Abstract
Background:
Obesity has become an epidemic in many countries and is supporting a billion dollar industry involved in promoting weight loss through diet, exercise and surgical procedures. Because of difficulties in maintaining body weight reduction, a pattern of weight cycling often occurs (so called ‘yo-yo' dieting) that may result in deleterious outcomes to health. There is controversy about cardiovascular benefits of yo-yo dieting, and an animal model is needed to better understand the contributions of major diet and body weight changes on heart and vascular functions. Our purpose is to determine the effects of weight cycling on cardiac function and atherosclerosis development in a mouse model.
Methods:
We used low-density lipoprotein receptor-deficient mice due to their sensitivity to metabolic syndrome and cardiovascular diseases when fed high-fat diets. Alternating ad libitum feeding of high-fat and low-fat (rodent chow) diets was used to instigate weight cycling during a 29-week period. Glucose tolerance and insulin sensitivity tests were done at 22 and 24 weeks, echocardiograms at 25 weeks and atherosclerosis and plasma lipoproteins assessed at 29 weeks.
Results:
Mice subjected to weight cycling showed improvements in glucose homeostasis during the weight loss cycle. Weight-cycled mice showed a reduction in the severity of atherosclerosis as compared with high-fat diet-fed mice. However, atherosclerosis still persisted in weight-cycled mice as compared with mice fed rodent chow. Cardiac function was impaired in weight-cycled mice and matched with that of mice fed only the high-fat diet.
Conclusion:
This model provides an initial structure in which to begin detailed studies of diet, calorie restriction and surgical modifications on energy balance and metabolic diseases. This model also shows differential effects of yo-yo dieting on metabolic syndrome and cardiovascular diseases.
Keywords: atherosclerosis, cardiac function, mouse, body weight cycling
Introduction
Obesity is a major health risk worldwide as ∼60% of adults of 20 years of age or older are overweight or obese.1, 2, 3 There is also a billion dollar industry promoting weight loss through diet products, books and services as well as available surgical approaches. Although many obese individuals are able to lose significant weight initially, maintaining body weight reduction has been difficult for nearly 99% of dieting individuals.4, 5 A major issue is that a consequence of weight loss is the decreased in daily energy expenditure6 and an unrelenting desire to increase food intake, resulting in a return of excessive body weight. It is not uncommon that individuals repeat this ‘yo-yo' pattern of repeated loss and regain of body weight during many years of their adult lifetime.
The effects of weight cycling on health outcomes in humans are controversial.7, 8, 9, 10, 11, 12, 13, 14, 15, 16 Some studies find increased health risks associated with weight cycling, while others do not. A major drawback of these studies is the lack of consistency in the definition of weight cycling. Differences exist in the duration and extent of weight loss, gender, the reason for weight loss, as well as the number of weight cycles associated with the outcomes. To have a better control of variables affecting health outcomes, weight cycling has been extensively studied in animals.17, 18, 19, 20, 21, 22, 23 Most of these studies were focused on the effect of weight cycling on metabolism, rate of weight gain during cycling phases, aging, adipose inflammation and body composition. However, data on the effects of weight cycling on cardiovascular diseases are lacking.
Cardiovascular diseases such as atherosclerosis and heart failure are associated with obesity and insulin resistance.24, 25 Thus, improving insulin resistance and decreasing body weight are likely to improve these outcomes. To test these possibilities, we developed a mouse model of weight cycling that will also be useful for the identification of particular nutritional components, caloric restriction regimes and surgical modification on cardiovascular diseases. To our knowledge, no one has developed such a mouse model and here, we focus on atherosclerosis and cardiac function outcomes during a diet-controlled weight cycling regime. We utilized the low-density lipoprotein receptor-deficient (LDLR−/−) mouse strain as it is sensitive to nutritional alterations with respect to metabolic syndrome and cardiovascular disease and mimics human lipoprotein levels.26, 27
Body weight cycling was achieved by alternating two types of diets. We found that although mice subjected to ‘yo-yo' dieting showed greater atherosclerosis as compared with mice maintained on a low-fat rodent chow diet, the extent of disease was improved as compared with high-fat diet-fed mice. However, cardiac function was reduced in ‘yo-yo' diet animals and matched that of mice fed with the high-fat diet, suggesting that either the heart does not recover from exposure to the high-fat diet or that acute diet changes rapidly that influences heart function. Overall, this model provides an initial structure in which to begin detailed studies of diet, calorie restriction and surgical modifications on energy balance and metabolic diseases.
Materials and methods
Mice
Male LDLR−/− mice, primarily on a C57BL/6 background, were purchased from the Jackson Laboratory (Bar Harbor, ME, USA; strain 002207) and housed four per cage. At 12 weeks of age, the mice were randomly assigned to three diet groups: an accelerated weight gain group that was fed a diet containing high-fat/high-sucrose (HFHS), a weight cycling group (YOYO) and a group that consumed normal rodent chow throughout (CHOW). All the three groups were fed ad libitum. The group designated YOYO went through three weight cycles of weight gain and weight loss instituted by altering the HFHS for rodent chow diets. Cycle 1 lasted from 0–8 weeks with 4 weeks of HFHS diet feeding followed by 4 weeks of rodent chow. Cycle 2 was from 8–18 weeks with 5 weeks of HFHS diet feeding followed by 5 weeks of rodent chow. Cycle 3 had a weight gain period of 7 weeks to accommodate an additional testing followed by 4 weeks of rodent chow feeding. Weight loss was achieved by changing the diet from the HFHS (5.45 kcal g−1) to standard rodent chow (3.02 kcal g−1). The HFHS diet contained 35.5% fat (primarily lard), 20% protein and 36.6% carbohydrate (primarily sucrose) (no. F1850; Bioserve, Frenchtown, NJ, USA).
At necropsy, the mice were fasted for 4 h in the morning, bled from the retro-orbital sinus into tubes containing 1 mℳ EDTA, killed by cervical dislocation and the tissues were collected and stored at –80 °C until analyses. The mice were maintained in a specific pathogen-free animal facility at the University of Washington at 25 °C with a fixed 12-h light and dark cycle. All procedures were done in accordance with the current National Institutes of Health guidelines and approved by the Animal Care and Use Committee of the University of Washington.
