Abstract
Salivary gland carcinomas metastasize to distant organs in 20% of salivary gland malignancies. Applying immunohistochemistry (IHC) measures, salivary gland tumors showed a wide range of oncogene markers expression, including the human epidermoid receptor 2 (Her2/neu), which could be targeted with monoclonal antibody. Treating salivary gland tumors, which have Her2/neu over-expression, with trastuzumab was reported in a few case reports. We report a 61-year-old Caucasian male, with a history of salivary gland tumor, who presented after 20 years of complete surgical resection with kidney mass. He was treated as primary renal cell carcinoma, unclassified, with nephrectomy and adjuvant clinical trail where he received placebo. Subsequently, he developed multiple hepatic lesions and retroperitoneal mesenteric lymphadenopathy; CT-guided biopsy revealed adenocarcinoma with Her2/neu, 3+ by IHC. The patient was treated successfully with trastuzumab with near-complete response.
Keywords: Metastasis, salivary gland tumor, salivary glands, trastuzumab
INTRODUCTION
Most salivary gland tumors are benign neoplasms.[1,2] They are classified depending on their histological appearance into several major groups: adenomas, carcinomas, nonepithelial tumors, malignant lymphomas, secondary tumors, unclassified tumors, and tumor-like lesions, which all have sub-classifications.[3] Eighty percent of salivary gland tumors occur in the parotid gland, where the majority are benign; 9% occur in the submandibular gland, where the majority are malignant; minor intraoral salivary glands tumor are rare and mostly malignant.[1] Pleomorphic adenoma is the most common salivary gland benign tumor and accounts for 70% of parotid tumors, whereas mucoepidermoid carcinoma is the most common malignant tumor. Adenolymphoma (Warthin's tumor) is the second most common benign tumor in the parotid salivary gland.[1,4] Salivary gland's malignant tumors metastasize to distant organs, such as lung, bone, or other visceral organs in 20% of cases; metastases to regional lymph nodes indicate poor prognosis and it is associated with reduced survival.[1,5]
Investigated by immunohistochemistry (IHC), salivary gland tumors showed to have a wide range of oncogene markers: hormonal receptors and tyrosine kinase receptors, i.e. c-kit, human epidermoid receptor 2 (Her2/neu), endothelial growth factor receptor.[6] Her2/neu oncogene has been shown to be over-expressed in salivary duct carcinomas (44%), adenocarcinomas (21%), adenoid cystic carcinomas (2%), myoepithelial carcinomas, mucoepidermoid carcinomas, and other histotypes, at both the protein and gene levels.[6,7] Targeting Her2/neu pathway in cancers that overexpress Her2/neu-like breast cancer and gastric adenocarcinoma is well-investigated and approved. This includes a monoclonal antibody therapy, trastuzumab, and specific Her2/neu and Her1 tyrosine kinase inhibitor, lapatinib.
CASE REPORT
We are reporting a 62-year-old Caucasian male with a history of resected benign parotid gland tumor on 1985. After 22 years of resection, he presented with right kidney mass which was suspicious for renal cell carcinoma. He underwent right nephrectomy with a pathological diagnosis of renal cell carcinoma of unclassified histology. Afterward, the patient was enrolled in the adjuvant Econ 2805 study in the placebo arm; subsequently, he relapsed with retroperitoneal lymphadenopathy. Following this, the patient was started on sunitinib therapy, a receptor tyrosine kinase inhibitor, in 2009 for metastatic renal cell carcinoma, though patient's tumor continued to progress. He was sent to be evaluated for a phase-I trial; during the evaluation, multiple pathologists evaluated his renal tumor histopathology studies and core biopsies of the salivary gland resection were compared to his nephrectomy ones. They concluded that the renal tumor is a metastasis from the salivary gland carcinoma with adenocarcinoma features.
Subsequently, the patient developed severe right upper quadrant abdominal pain. Follow-up diagnostic studies with positron emission tomography (PET) scan showed disease progression with multiple bilateral hepatic lesions and retroperitoneal lymphadenopathy [Figure 1], with elevated liver enzymes. The tumor was tested for Her2/neu, which was strongly positive, +3 by IHC. Then, the patient was started on weekly trastuzumab (4 mg/kg loading dose, then 2 mg/kg maintenance) and paclitaxel 80 mg/m2.[8]
Figure 1.
