Abstract
Background
Protein kinase C (PKC) serves as the receptor for tumor-promoting phorbol esters, which are potent activators of conventional (c) and novel (n) PKCs. We recently showed that these activators induced selective upregulation of PKCη in breast cancer cells. The objective of this study is to understand unique regulation of PKCη and its importance in breast cancer.
Methods
The levels of PKC isozymes were monitored in breast cancer cells following treatment with inhibitors of kinases, proteasome and proteases by Western blotting. PKCε was introduced by adenoviral delivery. PKCη and PDK1 were depleted by siRNA silencing. Cell growth was determined by the MTT or clonal assay.
Results
The general PKC inhibitors Gö 6983 and bisindolylmaleimide but not cPKC inhibitor Gö 6976 led to substantial PKCη downregulation, which was partly rescued by the introduction of nPKCε. Inhibition of phosphoinositide-dependent kinase-1 (PDK1) by Ly294002 or knockdown of PDK1 also led to downregulation of basal PKCη but had no effect on PKC activator-induced upregulation of PKCη. Proteasome inhibitors blocked PKCη downregulation triggered by PDK1 inhibition/depletion but not by Gö 6983. PKCη level increased in malignant but not in non-tumorigenic or pre-malignant cells in the progressive MCF-10A series associated with activated PDK1, and knockdown of PKCη inhibited breast cancer cell growth and clonogenic survival.
Conclusion
Upregulation of PKCη contributes to breast cancer cell growth and targeting either PKCε or PDK1 triggers PKCη downregulation but involves two distinct mechanisms.
General significance
The status of PKCη may serve as a potential biomarker for breast cancer malignancy.
Keywords: PKC, PDK1, downregulation, breast cancer
1. Introduction
Protein kinase C, a family of phospholipid-dependent serine/threonine kinases, plays a critical role in growth factor signal transduction pathways [1, 2]. The PKC family is classified into conventional (α, βI, βII, γ), novel (δ, ε, η, θ) and atypical PKCs (ζ, λ/τ) based on their structural features and cofactor sensitivity [1, 2]. Diacylglycerol (DAG) and Ca2+ are required for the activity of conventional PKCs whereas novel PKCs are insensitive to Ca2+ but dependent on DAG; atypical PKCs are insensitive to both Ca2+ and DAG [1, 2].
Tumor-promoting phorbol esters are potent activators of PKCs and can substitute for DAG. However, sustained activation by phorbol esters leads to downregulation of PKCs causing termination of the PKC signaling. Calcium-activated proteases, such as calpains and ubiquitin proteasome-mediated pathways have been implicated in PKC activator-induced downregulation of PKCs. Calpains are believed to downregulate cPKCs [3, 4] whereas PKCα, -δ and -ε were shown to be degraded via proteasome-mediated pathway [5-7]. Downregulation of PKCs has important implications in regulating long-term cellular responses such as cell proliferation, differentiation and tumor promotion [1, 8, 9].
PKCη is a member of the novel PKC family and shares highest homology with PKCε [10]. However, the regulation of PKCη appears to be unique. We have recently shown that several different PKC activators, including tumor-promoting phorbol esters, induce upregulation rather than downregulation of PKCη [11]. Moreover, phosphorylation of PKCη by novel PKCε appears to be responsible for PKC activator-induced upregulation of PKCη [11]. Depending on the cellular context, inhibition of PKCη could either promote or suppress tumor promotion [12-19]. Since PKCη is upregulated by tumor promoters, we examined the mechanism of PKCη downregulation. Our results indicate that inhibition of either PKC or PDK1 induces PKCη downregulation but involves two distinct mechanisms. While inhibition of PKC resulted in PKCη downregulation via proteasome-independent pathway, inhibition of PDK1 led to PKCη downregulation via proteasome-dependent pathway. We also showed that PKCη level is increased in malignant but not in non-tumorigenic or pre-malignant cells in the progressive MCF-10A series and knockdown of PKCη inhibited breast cancer cell growth.
2. Materials and Methods
2.1. Materials
PDBu was purchased from Alexis Biochemicals (San Diego, CA). Ly294002 was purchased from Calbiochem (San Diego, CA) and Cell Signaling Technology, Inc (Danvers, MA). Gö 6983, Gö 6976, KT5720, Rottlerin, PD98059, BIM, U0126, MG-132, lactacystin, calpeptin and cathepsin inhibitor I were obtained from Calbiochem (San Diego, CA). Polyclonal antibodies to PKCη, PKCδ, PKCε and monoclonal antibody to GAPDH were obtained from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA). Monoclonal antibody to PKCα was obtained from Upstate Biotechnology (Lake Placid, NY). Polyclonal antibody against PDK1 was purchased from Cell Signaling Technology, Inc. (Danvers, MA). Monoclonal antibody against actin was obtained from Sigma (St. Louis, MO). Horseradish-peroxidase-conjugated donkey anti-rabbit and goat anti-mouse secondary antibodies were purchased from Jackson ImmunoResearch Laboratories, Inc. (West Grove, PA). Control non-targeting siRNA and siRNA specific for PDK1 and PKCη were obtained from Dharmacon (Lafayette, CO). Poly(vinylidenedifluoride) membrane was from Millipore (Bedford, MA) and enhanced chemiluminescence detection kit was from Amersham (Arlington Heights, IL).