Glucose and insulin tolerance tests
After a 6-h fast, the mice were intraperitoneally injected with 1 mg glucose per gram body weight (week 22) or injected intraperitoneally with 1 mU Humulin R insulin per kilogram body weight (week 24) (Elli Lilly, Indianapolis, IN, USA). Blood was collected by tail nick and glucose was monitored using a glucometer (OneTouch Ultra; LifeScan Inc., Milpitas, CA, USA) before and serially after glucose or insulin administration. Approximately 50 μl of blood was collected at the 30-min time point of the glucose tolerance test for plasma insulin measurement. Plasma insulin levels were measured using the Linco insulin ELISA (Millipore, St Charles, MO, USA; catalog EZRMI-13 K). HOMA-IR (homeostasis model assessment scores of insulin resistance) values were calculated from fasting glucose and insulin levels as: HOMA-IR=insulin (mU l−1) × glucose (mmol l−1)/22.5.28
Plasma lipid and lipoprotein analyses
Plasma total cholesterol levels were determined with a colorimetric kit (Diagnostic Chemicals Ltd, Oxford, CT, USA) with cholesterol standards (Sigma-Aldrich Corp., St Louis, MO, USA). Plasma triglyceride levels were determined calorimetrically after the removal of free glycerol (Roche Diagnostics, Indianapolis, IN, USA). Plasma lipoproteins were separated by high-resolution size-exclusion chromatography (Superose 6 column; Amersham Biosciences, Piscataway, NJ, USA). A 100-μl aliquot of plasma pooled from each diet group was separated at a flow rate of 0.2 ml per min with phosphate-buffered saline. Aliquots (100 μl) from each 0.5-ml fraction were used for cholesterol and triglyceride determinations.
Body composition analysis
Body composition was performed on conscious immobilized mice using quantitative magnetic resonance imaging at 24 weeks (EchoMRI 3-in-1 machine whole-body composition analyzer; Echo MRI, LLC., Houston, TX, USA).29 Body weights of mice were taken immediately before quantitative magnetic resonance imaging assessments. Percent body compositions were determined from the total sum of lean, fat and water mass.
Quantification of atherosclerosis
Atherosclerosis was evaluated by analyzing serial sections at the aortic root and by en face analysis of the aorta. Lesion sizes were quantified in the aortic root essentially as described.30 In brief, the upper sections of the hearts were fixed overnight in 10% neutral-buffered formalin and embedded in paraffin the following day. Every other section (5-μm thick) through the aortic root was taken for analyses. Aortic root lesion area was quantified, beginning at the termination of the aortic valve and spanning 400 μm of the ascending aorta. A subset of five sections from each animal spanning the region was stained with Movat's pentachrome stain and photographed. Lesions were quantified using ImageJ software (National Institutes of Health; http://rsbweb.nih.gov/ij/). For en face analyses, the thoracic and abdominal aortas were cut longitudinally, pinned flat on black wax and photographed. Total surface areas and lesion areas were quantified using Image J software.
Cardiac function/echocardiography
At 25 weeks, the animals on different diet cycles underwent echocardiography to measure the chamber dimensions and fractional shortening as previously described.31, 32 In brief, the animals were lightly sedated using 0.5–1% inhaled isoflurane, and echocardiographic images were obtained in parasternal long axis and short axis views to acquire M-mode images to measure the left ventricular end-diastolic (LVEDD) and end-systolic (LVESD) dimensions.31, 32, 33 Data were averaged from three to five cardiac cycles. Fractional shortening was then calculated using the following equation fractional shortening%=(LVEDDave−LVESDave)/LVEDDave × 100%. Ejection fraction was determined by tracing the endocardial borders of the left ventricle at end-diastole and at end-systole of the parasternal long axis view to calculate the volume using modified Simpson method of discs. All measurements were made by a single-blinded reader and are in accordance with guidelines approved by the American Society of Echocardiography.
Statistical analysis
Data are presented as mean±s.e. and statistical significance was established at P<0.05. Two-tailed t-tests were done using the Prism statistical programming package (La Jolla, CA, USA) and two-way ANOVA was used to evaluate main effects and interactions involving genotype and diet status.
Results
Weight cycling and body composition
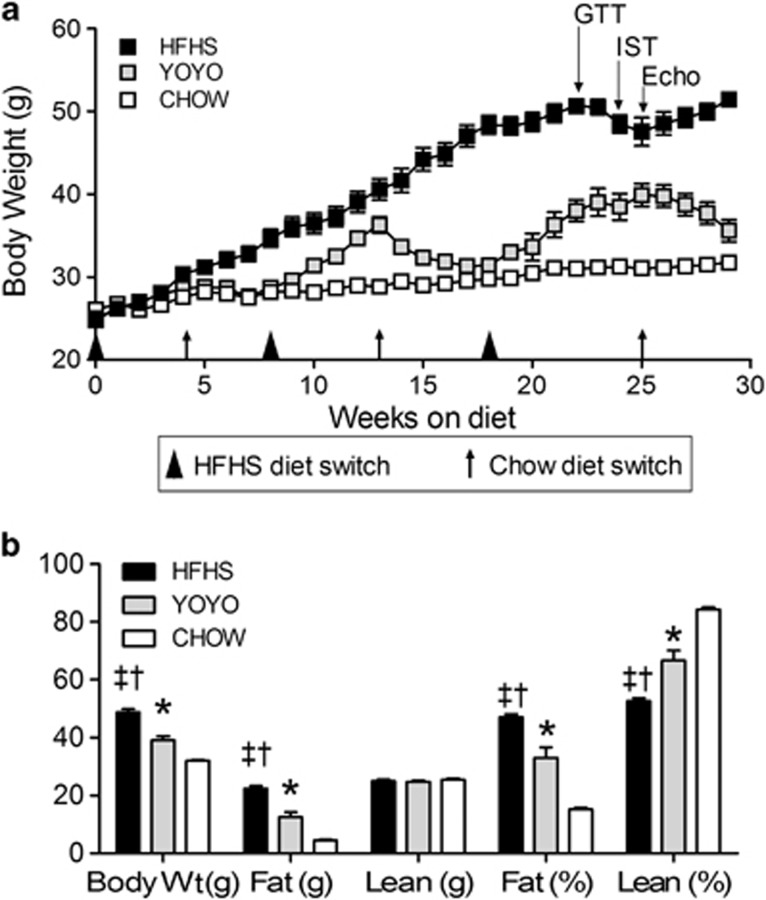
LDLR−/− mice were chosen for study due to their positive responses to high-fat diets with respect to body weight, insulin resistance, hyperlipidemia and atherosclerosis.26, 27 These mice are on the C57BL/6 background. Mice fed only rodent chow (CHOW) showed little weight gain (25–32 g; 28%), representing basal animal growth (Figure 1a). Mice maintained on the high-fat diet (HFHS) doubled their initial weight (25 g) to 51 g by 30 weeks (200% weight gain) as seen previously.26 The mice in the YOYO group showed robust fluctuations in body weight. For each of the three cycles, mice gained ∼20% of their starting cycle weight (0–4 weeks, 15.4±5.1% 8–13 weeks, 26.6±9.03% and 18–25 weeks, 26.9±9.1%). The amounts of weight gained were greater than weight losses (4–8 weeks, 0.5% 13–18 weeks, 13.2% and 25–29 weeks, 10.7%). Overall, three cycles of weight gain and reduction were achieved for the YOYO group during 29 weeks of study.