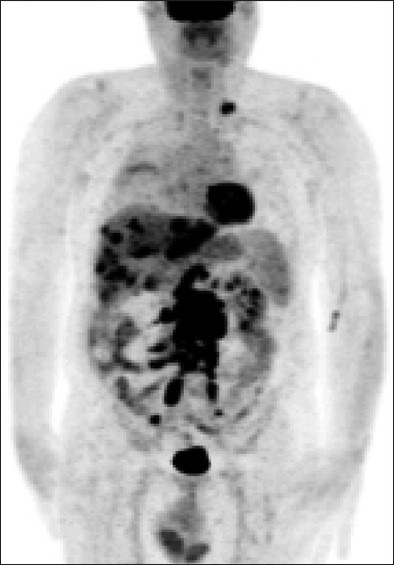
Pre-treatment; positron emission tomography scan showing multiple metastatic bilateral hepatic lesions and retroperitoneal lymphadenopathy from primary salivary gland carcinoma
The abdominal pain he was having and the elevated liver enzymes resolved after the first dose of trastuzumab and paclitaxel. After 6 weeks of treatment, diagnostic studies of the abdomen showed decreased size of the metastatic disease in the liver and retroperitoneal lymph nodes. The patient was continued on a maintenance weekly trastuzumab. Three months later, repeated scanning showed continuous response to therapy. Follow-up PET-scanning 1 year later revealed complete resolution of the previously seen liver lesions [Figure 2]. To date, the patient is alive, stable, and receiving weekly Trastuzumab.
Figure 2.
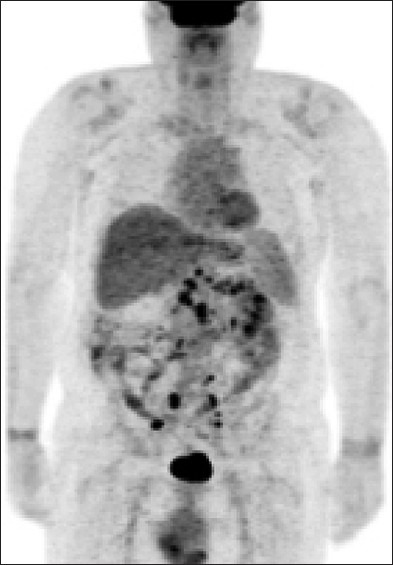
Post treatment; positron emission tomography scan performed 1-year after the beginning of the treatment which reveals complete resolution of the previously seen liver lesions
DISCUSSION
Salivary gland adenocarcinoma is one of the malignant subtypes of the salivary gland tumors; the over-expression of Her2/neu protein in salivary gland adenocarcinomas is reported in around 20% of cases.[6] Her2/neu is one of the epidermal growth factor (EGF) tyrosine kinase receptors. Trastuzumab, a humanized monoclonal antibody, binds with high affinity to the extracellular domain of Her2/neu, inhibiting the proliferation of human tumor cells that over-express Her2/neu. The predictive value of these findings has been suggested in phase II studies of trastuzumab monotherapy in advanced Her2 malignant salivary gland tumors. Our case explains the potential benefit of using targeted therapy in Her2/neu over-expressing salivary gland adenocarcinoma.
Currently, there are no standard treatment guidelines for metastatic salivary gland cancers; however, two phase II trials of Her2/neu-targeted therapies in salivary carcinomas are promising [Table 1]. Agulnik et al. reported a trial of lapatinib in 62 salivary tumor patients with EGFR and Her2/neu expression, the outcome being either stable or progressed disease with no obvious improvements, knowing that adenocarcinoma accounted for only 11% of tumors.[12] Similarly, Haddad et al. reported the use of trastuzumab in 14 patients, with Her2/neu over-expression. Fifty percent of them had adenocarcinoma, who were improved partially with the treatment.[13] Our literature search revealed three recent case reports of Her2/neu over-expressing salivary gland carcinomas, which were treated with trastuzumab [Table 1]. Sharon et al. recently reported a complete response in a patient with carcinoma ex-pleomorphic adenoma with multiple bone metastases treated with trastuzumab, capecitabine, and zoledronic acid, and maintained stabilized over a 2-year period.[9] Nashed et al. recently reported another durable complete remission of a metastatic salivary duct carcinoma treated with a combination of docetaxel and trastuzumab after an initial stabilization with trastuzumab for 5 months.[10] A third case was reported by Prat et al. at which the patient was having salivary duct carcinoma with extensive cervical lymph node involvement and multiple pulmonary metastatic lesions; systemic treatment with paclitaxel, carboplatin, and trastuzumab was initiated. Complete response was observed 3 months later and the patient was continued on maintenance trastuzumab therapy.[11] In our case, we report a patient with Her2/neu positive metastatic adenocarcinoma who had a complete clinical response to initial treatment with trastuzumab in combination with paclitaxel, followed by maintenance trastuzumab.
Table 1.