2.2. Cell culture
MCF-7 and T47D cells were maintained in RPMI 1640 medium supplemented with 10% fetal bovine serum and 2 mM glutamine. Cells were kept in a humidified incubator at 37°C with 95% air and 5% CO2. The MCF-10A series developed by Dr. Fred Miller and colleagues [20], was obtained from the Barbara Ann Karmanos Cancer Institute (Detroit, MI). They were cultured as described previously [21].
2.3. Transfection
Control non-targeting siRNA or SMARTpool siRNA against PDK1 or PKCη were introduced into cells using Lipofectamine RNAiMax (Invitrogen, Carlsbad, CA) and manufacturer's protocol. Cells were treated as indicated in the text and processed for Western blot analysis.
2.4. Immunoblot analysis
Cells were lysed in extraction buffer containing 1 mM DTT, protease inhibitors and phosphatase inhibitors. Equal amounts of protein were separated by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and transferred on PVDF membranes. Western blot analysis was performed as described previously [22].
2.5. Clonogenic assay
Cells transfected with or without control non-targeting or PKCη siRNA were cultured at 37°C in a humidified incubator with 5% CO2 until there were at least 50 cells per colony. At the end of the incubation, the cells were washed with PBS and incubated with 0.025% crystal violet solution for 15 minutes. Colonies were counted using ImageJ software (http://rsbweb.nih.gov/ij/), and the plate was photographed using the BioChemi System (BioImaging System, UVP, Upland, CA).
2.6. MTT assay
Cells transfected with or without control non-targeting or PKCη siRNA were plated in microtiter plates and incubated at 37°C and 5% CO2. After 4 days, the number of viable cells was determined using 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetra-zolium bromide (MTT) as described previously [23].
2.7. Statistical analysis
Statistical significance was determined by Student's t test by using PASW Statistics (SPSS, Inc.). p values < 0.05 was considered statistically significant.
3. Results
3.1. Inhibitors of PKC and PI3K induce downregulation of PKCη
We have previously shown that the regulation of PKCη is unique since it is upregulated rather than downregulated by PKC activators in breast cancer cells [11]. In the present study, we examined if inhibition of PKC induces its downregulation. Figure 1A shows that the general PKC inhibitor bisindolylmaleimide caused substantial downregulation of PKCη whereas rottlerin, which inhibits novel PKCδ, had no effect; the conventional PKC inhibitor Gö 6976 caused only a modest decrease in PKCη level in MCF-7 cells. The PI3K inhibitor Ly294002 also induced PKCη downregulation whereas rapamycin, PD98059 and KT5720, which inhibit mTOR, MAPK and PKA, respectively had little or no effect (Fig. 1A). To determine if the ability of PKC or PI3K inhibitor to downregulate PKCη is a general phenomenon, we tested several different breast cancer cell lines. Figure 1B shows that similar to MCF-7 cells, bisindolylmaleimide and Ly294002 induced substantial downregulation of PKCη in T47D cells whereas mTOR inhibitor rapamycin had a modest effect and MEK inhibitor U0126 had no effect. These results suggest that both PKC and PI3K pathways are involved in regulating PKCη level.
Fig. 1.

Effects of kinase inhibitors on PKCη levels. MCF-7 (A) or T47D (B) cells were treated with or without 10 μM BIM, 10 μM rottlerin, 1 μM Gö 6976, 25 μM Ly294002, 50 nM rapamycin, 50 μM PD98059, 10 μM U0126 or 2 μM KT5720 for 15 h. Western blot analysis was performed with total cellular extracts using the indicated antibodies. Actin was used as a loading control.
3.2. PKC and PI3K inhibitors induce PKCη downregulation via two distinct pathways
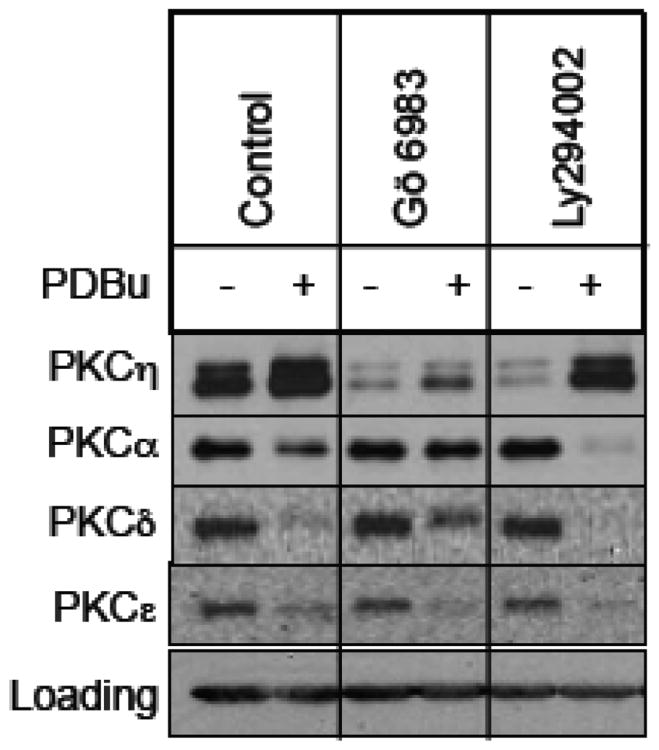
Since PKC activators induce upregulation of PKCη, we examined if the general PKC inhibitor Gö 6983 or the PI3K inhibitor Ly294002 could block PKC activator-induced upregulation of PKCη. Figure 2 shows that while the PKC activator PDBu induced downregulation of PKCα, -δ and -ε, it caused upregulation of PKCη. Consistent with the notion that PKC activity is required for PKC activator-induced downregulation of PKCs [5, 6, 24], the general PKC inhibitor Gö 6983 prevented PDBu-induced downregulation of PKCα and PKCδ. However, Gö 6983 was able to induce PKCη downregulation in PDBu-treated cells. The PI3K inhibitor Ly294002 caused a substantial decrease in basal PKCη level but the constitutive levels of PKCα, -δ and -ε were not altered by the Ly294002 treatment. In contrast to Gö 6983, Ly294002 was unable to induce PKCη downregulation in PDBu-treated cells. These results suggest that Gö 6983 and Ly294002 induced PKCη downregulation via two distinct pathways.
