sereika SM, Ewing LJ, Burke LE. Randomized clinical trials of weight loss maintenance: a review. J Cardiovasc Nurs. 2009;24:58–80. doi: 10.1097/01.JCN.0000317471.58048.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coons MJ, Demott A, Buscemi J, Duncan JM, Pellegrini CA, Steglitz J, et al. Technology interventions to curb obesity: a systematic review of the current literature. Curr Cardiovasc Risk Rep. 2012;6:120–134. doi: 10.1007/s12170-012-0222-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yank V, Stafford RS, Rosas LG, Ma J. Baseline reach and adoption characteristics in a randomized controlled trial of two weight loss interventions translated into primary care: A structured report of real-world applicability. Contemp Clin Trials. 2013;34:126–135. doi: 10.1016/j.cct.2012.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]