Figure 1.
Body weight and body composition. Mice were divided into three groups and either maintained on rodent chow (CHOW), a high-fat-high sucrose diet (HFHS) or fed alternately between the two diets (YOYO). Diet compositions are given in the text. (a) Body weight for mice fed test diets for a duration of 29 weeks. (b) Body composition in terms of actual (g) and relative (%) body weight and fat and lean mass at necropsy (29 weeks) as described in the text. For all panels, n=4–8 per group and data are presented as mean±s.e.m.; *P<0.05 between CHOW and YOYO groups; †P<0.05 between CHOW and HFHS; ‡P<0.05 between HFHS and YOYO.
Body compositions were determined using quantitative magnetic resonance imaging (Figure 1b) at week 24. Body weights between the groups were significantly different primarily due to the absolute levels (g) of body fat (Figure 1b). At this time point, the HFHS group showed significantly more fat mass (23.4±0.9 g) as compared with both the YOYO (12.4±2.1 g; P<0.002) and CHOW groups (4.8±0.2 g; P<0.0000002). No significant differences in absolute lean mass (g) were seen between the groups.
Weight cycling partially improved glucose intolerance
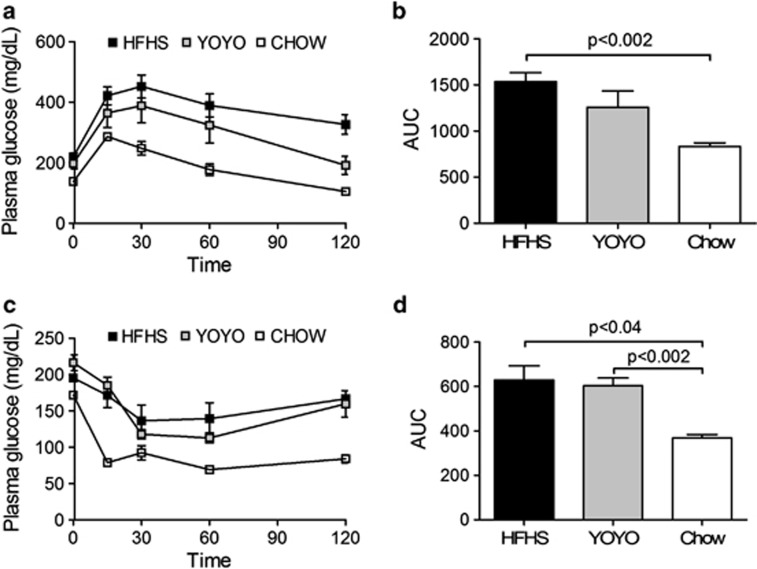
Glucose homeostasis was assessed by intraperitoneal glucose tolerance and insulin sensitivity tests6 during weeks 22 and 24, respectively (Figure 2a). For this time point, YOYO mice showed body weights intermediate between HFHS and CHOW mice. Initial glucose excursions (0–15 min) showed no differences between the groups, suggesting equivalent pancreatic beta cell release of insulin. The primary return to initial glucose phase (30–120 min) was faster for CHOW mice as compared with HFHS and YOYO diet groups, and this was also reflected in the overall area-under-the-curve (Figure 2b). For insulin sensitivity (Figure 2c), CHOW mice exhibited markedly reduced blood glucose levels as compared with the other groups, demonstrating increased insulin sensitivity, consistent with the intraperitoneal glucose tolerance test data. The response of the YOYO group to insulin injection demonstrated reduced insulin sensitivity as compared with the CHOW group (Figures 2c and d), likely owing to this time point being near the peak of the cycle 3 weight gain.
Figure 2.
Glucose homeostasis for HFHS, YOYO and CHOW groups of mice. (a) Interperitoneal glucose tolerance test (IPGTT) performed at 22 weeks of diet feeding and (b) data are compared as area under the curve (AUC) for the IPGTT results. (c) Insulin sensitivity test (IST) performed at 24 weeks of diet feeding and (d) IST results presented as AUC. Blood glucose levels were measured after a 4-h fast (n=4–8 per group) and data are presented as mean±s.e.m. Significant differences between groups are shown in the AUC curves.
At 29 weeks, HFHS mice showed robust metabolic syndrome traits, including insulin resistance and hyperlipidemia as compared with YOYO and CHOW mice (Table 1). At this time point, YOYO mice had been fed the rodent chow diet and showed body weight comparable to CHOW mice (Figure 1a). Plasma glucose and insulin levels were significantly elevated for HFHS mice as compared with the other groups. The ratio of glucose to insulin was reduced threefold in HFHS mice as compared with CHOW mice, and half the value as seen for the YOYO group, further indicating glucose intolerance for HFHS mice. The HOMA-IR also showed a marked insulin resistance phenotype for HFHS mice, which was fourfold greater as compared with YOYO and CHOW animals. However, YOYO mice did not completely regain insulin sensitivity as evidenced by a twofold elevation in HOMA as compared with CHOW mice.
Table 1. Plasma lipids, glucose and insulin levels, as well as assessments of insulin resistance (glucose/insulin and HOMA-IR).
| Traits | HFHS | YOYO | CHOW |
|---|---|---|---|
| Glucose (mg dl−1) | 187±6.4*,# | 151±12.0 | 122±3.9 |
| Insulin (ng ml−1) | 6.7±1.5*,# | 2.2±0.4 | 1.3±0.1 |
| G/I ratio | 36.1±7.5*,# | 75.7±12.5 | 97.6±13.0 |
| HOMA | 5.32±0.04*,# | 1.41±0.02 | 0.67±0.01 |
| Total cholesterol (mg dl−1) | 741±16.1*,# | 369±13.4† | 310±26.2 |
| Triglyceride (mg dl−1) | 262±29.6*,# | 105±7.3 | 98±13.4 |
Abbreviations: HFHS, high-fat/high-sucrose; HOMA-IR, homeostasis model assessment scores of insulin resistance.
Plasma was taken from mice fasted for 4 h in the morning at time of necropsy (29 weeks).
*P<0.05 between HFHS and YOYO.
#P<0.05 between HFHS and CHOW.
†P<0.05 between YOYO and CHOW.
Data are presented as mean±s.e.m.