Fig. 2.
Effect of PKC activators on PKCη downregulation by PKC and PI3K inhibitors. MCF-7 cells were pretreated with 1 μM PDBu for 15 min, followed by treatment with or without 1μM Gö 6983 or 25 μM Ly294002 for 15 h. Western blot analysis was performed with total cellular extracts using the indicated antibodies. Actin was used as a loading control.
3.3. Ly294002 induces PKCη downregulation via ubiquitin proteasome-mediated pathway
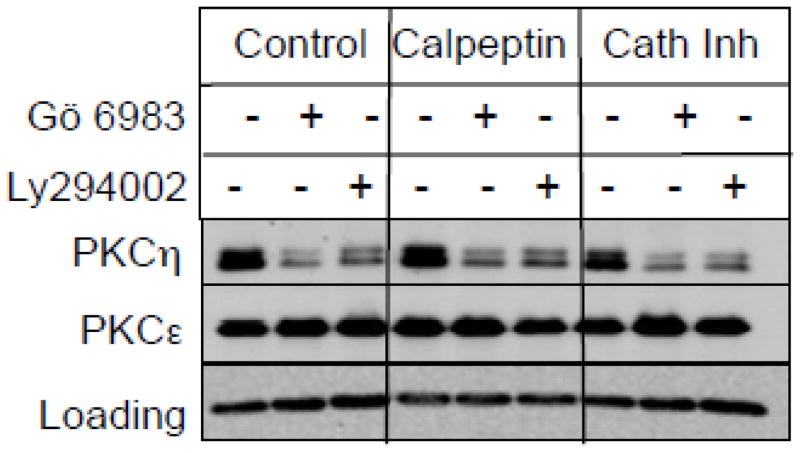
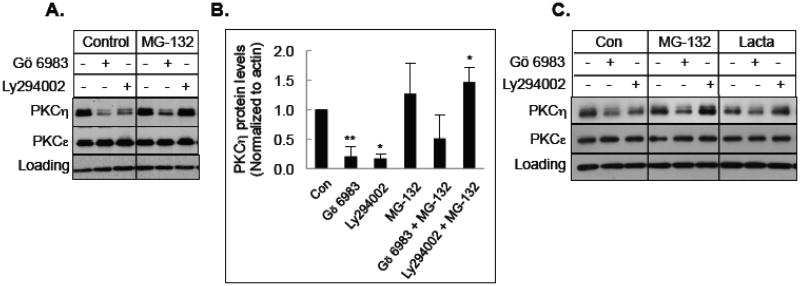
Both calcium-activated proteases, such as calpains as well as ubiquitin proteasome-mediated pathway have been implicated in the activation-induced downregulation of PKCs [4-6]. Therefore, we examined the ability of calpain inhibitor calpeptin, cathepsin inhibitor I and proteasome inhibitor MG-132 or lactacystin in preventing downregulation of PKCη by Gö 6983 or Ly294002. Figure 3 shows that neither calpain inhibitor nor cathepsin inhibitor blocked PKCη downregulation induced by either Gö 6983 or Ly294002. Although the proteasome inhibitor MG-132 prevented PKCη downregulation by Ly294002, it had only a modest effect on Gö 6983-mediated downregulation of PKCη (Figs. 4A & 4B). Similar results were observed in T47D cells where two different proteasome inhibitors MG-132 and lactacystin blocked Ly294002-mediated but not Gö 6983-mediated downregulation of PKCη (Fig. 4C). These results demonstrate that Ly294002 but not Gö 6983 induces PKCη downregulation via the proteasome-mediated pathway.
Fig. 3.
Effect of protease inhibitors on PKCη downregulation in MCF-7 cells. MCF-7 cells were pretreated with or without 50 μM calpeptin or 50 μM cathepsin inhibitor I for 30 min, followed by treatment with 1 μM Gö 6983 or 25 μM Ly294002 for 12 h. Western blot analysis was performed with total cellular extracts using the indicated antibodies. GAPDH was used as a loading control. Results are representative of 2 independent experiments.
Fig. 4.
