Abstract
Objective:
Previous experiments have demonstrated that acute sleep loss impairs glucose homeostasis and increases food intake in humans. The incretin hormone glucagon-like peptide 1 (GLP-1) enhances postprandial insulin secretion and promotes satiety. Hypothesizing that the detrimental metabolic effects of sleep curtailment imply alterations in GLP-1 signaling, we investigated 24-h serum total GLP-1 concentrations during total sleep deprivation (TSD) and a normal sleep/wake cycle (comprising ∼8 h of sleep) in 12 healthy young men.
Methods:
Sessions started at 1800 h, and subjects were provided with standardized meals. Assessments of serum GLP-1 took place in 1.5- to 3-h intervals, focusing on the response to breakfast intake (3.8 MJ).
Results:
Across conditions, 24-h concentration profiles of GLP-1 were characterized by the expected postprandial increases (P<0.001). Although there were no differences in magnitude between conditions (P>0.11), the postprandial GLP-1 peak response to breakfast intake was delayed by ∼90 min following sleep loss in comparison with regular sleep (P<0.02).
Conclusions:
Results indicate that acute TSD exerts a mild, but discernible effect on the postprandial dynamics of circulating GLP-1 concentrations in healthy men.
Keywords: glucagon-like peptide 1, hormonal regulation, sleep, sleep deprivation
Introduction
Epidemiological observations indicate that short sleep is associated with an increased risk to develop obesity and type 2 diabetes.1, 2 Respective experimental studies have provided evidence that acute sleep loss increases food intake3, 4 and impairs glucose tolerance and insulin sensitivity.5 The intestinal hormone glucagon-like peptide 1 (GLP-1) acts as an incretin, that is secreted after oral nutrient intake and enhances insulin release,6 and moreover reduces food intake in humans.7 Therefore, some of the detrimental metabolic sequelae of sleep curtailment might involve an effect on GLP-1 signaling. In a recent study in healthy male subjects, plasma GLP-1 concentrations were reduced in the afternoon following a night of fragmented in comparison with regular sleep.8 However, overall 24-h GLP-1 concentrations were not affected, which might have been due to the subtlety of the intervention characterized by a slight shift from time spent in rapid-eye movement (REM) sleep to time spent in sleep stage 2.8 In a study in men and women, three nights of sleep restriction to 4 h per night decreased GLP-1 levels on the subsequent afternoon in female participants only.9 Apart from these conflicting findings, surprisingly little is known about the effects of sleep loss on GLP-1 and other incretins. Against this background, we assessed the 24-h profile of serum GLP-1 in healthy young men under conditions of regular night sleep and total sleep deprivation (TSD).
Materials and Methods
Participants
Twelve healthy men (mean±s.e.m., age=21.9±0.7 years; body mass index=24.1±0.6 kg m−2) who had a regular sleep-wake rhythm during the 6 weeks before the experiments and were not on medication participated in the experiments. Acute illness was excluded by physical examination and routine laboratory testing. All subjects gave written informed consent to the study that conformed to the Declaration of Helsinki and was approved by the local ethics committee. The present data derive from samples obtained in a larger study that focused on the effects of sleep deprivation on energy expenditure.10 Note that the samples of two subjects of the original study did not yield satisfactory results because of technical failures.