Weight cycling improved plasma lipoproteins
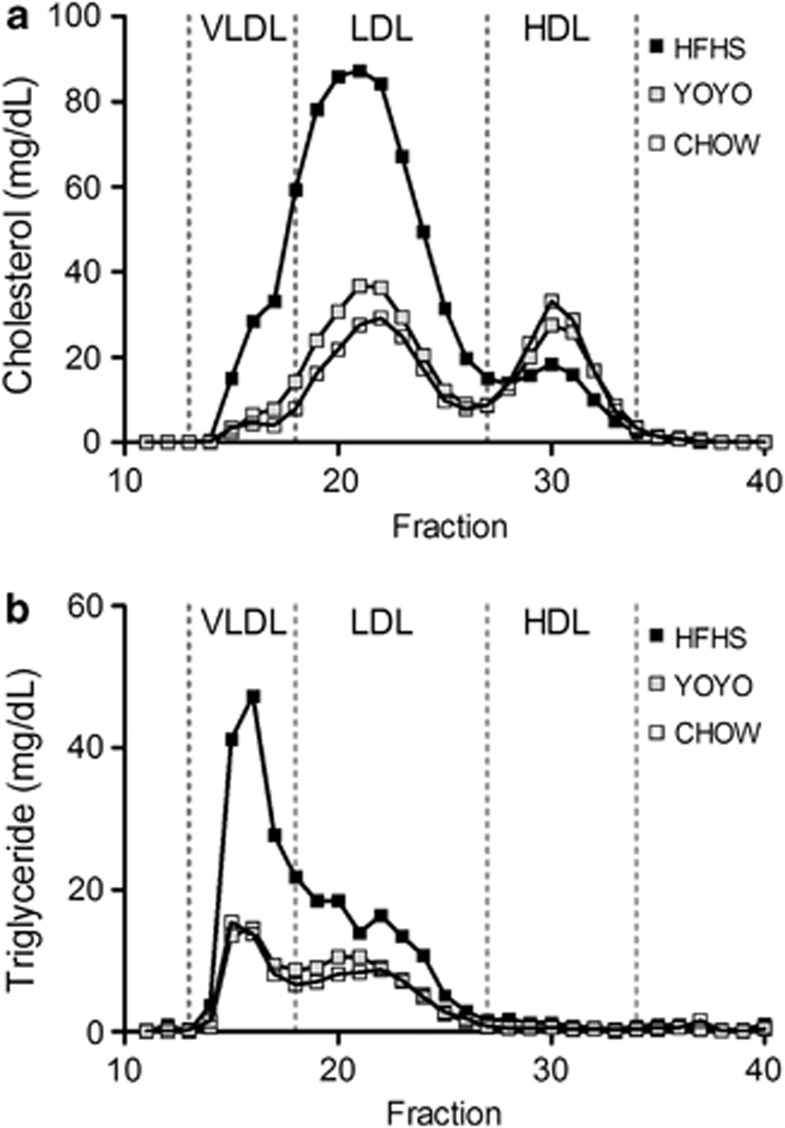
Plasma lipid parameters were evaluated only at the end of the study in order to avoid animal handling, which may interfere with body weight and physiological measurements of the heart and glucose homeostasis done at earlier time points. Plasma total cholesterol and triglyceride levels were twofold elevated for HFHS-fed mice as compared with YOYO and CHOW mice. Plasma total cholesterol levels were modestly but significantly higher for YOYO than CHOW mice. These results suggest that at this time, the YOYO group had not completely returned to the same metabolic state as CHOW mice.
Hyperlipidemia for HFHS mice was associated with a pro-atherogenic lipoprotein profile as assessed by fast performance liquid chromatography with elevated very-low-density lipoprotein and LDL levels and reduced high-density lipoprotein levels (Figures 3a and b) as compared with CHOW mice. Part of the LDL fraction was also enriched in triglycerides (Figure 3b). Interestingly, the elevation in total cholesterol seen for YOYO as compared with CHOW mice was due to higher LDL levels (Figure 3a). Overall, chronic feeding of the high-fat diet led to markedly elevated plasma lipids and a deleterious lipoprotein profile. Although not tested here due to experimental constraints, it is likely that YOYO mice also experienced durations of hyperlipidemia which were ameliorated during the weight loss phase of their feeding cycles.
Figure 3.
Plasma lipoprotein profiles for HFHS, YOYO and CHOW groups of mice. Plasma was taken following a 4-h fast at necropsy (29 weeks of diet feeding) and processed as described in the text. Fast performance liquid chromatography (FPLC) was performed as described in the text. (a) HFHS feeding resulted in an elevated very-low-density lipoprotein (VLDL) and LDL cholesterol, with a reduction in high-density lipoprotein (HDL) cholesterol as compared with YOYO and CHOW animals. (b) VLDL triglyceride levels were elevated in HFHS mice as compared with the other groups.
Weight cycling partially protects from atherosclerosis but not cardiac function
Variable effects of weight cycling on cardiovascular morbidity, including hypertension and heart failure have been observed in humans.9, 14, 34, 35 In animals, a few studies have assessed blood pressure and heart structure, showing that weight cycling in young animals can alter heart structure, but does not markedly influence blood pressure.36, 37 To add to this somewhat scarce data on heart disease outcomes, we examined two cardiovascular outcomes. First, we measured cardiac function in vivo using echocardiography just before the end of study. Second, we measured the development of atherosclerotic plaques in the aortic root and aorta histologically.