Effect of proteasome inhibitors on PKCη downregulation. A, MCF-7 cells were pretreated with 10 μM MG-132 for 30 min, followed by treatment with 1 μM Gö 6983 or 25 μM Ly294002 for 12 h. Western blot analysis was performed with total cellular extracts using the indicated antibodies. GAPDH was used as a loading control. B, Densitometric quantification of PKCη protein levels from 3 separate experiments corrected for loading. Data represents the mean +/- s.e.m. The asterisk (*) indicates significant difference of MG-132 treated cells from control or treatment with the inhibitors alone using Student's t-test. *, P<0.05; **, P<0.005 C, T47D cells were pretreated with 10 M MG-132 or 10 μM lactacystin for 30 min, followed by treatment with 1 μM Gö 6983 or 10 μM Ly294002 for 15 h. Western blot analysis was performed with total cellular extracts using the indicated antibodies. Actin was used as a loading control.
3.4. Inhibition of PDK1 and PKCε triggers PKCη downregulation
Since PDK1 is believed to phosphorylate PKCη at the activation loop [25] and Ly294002 inhibits PDK1 which acts downstream of PI3K, we examined if knockdown of PDK1 induces downregulation of PKCη. Figure 5A shows that similar to Ly294002, depletion of PDK1 by siRNA decreased basal PKCη level. The proteasome inhibitors MG-132 and lactacystin caused a modest increase in PKCη level in cells transfected with control non-targeting siRNA or in untransfected cells, and blocked PKCη downregulation by both Ly294002 and PDK1 knockdown (Fig. 5A). These results suggest that inhibition of PDK1 by Ly294002 targets PKCη to proteasome-mediated downregulation.
Fig. 5.
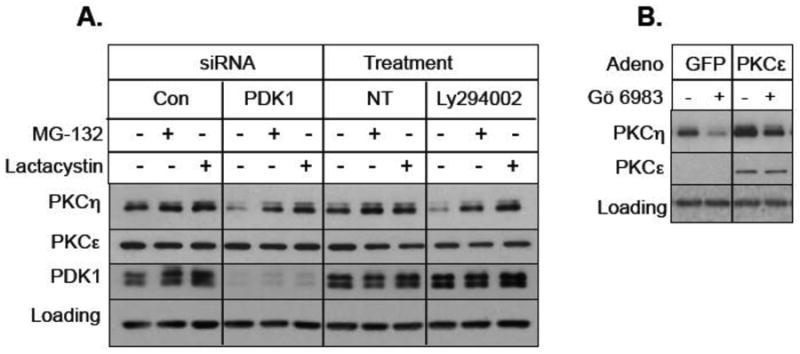
Effect of PDK1 and PKCε on PKCη downregulation. A, MCF-7 cells were transfected with control non-targeting siRNA, PDK1 siRNA or were left non-transfected (NT). Cells were then pre-treated with either 10 μM MG-132 or 10 μM lactacystin, followed by treatment with 25 μM Ly294002. Western blot analysis was performed with indicated antibodies. Actin was used as a loading control. B, T47D cells were infected with adenovirus vector containing GFP or PKCε construct and then treated with 1 μM Gö 6983 for 15 h. Western blot analysis was carried out with indicated antibodies.
Since we have previously shown that novel PKCε was responsible for PKC activator-induced upregulation of PKCη [11], we speculated that the general PKC inhibitor Gö 6983 triggers PKCη downregulation by inhibiting PKCε. Since T47D cells were more dependent on PKCε-mediated upregulation of PKCη [11], we infected T47D cells with adenoviral vector expressing either GFP or PKCε. Figure 5B shows that adenoviral delivery of PKCs but not GFP increased basal PKCs level and partially blocked Gö 6983-induced downregulation of PKCη. Taken together, these results suggest that inhibition of PDK1 and novel PKCε by Ly294002 and Gö 6983, respectively triggers PKCη downregulation via two distinct pathways.
3.5. PKCη is progressively increased in MCF-10A series and promotes breast cancer cell survival
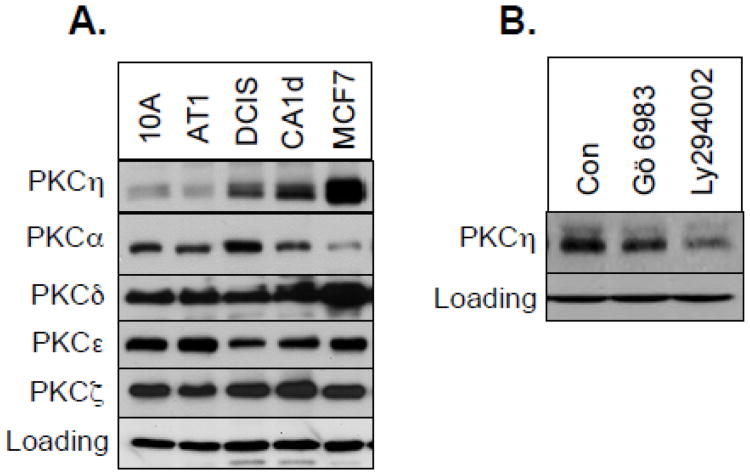
Since PKCη level is regulated by PKC and PI3K/PDK1 pathways that have been implicated in breast cancer [26-28], we compared PKCη level in non-tumorigenic MCF-10A cells and breast cancer cells. The level and activation status of PDK1 was increased in the progressive MCF-10A series [21] that includes spontaneously immortalized non-tumorigenic mammary epithelial MCF-10A cells, premalignant MCF-10AT cells, ductal carcinoma in situ (DCIS) and highly malignant MCF-10CA1d cells [20] . Figure 6A shows that PKCη is expressed only in malignant breast cancer cells but not in non-tumorigenic MCF-10A or premalignant MCF-10AT cells. The levels of other PKCs, such as PKCα, PKCδ, PKCε or PKCζ were not increased with the malignancy of the MCF-10A series. Similar to MCF-7 and T47D cells, both Gö 6983 and Ly294002 led to PKCη downregulation in CA1d cells (Fig. 6B).