Experimental protocol
Each subject took part in two 24-h laboratory sessions separated by 4 weeks (sleep and TSD) after participating in a separate adaptation night. From 1800 hours on, subjects rested in bed in a supine position until the next afternoon at 1300 hours, when they changed to a sitting position. In the sleep condition, subjects were allowed to sleep from 2300 to 0700 hours. Their sleep was registered polysomnographically (Nihon Kohden GmbH, Rosbach, Germany) and respective recordings were scored offline according to standard criteria.11 During TSD, subjects were kept awake during the entire 24-h period at an ambient light intensity of ∼300 lux, being allowed to watch non-arousing movies, play games or read under constant supervision. Physical activity and food intake were standardized and strictly controlled (see ref. 10 for details). Subjects ate meals at 1930 hours (∼1.7 MJ; carbohydrates, 0.7 MJ; fat, 0.5 MJ; protein, 0.5 MJ) and at 0830 hours (∼3.8 MJ; carbohydrates, 1.9 MJ; fat, 1.3 MJ; and protein, 0.6 MJ) and 1330 hours (∼4.5 MJ; carbohydrates, 1.9 MJ; fat, 1.9 MJ; and protein, 0.7 MJ) of the subsequent day. Breakfast at 0830 hours consisted of 600 ml liquid containing 112.8 g carbohydrate (Fresubin energy drink; Fresenius Kabi, Bad Homburg, Germany), consumed at a rate of 20 ml per min. At the end of the session, a test buffet of ∼16.5 MJ (carbohydrates, 8.4 MJ; fat, 6.1 MJ; and protein, 2.0 MJ) was offered from which subjects could eat ad libitum for 30 min. Water was freely provided, but additional food intake was not allowed.
Assessments
Peripheral blood was sampled in untreated EDTA-coated tubes in 1.5–3-h intervals across the 24-h period (cf. Figure 1a). During sleep, the blood was drawn via long thin tubes enabling blood collection from an adjacent room without disturbing the subject's sleep. All blood samples were immediately centrifuged (2000 g, 10 min) and kept frozen at −80 °C until analysis. Serum levels of total GLP-1 were determined using a radioimmunoassay (Millipore, Billerica, MA, USA), with a lowest detectable level of 3 pmol l−1 using a 300 μl extracted sample (inter-assay coefficient of variation, CV, 23% intra-assay CV, 22%).
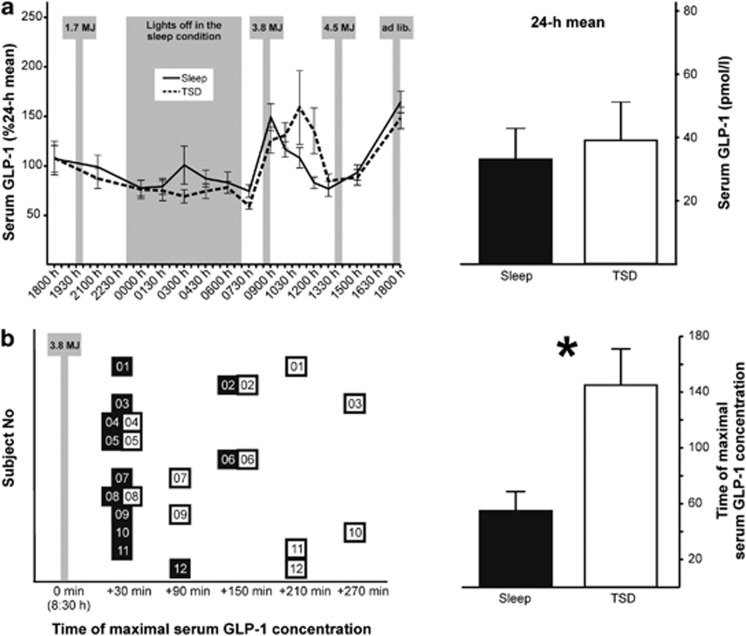
Figure 1.
Twenty-four hours serum concentrations of GLP-1 under conditions of a normal sleep/wake cycle and TSD. Data are mean values±s.e.m. (a) Left panel: serum GLP-1 concentrations during a regular 24 hour sleep-wake cycle (starting at 1800) and 24 hours of TSD, respectively, in 12 healthy men. Values were normalized by dividing individual data by the respective mean value across the 24-h sampling period. Right panel: serum concentrations of GLP-1 averaged across the respective 24 h-periods (black bar, regular sleep-wake cycle; white bar, TSD). (b) Postprandial delay of normalized GLP-1 peak concentrations during the 270 min following breakfast intake (black, sleep condition; white, TSD condition). Left panel shows individual responses with respective subject numbers, right panel indicates average delays in both conditions. Shaded areas indicate standardized meals and the respective calorific content as well as the nocturnal sleep/sleep deprivation period. ad lib., ad libitum. *P=0.017; paired Wilcoxon signed rank test.