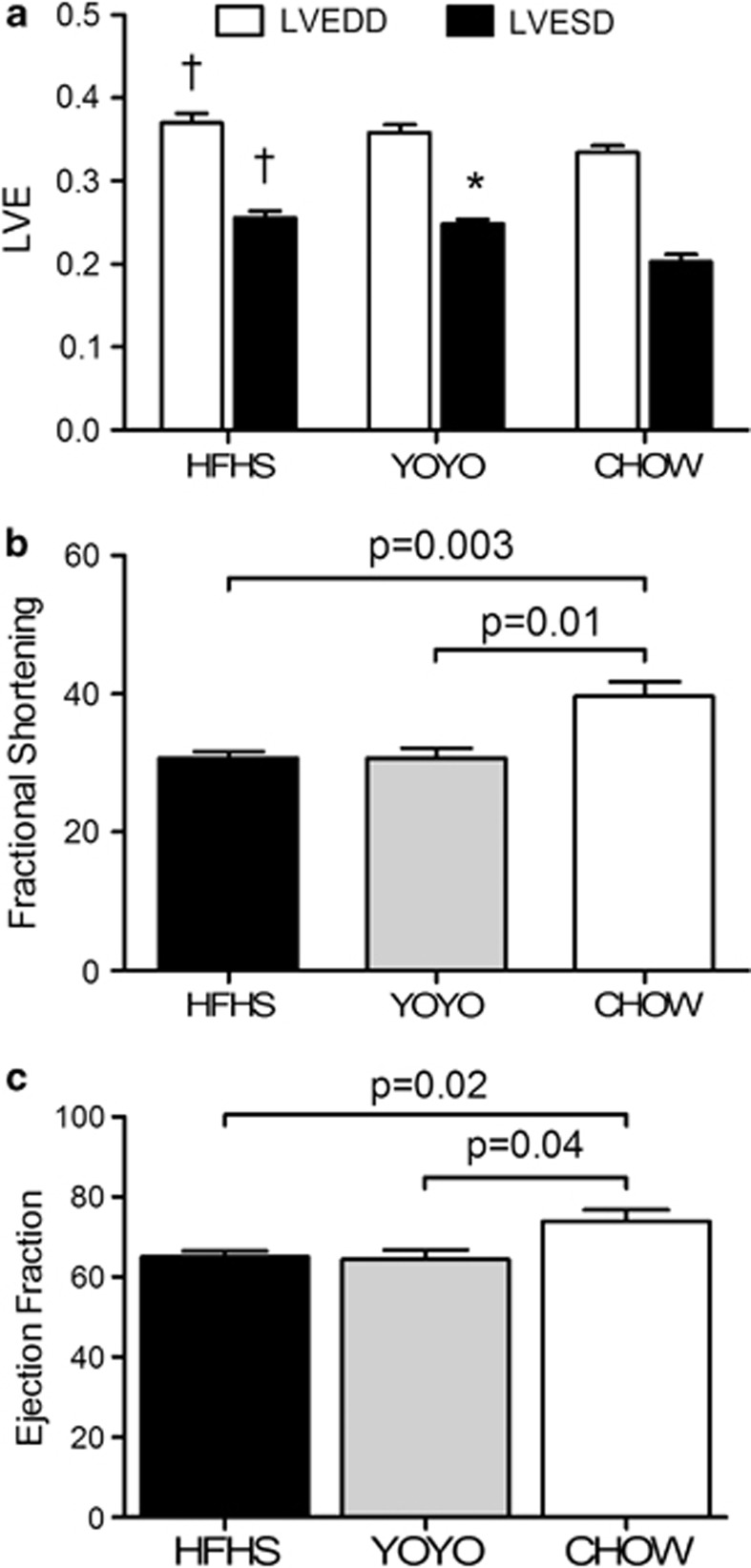
At week 25, cardiac function was assessed by echocardiography. Both groups exposed to the high-fat diet exhibited an impaired cardiac function to a similar degree (Figures 4a–c). LVED dimension was significantly (HFHS, 0.37±0.01; P<05) and modestly (YOYO) larger than for CHOW mice (Figure 4a). LVED dimension was also significantly larger for the two groups exposed to high-fat diets versus CHOW mice (P<0.05). The HFHS and YOYO groups also had significantly reduced fractional shortening and ejection fraction as compared with the chow group (Figures 4b and c). Thus, heart function is impaired by feeding the high-fat diet and if YOYO mice had improvements in heart function during the weight loss cycle, this was not retained upon re-feeding the high-fat diet.
Figure 4.
Cardiac function measures for HFHS, YOYO and CHOW mice. (a) Systolic and diastolic left ventricular end diameters, (b) fractional shortening and (c) ejection fraction (EF) are shown for mice at 26 weeks of diet feeding. N=4–8 per group and significant changes are shown in the figure. Data are presented as mean±s.e.m. For (a), *P<0.05 between YOYO and CHOW and †P<0.05 between HFHS and CHOW.
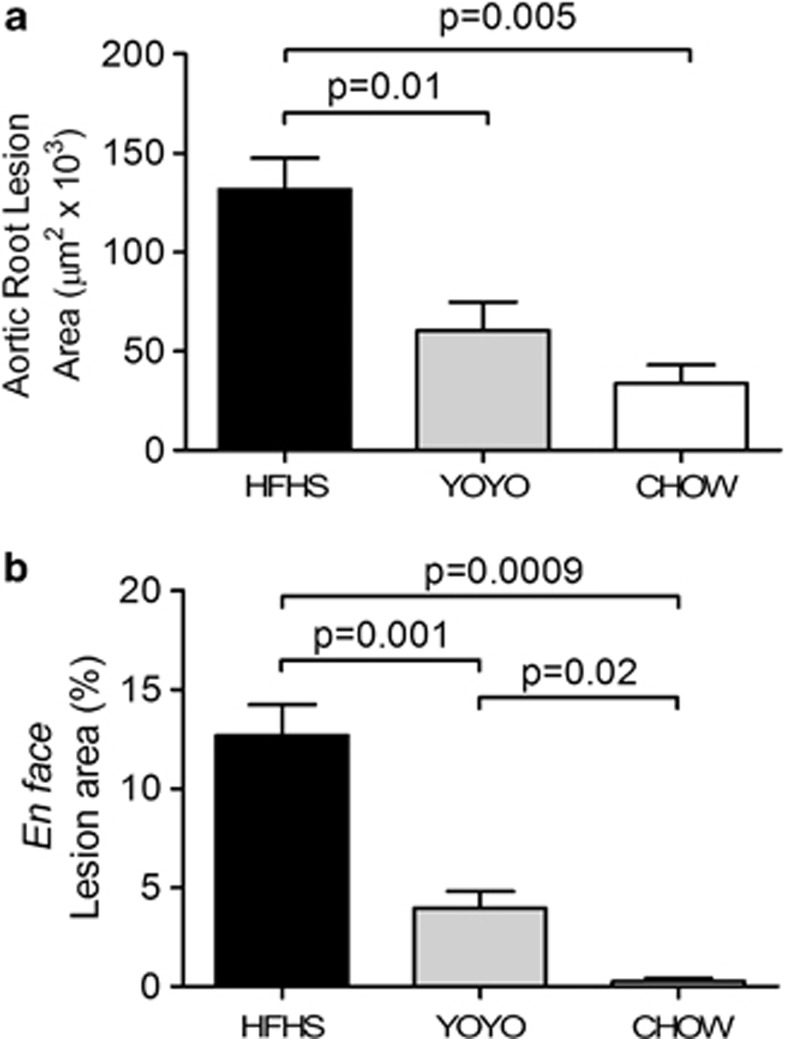
In contrast to heart function, weight cycling had a protective effect on atherosclerotic lesion development. Mice consuming the HFHS diet developed atherosclerotic lesions in the aortic root that were 65% larger than the lesions seen in YOYO mice and 74% larger than the lesions seen in the mice consuming the chow diet (Figure 5a, P<0.004). Lesion load in the aortic root was not statistically different between the YOYO and CHOW groups. Lesion development in the aortic arch and descending aorta as evaluated by en face methods showed significant differences among all the three groups, with CHOW mice showing the lowest and HFHS groups showing the highest extent of lesion areas (Figure 5b).