Fig. 6.
Comparison of PKCη levels in MCF10A series. A, Western blot analysis was performed with total cellular extracts from 10A, AT1, DCIS, CA1d and MCF-7 cells and probed with the indicated antibodies. B, CA1d cells were treated with or without 1 μM Gö 6983 or 25 μM Ly294002 for 16 h. Western blot analysis was performed with total cellular extracts using the indicated antibodies. Actin was used as a loading control.
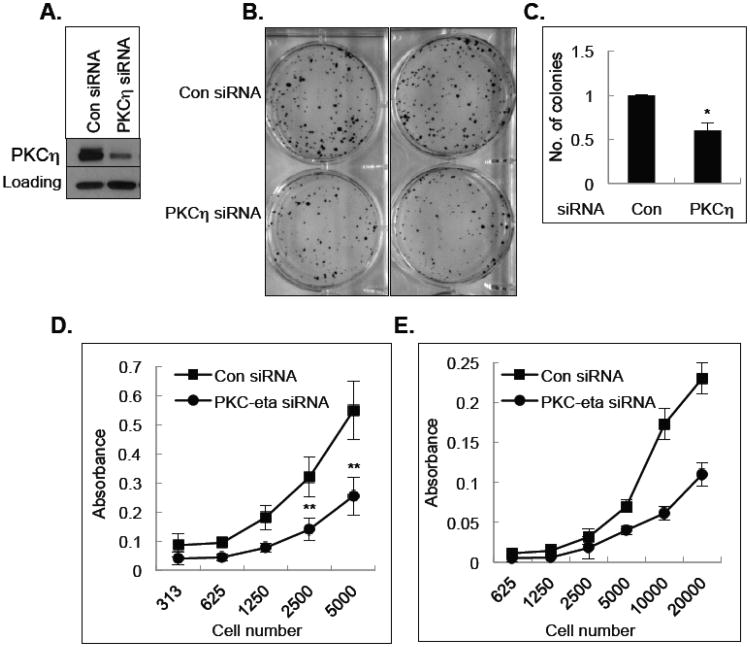
To determine the functional significance of PKCη upregulation in breast cancer cells, we determined the consequence of PKCη depletion on the viability of breast cancer cells. Depletion of PKCη by siRNA (Fig. 7A) decreased MCF-7 cell survival in a long-term clonogenic assay (Figs. 7B & 7C). Knockdown of PKCη also inhibited the growth of both T47D (Fig. 7D) and DCIS (Fig. 7E) cells. These results suggest that PKCη contributes to the viability of breast cancer cells.
Fig. 7.
Effect of PKCη on cell growth. A, MCF-7 cells were transfected with control non-targeting siRNA or PKCη siRNA and total cell extracts were used for Western blot analysis. Actin was used as a loading control. B, Clonogenic assay was performed with MCF-7 cells transfected with either control non-targeting siRNA or PKCη siRNA as described in the Materials and Methods. C, Quantification of the number of colonies as determined by the clonogenic assay. The results are representative of 3 independent experiments. *P < 0.05 using paired Student t test. D, MTT assay was performed with T47D cells transfected with control, non-targeting siRNA or PKCη siRNA as described under Materials and Methods. The results are representative of 3 independent experiments. **P < 0.01 using paired Student t test. E, MTT assay was performed with DCIS cells transfected with control non-targeting siRNA or PKCη siRNA as described under Materials and Methods.
4. Discussion
Because of the pivotal role of the PKC family members in signal transduction and cell regulation, there have been significant efforts in understanding their function and regulation. Among the novel PKCs, most of the studies thus far have been focused on PKCδ and -ε but little is known about the regulation of novel PKCη. Our results suggest that the regulation of PKCη is unique. We recently reported that persistent treatment with PKC activators induce upregulation of PKCη rather than downregulation which is seen with other phorbol ester-sensitive conventional and novel PKCs. In the present study, we show that downregulation of PKCη can be achieved via two distinct pathways. While inhibition of PKC induces PKCη downregulation via a proteasome-independent pathway, inhibition of PDK1 results in PKCη downregulation via a proteasome-dependent pathway. We also made a novel observation that PKCη is the only PKC isozyme which is upregulated in breast cancer cells compared to non-malignant cells and downregulation of PKCη inhibits breast cancer cell growth.