Statistical analysis
Data are means±s.e.m. GLP-1 values were normalized by dividing them by the respective 24-h individual mean value. Differences in GLP-1 concentrations between conditions were analyzed by repeated measures analysis of variance, including ‘Sleep/TSD' and ‘Time' as within-subject factors. Pairwise t-tests and Wilcoxon tests were used for post-hoc analyses of single time point values as well as for areas under the curve (AUC) calculated according to the trapezoidal rule. A two-sided P-value<0.05 was considered significant.
Results
Across experimental conditions, serum GLP-1 concentrations were relatively stable during the 24-h sampling period and displayed increases following meal intake (P<0.001 for the factor Time; Figure 1a, left panel). Overall, GLP-1 concentrations were not affected by TSD (P=0.83 for Sleep/TSD; P=0.11 for Sleep/TSD × Time; 24-h mean, TSD vs Sleep, 39.1±12.1 vs 33.1±9.7 pmol l−1; P=0.72; Figure 1a). Similarly, no difference between conditions was found for postprandial GLP-1 concentrations in response to standardized breakfast intake (P=0.18 for the difference in AUC0730–1300 hours). However, analyses of the respective temporal dynamics revealed that after sleep loss, GLP-1 concentrations peaked around 90 min later than in the regular sleep condition (145±26 min vs 55±14 min, P=0.017; Figure 1b).
Food intake from the ad libitum buffet at the end of the session did not differ between the TSD (7.7±0.6 MJ) and the sleep condition (7.1±1.0 MJ; P>0.52). In the sleep condition, subjects slept on average 411±12 min after an average sleep onset latency of 28±7 min, with slow-wave sleep accounting for 57±8 min and REM sleep for 80±7 min.
Discussion
We show that TSD for one night does not markedly affect the circulating concentrations of serum total GLP-1 in healthy men, but induces a temporal shift in the GLP-1 peak response to breakfast intake. Importantly, the timing and magnitude of the observed meal-associated increases are well comparable to previous findings in healthy subjects8, 12 and corroborate the notion that GLP-1 secretion is primarily regulated depending on food ingestion.6 As energy content and macronutrient composition of the meals provided in our study varied across the day, our results do not warrant conclusions on whether the timing of meals, in addition to meal size,13 regulates the magnitude of the GLP-1 response.
Our finding that sleep loss does not alter the amount of circulating GLP-1 in male subjects corroborates recent observations of reduced afternoon GLP-1 concentrations in women but not in men after sleep curtailment.9 In another recent study, however, sleep fragmentation decreased GLP-1 concentrations on the subsequent afternoon in male participants,8 an effect that might have eluded our attention because we obtained only two GLP-1 values in the afternoon. Still, complete sleep loss induced a marked delay in the postprandial response to breakfast intake. Considering that food intake and digestion are the major physiological stimulus of GLP-1 secretion,6 delayed gastric emptying after nocturnal wakefulness is one possible mechanism behind the shift in the GLP-1 response to breakfast. To the best of our knowledge, however, the relationship between sleep duration and gastric emptying has not yet been systematically studied, so this explanation requires further research.