Figure 5.
Atherosclerosis quantification for the three groups of mice. (a) Hearts were collected at necropsy and processed for quantification of aortic root atherosclerosis as described in the text. (b) Aortas (thoracic and abdominal) were cleaned of adventitial tissue and processed for en face quantification of lesion sizes as described in the text. N=4–8 and *P<0.05 between groups. Data are presented as mean±s.e.m.
Overall, cycling between high- and low-fat diets resulted in an improvement, but not amelioration, of atherosclerosis as compared with mice fed HFHS chronically. In contrast, cardiac function was not improved, although this could be due to the fact that these measures were taken while YOYO animals were in the high-fat diet phase of their cycle. Further work to repeat such measurements at other time points are in progress.
Discussion
This is the first report directly testing whether weight cycling adversely affects cardiovascular disease in mice. Mice subjected to weight cycling showed significant improvement in atherosclerotic load relative to mice allowed ad libitum access to a high-fat diet throughout the study. However, weight cycling failed to fully protect the mice from atherosclerosis. Further, HFHS mice had a significant reduction in cardiac function compared with chow-fed mice, and weight cycling offered no lasting protection from this phenotype.
Weight cycling is a common occurrence in this country due to the high incidence of obesity and the desire for weight reduction due to health and appearance issues. Although there is controversy in the literature about whether or not yo-yo dieting can lead to problems involving gall bladder and liver,15, 38 data are overall supportive that weight loss decreases risk factors associated with major diseases such as cancer.39 With respect to cardiovascular diseases, there is still controversy based on the idea that weight cycling, particularly in younger individuals, may result in fluctuations of heart rate, blood pressure, sympathetic activity, glomerular filtration rates and circulating glucose and lipid levels that may alter heart development and/or cause lipotoxic events influencing cardiac function.40, 41, 42 Thus, maintenance of weight loss remains an important problem and setting up an animal model in which we can eventually study the central and peripheral factors affecting appetite and energy expenditure as well as identify factors benefiting cardiovascular health is a first step toward delineating therapeutic treatments. We have shown that the LDLR−/− mouse model will provide a potentially important model for subsequent detail studies of the consequences of yo-yo dieting, with emphasis on cardiovascular outcomes.
We also demonstrated that for both atherosclerosis and cardiac function, the best outcome was seen for animals never exposed to the high-fat diet (CHOW group). It is known that hypercholesterolemia in mice aggravates atherosclerosis.43, 44 By the end of this study, the mice from the HFHS group reached plasma total cholesterol levels of >700 mg dl−1 and it is likely that cholesterol levels were high throughout the duration of study explaining their extensive lesion development, as seen previously.45 Given that the YOYO group was fed the high-fat diet for approximately half the study duration, we expected YOYO mice to have about half the lesion load as HFHS mice. Indeed, this was seen for aortic root lesion areas but not for the en face areas. It is known that atherosclerosis is initiated in the aortic root in both humans and mice with disease progressing outward through the aortic arch and descending aorta.46, 47 Thus, it may be that the rate of overall lesion development through the aorta was reduced in YOYO mice as compared with HFHS animals. In future studies, plasma lipids and lesion sizes will be monitored frequently to examine lesion initiation and development.
Two recent weight cycling studies in mice also monitored the features of insulin resistance and circulating lipid levels. Weight cycling in wild-type C57BL/6 mice was used to test the effects on lifespan.22 Lifespan was not reduced in weight-cycled mice between a high-fat (60% of energy from fat) versus low-fat control diet. For weight-cycled mice, the extent of glucose intolerance and levels of several hormones (insulin-like growth factor, leptin, resistin and gastric inhibitory polypeptide) were highest during the high-fat phase of the cycle. Cycled mice in this study gained ∼20% of total body weight during the high-fat feeding portion of the cycle as seen in our study. No differences in lean mass were seen between groups, thus agreeing with our findings. A second report addressed the issue of adipose tissue inflammation using three cycles between a high-fat and rodent chow-based low-fat diet during 6 months.17 Adipose tissue inflammation is elevated by diet-induced obesity.48 This study provided detailed analyses of rates of weight gain and losses, glucose metabolism, serum lipid levels, adipocyte morphology and adipose tissue inflammatory marker expression. Among the findings was that several inflammatory markers (adiponectin, interleukin-6) did not recuperate following the weight loss cycle phases. These are helpful studies and further demonstrate that weight cycling regimes utilizing high and low-fat diets do provide paradigms to robustly alter glucose and lipid metabolism.
There are limitations to our studies. Animals were not pair fed and diets were not well matched for total calories or nutrient content, but reflect extreme high versus low-fat diets used by humans. However, we did establish that this mouse strain was amenable to robust changes in body weight with diet changes and further studies are planned to explore individual nutrients and energy balance issues. In addition, additional time points will be needed during our study in order to follow changes in cardiac function. Acute administration of particular high-fat diets has been shown to influence cardiac function,49, 50, 51 and further studies are needed to monitor heart function during multiple phases of weight cycling to determine whether transient high-fat feeding leads to chronic heart dysfunction.
Acknowledgments
This study was funded by the National Institutes of Health RO1 HL098227 (R.C.L.). We thank the Mouse Metabolic Phenotyping Center (University of Washington, Seattle, WA) for help with body composition measurements, especially the help of Dr Kayoko Ogimoto.
The authors declare no conflict of interest.