PKCη has been implicated in both tumor promotion and tumor suppression. For example, PKCη expression was decreased in hepatocellular carcinoma [16], and PKCη deficiency enhanced susceptibility to tumor formation in a two-stage skin carcinogenesis model [12, 13]. Moreover, overexpression of PKCη induced differentiation in keratinocytes [17, 19]. In contrast, PKCη has been implicated in breast cancer [29], glioblastoma [15], lung cancer [14] and renal cell carcinoma [18]. In addition, overexpression of PKCη induced anchorage-independent growth in NIH3T3 cells [30] and provided proliferative advantage in astrocytic tumor cells [31]. The contrasting function of PKCη is not unique and several other PKC isoforms have been shown to exert opposite effects depending on the cell type [1, 9, 27, 32]. Thus, it is important to understand how PKCη is regulated in a particular cellular context in order to understand its function.
We recently reported that the regulation of PKCη in breast cancer cells is distinct from other PKC isozymes. While brief exposure to PKC activators, such as tumor-promoting phorbol esters leads to activation of conventional and novel PKCs, persistent treatment with these activators leads to their downregulation [1, 2]. We showed that prolonged treatment with several structurally and functionally distinct PKC activators caused upregulation rather than downregulation of PKCη and a general PKC inhibitor led to PKCη downregulation [11]. When we compared the effects of pharmacological inhibitors of several different kinases, we found that not only PKC inhibitors but also the PI3K inhibitor Ly294002 induced PKCη downregulation.
PKCs are phosphorylated by autophosphorylation and transphosphorylation by other members of the PKC family as well as PDK1 [33-35]. We recently reported that transphosphorylation of PKCη by novel PKCs is responsible for its upregulation [11]. Since two different general PKC inhibitors bisindolylmaleimide and Gö 6983 induced PKCη downregulation whereas the conventional PKC inhibitor Gö 6976 had only a modest effect, it is likely that inhibition of PKCη phosphorylation by novel PKCε leads to its downregulation. Our observation that adenoviral delivery of PKCε increased PKCη level both in control and Gö 6983-treated cells supports this notion. We, however, found that overexpression of PKCε was unable to completely prevent Gö 6983-mediated downregulation of PKCη, presumably because Gö 6983 could inhibit PKCε in PKCε-overexpressing cells albeit to a lesser extent compared to endogenous PKCε.
It has been reported that PDK1 directly phosphorylates PKCη resulting in its activation [25] but knockdown of PDK1 had little effect on PKC activator-induced upregulation of PKCη [11]. Although PDK1 was not required for PKC activator-induced upregulation of PKCη, depletion of PDK1 by siRNA or its inhibition by the PI3K inhibitor Ly294002 decreased basal PKCη level, suggesting that phosphorylation of PKCη by PDK1 also protects it from downregulation. However, the mechanism by which Gö 6983 and Ly294002 induced PKCη downregulation was distinct. While both Gö 6983 and Ly294002 decreased basal PKCη level, only Gö 6983 but not Ly294002 could induce PKCη downregulation in the presence of PDBu. Moreover, two distinct proteasome inhibitors MG-132 and lactacystin could prevent PKCη downregulation caused by Ly294002 or PDK1 knockdown but not by Gö 6983. These results suggest that while inhibition of PDK1 triggers PKCη downregulation via proteasome-dependent pathway, inhibition of PKC triggers PKCη downregulation via proteasome-independent pathway.
Both PKC and PI3K/PDK1/Akt signaling pathways play important roles in the development and progression of breast cancer [1, 26-28]. It has been reported that the level and activation status of PDK1 is progressively increased in the MCF-10A series [21] which includes spontaneously immortalized non-tumorigenic mammary epithelial MCF-10A, premalignant MCF-10AT and MCF10AT3G, ductal carcinoma in situ DCIS and fully malignant MCF10CA1a and MCF10CA1d cells [20, 21]. The MCF-10A series provides a unique cell culture model system to study the alterations in signaling molecules leading to breast cancer since these cells share common genetic background. We made a novel observation that PKCη is the only PKC isozyme that is progressively increased in the MCF-10 cell series but there was no correlation between the levels of conventional PKCα, novel PKCδ and -ε and atypical PKCζ and the malignant status of MCF10A-derived cells. While PKCη level correlated with the level/activation status of PDK1 in the MCF-10 cell series, there was no correlation between PKCη level and hormone receptor status. For example, it was difficult to detect PKCη in triple-negative MDA-MB-231 cells (data not shown). However, PKCη is regulated by similar mechanisms regardless of the hormone receptor status. Both PKC inhibitor Gö 6983 and PI3K/PDK1 inhibitor Ly294002 induced downregulation of PKCη in basal type MCF-10CA1d (Fig. 6B) and MCF-10CA1a cells (data not shown).
To determine the functional significance of PKCη in breast cancer cells we depleted PKCη using siRNA since a selective inhibitor of PKCη is currently not available. Silencing of PKCη by siRNA decreased the growth of both DCIS and T47D cells. Knockdown of PKCη also reduced the long-term clonogenic survival of MCF-7 cells, which express very high level of PKCη. Moreover, since PKCη is regulated by PKCε and PDK1 that play critical roles in breast cancer, PKCη may serve as an important target for breast cancer therapy.
Highlights.
Unique regulation of PKCη by nPKC and PDK1 involves two distinct mechanisms.
Upregulation of PKCη is associated with breast cancer malignancy.
PKCη contributes to breast cancer cell growth.
Acknowledgments
This work was supported by the grant CA071727S1 from the National Cancer Institute. Adenovirus containing PKCε was a kind gift from Dr. Ajit Verma of the University of Wisconsin Medical School.