Considering that GLP-1 infusion increases postprandial satiety in normal weight7 as well as obese humans,14 a delay in the postprandial GLP-1 response might affect food intake regulation and in particular impact inter-meal snacking that has been shown to be augmented after sleep loss.4, 15 Accordingly, our group has demonstrated that TSD enhances the brain's response to high-calorie food stimuli presented after a caloric preload.16 The tentative conclusion that alterations in postprandial GLP-1 dynamics might contribute to such effects of sleep loss is supported by findings in rats that the GLP-1 analog exendin-4 decreases the rewarding value of food.17 Nevertheless, as snack intake was not measured in our study and food consumption in the late afternoon (that is, some hours after the observed effects) did not differ between conditions, this interpretation clearly needs further corroboration.
GLP-1 enhances insulin secretion in response to oral glucose uptake, thus contributing to the limitation of postprandial glucose increases,6 but GLP-1 responses are blunted in type 2 diabetes.18 Sleep deprivation is known to severely interfere with glucoregulatory mechanisms, unleashing the increase in blood glucose levels after breakfast intake.19 The corollary that sleep loss-induced delays in postprandial GLP-1 secretion might contribute to this effect is supported by the slight increase in post breakfast glucose concentrations that emerged in the TSD in comparison with the sleep condition of the present study, as reported previously.10 Thus, although our results suggest that GLP-1 secretion is not primarily regulated in a sleep-dependent manner in healthy men, the contribution of subtle changes in postprandial GLP-1 signaling to the deteriorating effect of sleep loss on glucose homeostasis and food intake regulation deserves a closer look in future investigations.
Acknowledgments
This study was supported by Deutsche Forschungsgemeinschaft (DFG; SFB 654). Work by CB is supported by the Olle Engqvist Byggmästare foundation and Novo Nordisk foundation. HO is an Emmy Noether fellow of the DFG and a Lichtenberg fellow of the Volkswagen Foundation. The funding sources had no input in the design and conduct of this study, in the collection, analysis and interpretation of the data or in the preparation, review or approval of the manuscript. The authors' responsibilities were as follows: CB HO and MH designed the study; CB, JLB, HO, VO and MH analyzed data; and all authors critically revised the manuscript for important intellectual content and contributed to writing the manuscript. All authors had full access to all of the data and take responsibility for the integrity and accuracy of the data analysis.
The authors declare no conflict of interest.
References
- Knutson KL, Van Cauter E. Associations between sleep loss and increased risk of obesity and diabetes. Ann N Y Acad Sci. 2008;1129:287–304. doi: 10.1196/annals.1417.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi S, Nakagawa T, Yamamoto S, Mizoue T, Takahashi Y, Noda M, et al. Sleep duration and BMI in a sample of young adults. Short sleep duration in association with CT-scanned abdominal fat areas: the Hitachi Health Study. Int J Obes (Lond) 2013;37:129–134. doi: 10.1038/ijo.2012.17. [DOI] [PubMed] [Google Scholar]
- St-Onge MP, Roberts AL, Chen J, Kelleman M, O'Keeffe M, RoyChoudhury A, et al. Short sleep duration increases energy intakes but does not change energy expenditure in normal-weight individuals. Am J Clin Nutr. 2011;94:410–416. doi: 10.3945/ajcn.111.013904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogenkamp PS, Nilsson E, Nilsson VC, Chapman CD, Vogel H, Lundberg LS, et al. Acute sleep deprivation increases portion size and affects food choice in young men Psychoneuroendocrinology 2013. doi: pii:S0306-4530(13)00017-6. 10.1016/j.psyneuen.2013.01.012. [DOI] [PubMed]