Literature summary: summary of metastatic salivary gland neoplasms expressing Her2/neu oncogene markers and treated with trastuzumab
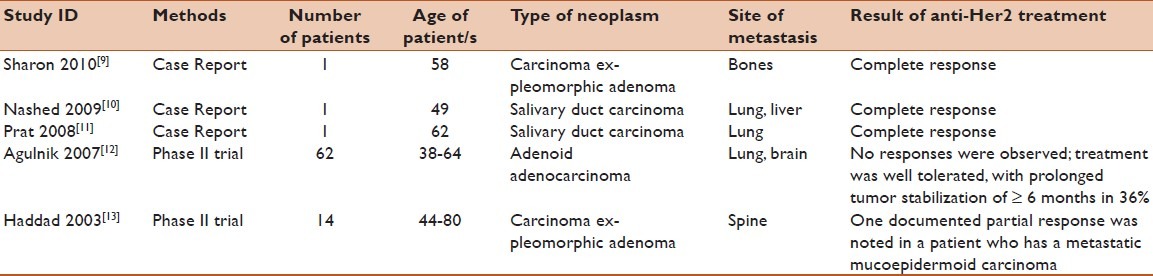
Interestingly, all four cases, including our case, over-expressed Her2/neu oncogene in different subtypes of salivary gland tumor, showed durable responses to trastuzumab in combination with standard chemotherapy, and were continued on trastuzumab as a maintenance therapy. Since Her2/neu oncogene is uncommonly expressed in adenocarcinoma and other salivary gland tumor histological subtypes, prospective clinical trial is unlikely to be conducted. In the light of this case, and the three published cases, Her2/neu oncogene expression should be evaluated in metastatic or locally advanced non-resectable salivary gland carcinomas; and if positive, trastuzumab alone or in combination with chemotherapy should be considered for the initial therapy in those patients, as well as for maintenance therapy.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Madani G, Beale T. Tumors of the salivary glands. Semin Ultrasound CT MR. 2006;27:452–64. doi: 10.1053/j.sult.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 2.Scianna JM, Petruzzelli GJ. Contemporary management of tumors of the salivary glands. Curr Oncol Rep. 2007;9:134–8. doi: 10.1007/s11912-007-0011-6. [DOI] [PubMed] [Google Scholar]
- 3.Seifert G, Sobin LH. The World Health Organization's Histological Classification of Salivary Gland Tumors. A commentary on the second edition. Cancer. 1992;70:379–85. doi: 10.1002/1097-0142(19920715)70:2<379::aid-cncr2820700202>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 4.Ellis GL. Lymphoid lesions of salivary glands: Malignant and benign. Med Oral Patol Oral Cir Bucal. 2007;12:E479–85. [PubMed] [Google Scholar]
- 5.Bhattacharyya N, Fried MP. Nodal metastasis in major salivary gland cancer: Predictive factors and effects on survival. Arch Otolaryngol Head Neck Surg. 2002;128:904–8. doi: 10.1001/archotol.128.8.904. [DOI] [PubMed] [Google Scholar]
- 6.Locati LD, Perrone F, Losa M, Mela M, Casieri P, Orsenigo M, et al. Treatment relevant target immunophenotyping of 139 salivary gland carcinomas (SGCs) Oral Oncol. 2009;45:986–90. doi: 10.1016/j.oraloncology.2009.05.635. [DOI] [PubMed] [Google Scholar]
- 7.Elledge R. Current concepts in research related to oncogenes implicated in salivary gland tumourigenesis: A review of the literature. Oral Dis. 2009;15:249–54. doi: 10.1111/j.1601-0825.2009.01529.x. [DOI] [PubMed] [Google Scholar]
- 8.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 9.Sharon E, Kelly RJ, Szabo E. Sustained response of carcinoma ex pleomorphic adenoma treated with trastuzumab and capecitabine. Head Neck Oncol. 2010;2:12. doi: 10.1186/1758-3284-2-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nashed M, Casasola RJ. Biological therapy of salivary duct carcinoma. J Laryngol Otol. 2009;123:250–2. doi: 10.1017/S0022215108002314. [DOI] [PubMed] [Google Scholar]
- 11.Prat A, Parera M, Reyes V, Peralta S, Cedrés S, Andreu J, et al. Successful treatment of pulmonary metastatic salivary ductal carcinoma with trastuzumab-based therapy. Head Neck. 2008;30:680–3. doi: 10.1002/hed.20714. [DOI] [PubMed] [Google Scholar]
- 12.Agulnik M, Cohen EW, Cohen RB, Chen EX, Vokes EE, Hotte SJ, et al. Phase II study of lapatinib in recurrent or metastatic epidermal growth factor receptor and/or erbB2 expressing adenoid cystic carcinoma and non adenoid cystic carcinoma malignant tumors of the salivary glands. J Clin Oncol. 2007;25:3978–84. doi: 10.1200/JCO.2007.11.8612. [DOI] [PubMed] [Google Scholar]
- 13.Haddad R, Colevas AD, Krane JF, Cooper D, Glisson B, Amrein PC, et al. Herceptin in patients with advanced or metastatic salivary gland carcinomas.A phase II study. Oral Oncol. 2003;39:724–7. doi: 10.1016/s1368-8375(03)00097-6. [DOI] [PubMed] [Google Scholar]