Abbreviations
- BIM
Bisindolylmaleimide
- DAG
diacylglycerol
- ILV
indolactam V
- MAPK
mitogen-activated protein kinase
- MTT
3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide
- PKA
protein kinase A
- PI3K
phosphatidylinositol 3-kinase
- PDK1
phosphoinositide-dependent kinase-1
- PKC
protein kinase C
- aPKC
atypical PKC
- cPKC
conventional PKC
- nPKC
novel PKC
- PDBu
Phorbol 12,13-dibutyrate
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Griner EM, Kazanietz MG. Protein kinase C and other diacylglycerol effectors in cancer. Nat Rev Cancer. 2007;7:281–294. doi: 10.1038/nrc2110. [DOI] [PubMed] [Google Scholar]
- 2.Steinberg SF. Structural basis of protein kinase C isoform function. Physiol Rev. 2008;88:1341–1378. doi: 10.1152/physrev.00034.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pontremoli S, Melloni E, Damiani G, Salamino F, Sparatore B, Michetti M, Horecker BL. Effects of a monoclonal anti-calpain antibody on responses of stimulated human neutrophils. Evidence for a role for proteolytically modified protein kinase C. The Journal of biological chemistry. 1988;263:1915–1919. [PubMed] [Google Scholar]
- 4.Savart M, Letard P, Bultel S, Ducastaing A. Induction of protein kinase C down-regulation by the phorbol ester TPA in a calpain/protein kinase C complex. Int J Cancer. 1992;52:399–403. doi: 10.1002/ijc.2910520312. [DOI] [PubMed] [Google Scholar]
- 5.Lee HW, Smith L, Pettit GR, Smith JB. Bryostatin 1 and phorbol ester down-modulate protein kinase C-alpha and -epsilon via the ubiquitin/proteasome pathway in human fibroblasts. Mol Pharmacol. 1997;51:439–447. [PubMed] [Google Scholar]
- 6.Lu Z, Liu D, Hornia A, Devonish W, Pagano M, Foster DA. Activation of protein kinase C triggers its ubiquitination and degradation. Mol Cell Biol. 1998;18:839–845. doi: 10.1128/mcb.18.2.839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee HW, Smith L, Pettit GR, Vinitsky A, Smith JB. Ubiquitination of protein kinase C-alpha and degradation by the proteasome. The Journal of biological chemistry. 1996;271:20973–20976. [PubMed] [Google Scholar]
- 8.Basu A. The potential of protein kinase C as a target for anticancer treatment. Pharmacol Ther. 1993;59:257–280. doi: 10.1016/0163-7258(93)90070-t. [DOI] [PubMed] [Google Scholar]
- 9.Basu A, Pal D. Two faces of protein kinase Cdelta: the contrasting roles of PKCdelta in cell survival and cell death. ScientificWorldJournal. 2010;10:2272–2284. doi: 10.1100/tsw.2010.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bacher N, Zisman Y, Berent E, Livneh E. Isolation and characterization of PKC-L, a new member of the protein kinase C-related gene family specifically expressed in lung, skin, and heart. Mol Cell Biol. 1991;11:126–133. doi: 10.1128/mcb.11.1.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pal D, Outram SP, Basu A. Novel regulation of protein kinase C-eta. Biochemical and biophysical research communications. 2012;425:836–841. doi: 10.1016/j.bbrc.2012.07.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chida K, Hara T, Hirai T, Konishi C, Nakamura K, Nakao K, Aiba A, Katsuki M, Kuroki T. Disruption of protein kinase Ceta results in impairment of wound healing and enhancement of tumor formation in mouse skin carcinogenesis. Cancer Res. 2003;63:2404–2408. [PubMed] [Google Scholar]
- 13.Kashiwagi M, Ohba M, Chida K, Kuroki T. Protein kinase C eta (PKC eta): its involvement in keratinocyte differentiation. J Biochem. 2002;132:853–857. doi: 10.1093/oxfordjournals.jbchem.a003297. [DOI] [PubMed] [Google Scholar]
- 14.Krasnitsky E, Baumfeld Y, Freedman J, Sion-Vardy N, Ariad S, Novack V, Livneh E. PKCeta is a novel prognostic marker in non-small cell lung cancer. Anticancer Res. 2012;32:1507–1513. [PubMed] [Google Scholar]
- 15.Hussaini IM, Carpenter JE, Redpath GT, Sando JJ, Shaffrey ME, Vandenberg SR. Protein kinase C-eta regulates resistance to UV- and gamma-irradiation-induced apoptosis in glioblastoma cells by preventing caspase-9 activation. Neuro Oncol. 2002;4:9–21. doi: 10.1093/neuonc/4.1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lu HC, Chou FP, Yeh KT, Chang YS, Hsu NC, Chang JG. Analysing the expression of protein kinase C eta in human hepatocellular carcinoma. Pathology. 2009;41:626–629. doi: 10.3109/00313020903273076. [DOI] [PubMed] [Google Scholar]
- 17.Cabodi S, Calautti E, Talora C, Kuroki T, Stein PL, Dotto GP. A PKC-eta/Fyn-dependent pathway leading to keratinocyte growth arrest and differentiation. Mol Cell. 2000;6:1121–1129. doi: 10.1016/s1097-2765(00)00110-6. [DOI] [PubMed] [Google Scholar]