- Spiegel K, Leproult R, Van Cauter E. Impact of sleep debt on metabolic and endocrine function. Lancet. 1999;354:1435–1439. doi: 10.1016/S0140-6736(99)01376-8. [DOI] [PubMed] [Google Scholar]
- Holst JJ. The physiology of glucagon-like peptide 1. Physiol Rev. 2007;87:1409–1439. doi: 10.1152/physrev.00034.2006. [DOI] [PubMed] [Google Scholar]
- Flint A, Raben A, Astrup A, Holst JJ. Glucagon-like peptide 1 promotes satiety and suppresses energy intake in humans. J Clin Invest. 1998;101:515–520. doi: 10.1172/JCI990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonnissen HK, Hursel R, Rutters F, Martens EA, Westerterp-Plantenga MS. Effects of sleep fragmentation on appetite and related hormone concentrations over 24 h in healthy men. Br J Nutr. 2012;8:1–9. doi: 10.1017/S0007114512001894. [DOI] [PubMed] [Google Scholar]
- St-Onge MP, O'Keeffe M, Roberts AL, Roychoudhury A, Laferrère B. Short sleep duration, glucose dysregulation and hormonal regulation of appetite in men and women. Sleep. 2012;35:1503–1510. doi: 10.5665/sleep.2198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benedict C, Hallschmid M, Lassen A, Mahnke C, Schultes B, Schiöth HB, et al. Acute sleep deprivation reduces energy expenditure in healthy men. Am J Clin Nutr. 2011;93:1229–1236. doi: 10.3945/ajcn.110.006460. [DOI] [PubMed] [Google Scholar]
- Rechtschaffen A, Kales A. A manual of standardized terminology, techniques and scoring system for sleep of human subjects. US Government Printing Office: Washington, DC, USA; 1968. [Google Scholar]
- Orskov C, Wettergren A, Holst JJ. Secretion of the incretin hormones glucagon-like peptide-1 and gastric inhibitory polypeptide correlates with insulin secretion in normal man throughout the day. Scand J Gastroenterol. 1996;31:665–670. doi: 10.3109/00365529609009147. [DOI] [PubMed] [Google Scholar]
- Vilsbøll T, Holst JJ. Incretins, insulin secretion and type 2 diabetes mellitus. Diabetologia. 2004;47:357–366. doi: 10.1007/s00125-004-1342-6. [DOI] [PubMed] [Google Scholar]
- Näslund E, Gutniak M, Skogar S, Rössner S, Hellström PM. Glucagon-like peptide 1 increases the period of postprandial satiety and slows gastric emptying in obese men. Am J Clin Nutr. 1998;68:525–530. doi: 10.1093/ajcn/68.3.525. [DOI] [PubMed] [Google Scholar]
- Nedeltcheva AV, Kilkus JM, Imperial J, Kasza K, Schoeller DA, Penev PD. Sleep curtailment is accompanied by increased intake of calories from snacks. Am J Clin Nutr. 2009;89:126–133. doi: 10.3945/ajcn.2008.26574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benedict C, Brooks SJ, O'Daly OG, Almèn MS, Morell A, Åberg K, et al. Acute sleep deprivation enhances the brain's response to hedonic food stimuli: an fMRI study. J Clin Endocrinol Metab. 2012;97:E443–E447. doi: 10.1210/jc.2011-2759. [DOI] [PubMed] [Google Scholar]
- Dickson SL, Shirazi RH, Hansson C, Bergquist F, Nissbrandt H, Skibicka KP. The glucagon-like peptide 1 (GLP-1) analogue, exendin-4, decreases the rewarding value of food: a new role for mesolimbic GLP-1 receptors. J Neurosci. 2012;32:4812–4820. doi: 10.1523/JNEUROSCI.6326-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilsbøll T, Krarup T, Sonne J, Madsbad S, Vølund A, Juul AG, et al. Incretin secretion in relation to meal size and body weight in healthy subjects and people with type 1 and type 2 diabetes mellitus. J Clin Endocrinol Metab. 2003;88:2706–2713. doi: 10.1210/jc.2002-021873. [DOI] [PubMed] [Google Scholar]
- Schmid SM, Hallschmid M, Jauch-Chara K, Wilms B, Lehnert H, Born J, et al. Disturbed glucoregulatory response to food intake after moderate sleep restriction. Sleep. 2011;34:371–377. doi: 10.1093/sleep/34.3.371. [DOI] [PMC free article] [PubMed] [Google Scholar]