References
- Kelly T, Yang W, Chen CS, Reynolds K, He J. Global burden of obesity in 2005 and projections to 2030. Int J Obes. 2008;32:1431–1437. doi: 10.1038/ijo.2008.102. [DOI] [PubMed] [Google Scholar]
- Organization WH. Global Health Risks: Mortality and Burden of Disease Attributable to Selected Major Risks. World Health Organization: Geneva, Switzerland; 2009. [Google Scholar]
- Malik VS, Willett WC, Hu FB. Global obesity: trends, risk factors and policy implications. Nat Rev Endocrinol. 2013;9:13–27. doi: 10.1038/nrendo.2012.199. [DOI] [PubMed] [Google Scholar]
- Franz MJ, VanWormer JJ, Crain AL, Boucher JL, Histon T, Caplan W, et al. NP: Weight-loss outcomes: a systematic review and meta-analysis of weight-loss clinical trials with a minimum 1-year follow-up. JAm Diet Assoc. 2007;107:1755–1767. doi: 10.1016/j.jada.2007.07.017. [DOI] [PubMed] [Google Scholar]
- Witkamp RF. Current and future drug targets in weight management. Pharma Res. 2011;28:1792–1818. doi: 10.1007/s11095-010-0341-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Froidevaux F, Schutz Y, Christin L, Jequier E. Energy expenditure in obese women before and during weight loss, after refeeding, and in the weight-relapse period. Am J Clin Nutr. 1993;57:35–42. doi: 10.1093/ajcn/57.1.35. [DOI] [PubMed] [Google Scholar]
- Cereda E, Malavazos AE, Caccialanza R, Rondanelli M, Fatati G, Barichella M. Weight cycling is associated with body weight excess and abdominal fat accumulation: a cross-sectional study. Clin Nutr. 2011;30:718–723. doi: 10.1016/j.clnu.2011.06.009. [DOI] [PubMed] [Google Scholar]
- Field AE, Byers T, Hunter DJ, Laird NM, Manson JE, Williamson DF, et al. Weight cycling, weight gain, and risk of hypertension in women. Am J Epidemiol. 1999;150:573–579. doi: 10.1093/oxfordjournals.aje.a010055. [DOI] [PubMed] [Google Scholar]
- Field AE, Malspeis S, Willett WC. Weight cycling and mortality among middle-aged or older women. Arch Intern Med. 2009;169:881–886. doi: 10.1001/archinternmed.2009.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooper LE, Foster-Schubert KE, Weigle DS, Sorensen B, Ulrich CM, McTiernan A. Frequent intentional weight loss is associated with higher ghrelin and lower glucose and androgen levels in postmenopausal women. Nutr Res. 2010;30:163–170. doi: 10.1016/j.nutres.2010.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kajioka T, Tsuzuku S, Shimokata H, Sato Y. Effects of intentional weight cycling on non-obese young women. Metabolism. 2002;51:149–154. doi: 10.1053/meta.2002.29976. [DOI] [PubMed] [Google Scholar]
- Lee JS, Visser M, Tylavsky FA, Kritchevsky SB, Schwartz AV, Sahyoun N, et al. Weight loss and regain and effects on body composition: the Health, Aging, and Body Composition Study. J Gerontol A Biol Sci Med Sci. 2010;65:78–83. doi: 10.1093/gerona/glp042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lissner L, Odell PM, D'Agostino RB, Stokes J, 3rd, Kreger BE, Belanger AJ, et al. Variability of body weight and health outcomes in the Framingham population. N Engl J Med. 1991;324:1839–1844. doi: 10.1056/NEJM199106273242602. [DOI] [PubMed] [Google Scholar]
- Schulz M, Liese AD, Boeing H, Cunningham JE, Moore CG, Kroke A. Associations of short-term weight changes and weight cycling with incidence of essential hypertension in the EPIC-Potsdam Study. J Hum Hypertens. 2005;19:61–67. doi: 10.1038/sj.jhh.1001776. [DOI] [PubMed] [Google Scholar]
- Wakui S, Odagiri Y, Takamiya T, Inoue S, Kato R, Ohya Y, et al. Relation between self-reported weight cycling history, dieting and bio-behavioral health in Japanese adult males. Environ Health Prev Med. 2002;6:248–255. doi: 10.1007/BF02897977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wannamethee SG, Shaper AG, Walker M. Weight change, weight fluctuation, and mortality. ArchIntern Med. 2002;162:2575–2580. doi: 10.1001/archinte.162.22.2575. [DOI] [PubMed] [Google Scholar]
- Barbosa-da-Silva S, Fraulob-Aquino JC, Lopes JR, Mandarim-de-Lacerda CA, Aguila MB. Weight cycling enhances adipose tissue inflammatory responses in male mice. PloS One. 2012;7:e39837. doi: 10.1371/journal.pone.0039837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brownell KD, Greenwood MR, Stellar E, Shrager EE. The effects of repeated cycles of weight loss and regain in rats. Physiol Behav. 1986;38:459–464. doi: 10.1016/0031-9384(86)90411-7. [DOI] [PubMed] [Google Scholar]
- Cleary MP. Consequences of restricted feeding/refeeding cycles in lean and obese female Zucker rats. J Nutr. 1986;116:290–303. doi: 10.1093/jn/116.2.290. [DOI] [PubMed] [Google Scholar]
- Gray DS, Fisler JS, Bray GA. Effects of repeated weight loss and regain on body composition in obese rats. Am J Clin Nutr. 1988;47:393–399. doi: 10.1093/ajcn/47.3.393. [DOI] [PubMed] [Google Scholar]
- Jen KL, Lu H, Savona L, Watkins A, Shaw M. Long-term weight cycling reduces body weight and fat free mass, but not fat mass in female Wistar rats. Int J Obes Relat Metab Disord. 1995;19:699–708. [PubMed] [Google Scholar]
- List EO, Berryman DE, Wright-Piekarski J, Jara A, Funk K, Kopchick JJ.The effects of weight cycling on lifespan in male C57BL/6J mice Int J Obese-pub ahead of print 11 December 2012; doi: 10.1038/ijo.2012.203 [DOI] [PMC free article] [PubMed]
- Rozen R, Brigant L, Apfelbaum M. Effects of cycles of food restriction followed by ad libitum refeeding on body composition and energy expenditure in obese rats. Am J Clin Nutr. 1994;59:560–565. doi: 10.1093/ajcn/59.3.560. [DOI] [PubMed] [Google Scholar]
- Blecker S, Herbert R, Brancati FL. Comorbid diabetes and end-of-life expenditures among Medicare beneficiaries with heart failure. J Card Fail. 2012;18:41–46. doi: 10.1016/j.cardfail.2011.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingelsson E, Sullivan LM, Murabito JM, Fox CS, Benjamin EJ, Polak JF, et al. Prevalence and prognostic impact of subclinical cardiovascular disease in individuals with the metabolic syndrome and diabetes. Diabetes. 2007;56:1718–1726. doi: 10.2337/db07-0078. [DOI] [PubMed] [Google Scholar]
- Schreyer SA, Vick C, Lystig TC, Mystkowski P, LeBoeuf RC. LDL receptor but not apolipoprotein E deficiency increases diet-induced obesity and diabetes in mice. Am J Physiol Endocrinol Metab. 2002;282:E207–E214. doi: 10.1152/ajpendo.2002.282.1.E207. [DOI] [PubMed] [Google Scholar]
- Towler DA, Bidder M, Latifi T, Coleman T, Semenkovich CF. Diet-induced diabetes activates an osteogenic gene regulatory program in the aortas of low density lipoprotein receptor-deficient mice. J Biol Chem. 1998;273:30427–30434. doi: 10.1074/jbc.273.46.30427. [DOI] [PubMed] [Google Scholar]
- Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- Tinsley FC, Taicher GZ, Heiman ML. Evaluation of a quantitative magnetic resonance method for mouse whole body composition analysis. Obesity Res. 2004;12:150–160. doi: 10.1038/oby.2004.20. [DOI] [PubMed] [Google Scholar]
- Kunjathoor VV, Wilson DL, LeBoeuf RC. Increased atherosclerosis in streptozotocin-induced diabetic mice. J Clin Invest. 1996;97:1767–1773. doi: 10.1172/JCI118604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregorevic P, Allen JM, Minami E, Blankinship MJ, Haraguchi M, Meuse L, et al. rAAV6-microdystrophin preserves muscle function and extends lifespan in severely dystrophic mice. Nat Med. 2006;12:787–789. doi: 10.1038/nm1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minami E, Castellani C, Malchodi L, Deem J, Bertko K, Meznarich J, et al. The role of macrophage-derived urokinase plasminogen activator in myocardial infarct repair: urokinase attenuates ventricular remodeling. J Mol Cell Cardiol. 2010;49:516–524. doi: 10.1016/j.yjmcc.2010.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins K, O'Cathain A. The continuum of patient satisfaction—from satisfied to very satisfied. Soc Sci Med. 2003;57:2465–2470. doi: 10.1016/s0277-9536(03)00098-4. [DOI] [PubMed] [Google Scholar]
- Lavie CJ, Milani RV, Ventura HO. Obesity and cardiovascular disease: risk factor, paradox, and impact of weight loss. J Am Coll Cardiol. 2009;53:1925–1932. doi: 10.1016/j.jacc.2008.12.068. [DOI] [PubMed] [Google Scholar]
- Allison DB, Zannolli R, Faith MS, Heo M, Pietrobelli A, VanItallie TB, et al. Weight loss increases and fat loss decreases all-cause mortality rate: results from two independent cohort studies. Int J Obes Relat Metab Disord. 1999;23:603–611. doi: 10.1038/sj.ijo.0800875. [DOI] [PubMed] [Google Scholar]
- Contreras RJ, Williams VL. Dietary obesity and weight cycling: effects on blood pressure and heart rate in rats. Am J Physiol. 1989;256:R1209–R1219. doi: 10.1152/ajpregu.1989.256.6.R1209. [DOI] [PubMed] [Google Scholar]
- Ernsberger P, Koletsky RJ, Baskin JS, Collins LA. Consequences of weight cycling in obese spontaneously hypertensive rats. Am J Physiol. 1996;270:R864–R872. doi: 10.1152/ajpregu.1996.270.4.R864. [DOI] [PubMed] [Google Scholar]
- Tsai CJ, Leitzmann MF, Willett WC, Giovannucci EL. Weight cycling and risk of gallstone disease in men. Arch Intern Med. 2006;166:2369–2374. doi: 10.1001/archinte.166.21.2369. [DOI] [PubMed] [Google Scholar]
- Thompson HJ, McTiernan A. Weight cycling and cancer: weighing the evidence of intermittent caloric restriction and cancer risk. Cancer Prev Res. 2011;4:1736–1742. doi: 10.1158/1940-6207.CAPR-11-0133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin CH, Kurup S, Herrero P, Schechtman KB, Eagon JC, Klein S, et al. Myocardial oxygen consumption change predicts left ventricular relaxation improvement in obese humans after weight loss. Obesity. 2011;19:1804–1812. doi: 10.1038/oby.2011.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montani JP, Viecelli AK, Prevot A, Dulloo AG. Weight cycling during growth and beyond as a risk factor for later cardiovascular diseases: the 'repeated overshoot' theory. Int J Obes. 2006;30 (Suppl 4:S58–S66. doi: 10.1038/sj.ijo.0803520. [DOI] [PubMed] [Google Scholar]
- Rider OJ, Cox P, Tyler D, Clarke K, Neubauer S.Myocardial substrate metabolism in obesity Int J Obese-pub ahead of print 16 October 2012; doi: 10.1038/ijo.2012.170 [DOI] [PubMed]
- Daugherty A, Lu H, Howatt DA, Rateri DL. Modes of defining atherosclerosis in mouse models: relative merits and evolving standards. Methods Mol Biol. 2009;573:1–15. doi: 10.1007/978-1-60761-247-6_1. [DOI] [PubMed] [Google Scholar]
- Getz GS, Reardon CA. Diet and murine atherosclerosis. Arterioscler Thromb Vasc Biol. 2006;26:242–249. doi: 10.1161/01.ATV.0000201071.49029.17. [DOI] [PubMed] [Google Scholar]
- Schreyer SA, Lystig TC, Vick CM, LeBoeuf RC. Mice deficient in apolipoprotein E but not LDL receptors are resistant to accelerated atherosclerosis associated with obesity. Atherosclerosis. 2003;171:49–55. doi: 10.1016/j.atherosclerosis.2003.07.010. [DOI] [PubMed] [Google Scholar]
- McGill HC, Jr., McMahan CA, Zieske AW, Tracy RE, Malcom GT, Herderick EE, et al. Association of Coronary Heart Disease Risk Factors with microscopic qualities of coronary atherosclerosis in youth. Circulation. 2000;102:374–379. doi: 10.1161/01.cir.102.4.374. [DOI] [PubMed] [Google Scholar]
- VanderLaan PA, Reardon CA, Getz GS. Site specificity of atherosclerosis: site-selective responses to atherosclerotic modulators. Arterioscler Thromb Vasc Biol. 2004;24:12–22. doi: 10.1161/01.ATV.0000105054.43931.f0. [DOI] [PubMed] [Google Scholar]
- Lumeng CN, Saltiel AR. Inflammatory links between obesity and metabolic disease. J Clin Invest. 2011;121:2111–2117. doi: 10.1172/JCI57132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harmancey R, Wilson CR, Wright NR, Taegtmeyer H. Western diet changes cardiac acyl-CoA composition in obese rats: a potential role for hepatic lipogenesis. J Lipid Res. 2010;51:1380–1393. doi: 10.1194/jlr.M001230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson CR, Tran MK, Salazar KL, Young ME, Taegtmeyer H. Western diet, but not high fat diet, causes derangements of fatty acid metabolism and contractile dysfunction in the heart of Wistar rats. Biochem J. 2007;406:457–467. doi: 10.1042/BJ20070392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright JJ, Kim J, Buchanan J, Boudina S, Sena S, Bakirtzi K, et al. Mechanisms for increased myocardial fatty acid utilization following short-term high-fat feeding. Cardiovas Res. 2009;82:351–360. doi: 10.1093/cvr/cvp017. [DOI] [PMC free article] [PubMed] [Google Scholar]