- 18.Brenner W, Farber G, Herget T, Wiesner C, Hengstler JG, Thuroff JW. Protein kinase C eta is associated with progression of renal cell carcinoma (RCC) Anticancer Res. 2003;23:4001–4006. [PubMed] [Google Scholar]
- 19.Ohba M, Ishino K, Kashiwagi M, Kawabe S, Chida K, Huh NH, Kuroki T. Induction of differentiation in normal human keratinocytes by adenovirus-mediated introduction of the eta and delta isoforms of protein kinase C. Mol Cell Biol. 1998;18:5199–5207. doi: 10.1128/mcb.18.9.5199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Santner SJ, Dawson PJ, Tait L, Soule HD, Eliason J, Mohamed AN, Wolman SR, Heppner GH, Miller FR. Malignant MCF10CA1 cell lines derived from premalignant human breast epithelial MCF10AT cells. Breast cancer research and treatment. 2001;65:101–110. doi: 10.1023/a:1006461422273. [DOI] [PubMed] [Google Scholar]
- 21.Kim SH, Miller FR, Tait L, Zheng J, Novak RF. Proteomic and phosphoproteomic alterations in benign, premalignant and tumor human breast epithelial cells and xenograft lesions: biomarkers of progression. Int J Cancer. 2009;124:2813–2828. doi: 10.1002/ijc.24278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Basu A, Lu D, Sun B, Moor AN, Akkaraju GR, Huang J. Proteolytic activation of protein kinase C-epsilon by caspase-mediated processing and transduction of antiapoptotic signals. The Journal of biological chemistry. 2002;277:41850–41856. doi: 10.1074/jbc.M205997200. [DOI] [PubMed] [Google Scholar]
- 23.Basu A. The involvement of novel protein kinase C isozymes in influencing sensitivity of breast cancer MCF-7 cells to tumor necrosis factor-alpha. Mol Pharmacol. 1998;53:105–111. doi: 10.1124/mol.53.1.105. [DOI] [PubMed] [Google Scholar]
- 24.Basu A, Sridharan S, Persaud S. Regulation of protein kinase C delta downregulation by protein kinase C epsilon and mammalian target of rapamycin complex 2. Cell Signal. 2009;21:1680–1685. doi: 10.1016/j.cellsig.2009.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Balendran A, Hare GR, Kieloch A, Williams MR, Alessi DR. Further evidence that 3-phosphoinositide-dependent protein kinase-1 (PDK1) is required for the stability and phosphorylation of protein kinase C (PKC) isoforms. FEBS Lett. 2000;484:217–223. doi: 10.1016/s0014-5793(00)02162-1. [DOI] [PubMed] [Google Scholar]
- 26.Gorin MA, Pan Q. Protein kinase C epsilon: an oncogene and emerging tumor biomarker. Mol Cancer. 2009;8:9. doi: 10.1186/1476-4598-8-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Basu A, Sivaprasad U. Protein kinase Cepsilon makes the life and death decision. Cell Signal. 2007;19:1633–1642. doi: 10.1016/j.cellsig.2007.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Miller TW, Rexer BN, Garrett JT, Arteaga CL. Mutations in the phosphatidylinositol 3-kinase pathway: role in tumor progression and therapeutic implications in breast cancer. Breast Cancer Res. 2011;13:224. doi: 10.1186/bcr3039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Masso-Welch PA, Winston JS, Edge S, Darcy KM, Asch H, Vaughan MM, Ip MM. Altered expression and localization of PKC eta in human breast tumors. Breast Cancer Res Treat. 2001;68:211–223. doi: 10.1023/a:1012265703669. [DOI] [PubMed] [Google Scholar]
- 30.Nomoto S, Watanabe Y, Ninomiya-Tsuji J, Yang LX, Nagai Y, Kiuchi K, Hagiwara M, Hidaka H, Matsumoto K, Irie K. Functional analyses of mammalian protein kinase C isozymes in budding yeast and mammalian fibroblasts. Genes Cells. 1997;2:601–614. doi: 10.1046/j.1365-2443.1997.1470346.x. [DOI] [PubMed] [Google Scholar]
- 31.Hussaini IM, Karns LR, Vinton G, Carpenter JE, Redpath GT, Sando JJ, VandenBerg SR. Phorbol 12-myristate 13-acetate induces protein kinase ceta-specific proliferative response in astrocytic tumor cells. J Biol Chem. 2000;275:22348–22354. doi: 10.1074/jbc.M003203200. [DOI] [PubMed] [Google Scholar]
- 32.Reyland ME. Protein kinase C isoforms: Multi-functional regulators of cell life and death. Front Biosci. 2009;14:2386–2399. doi: 10.2741/3385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rybin VO, Sabri A, Short J, Braz JC, Molkentin JD, Steinberg SF. Cross-regulation of novel protein kinase C (PKC) isoform function in cardiomyocytes. Role of PKC epsilon in activation loop phosphorylations and PKC delta in hydrophobic motif phosphorylations. J Biol Chem. 2003;278:14555–14564. doi: 10.1074/jbc.M212644200. [DOI] [PubMed] [Google Scholar]
- 34.Gould CM, Newton AC. The life and death of protein kinase C. Curr Drug Targets. 2008;9:614–625. doi: 10.2174/138945008785132411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Parekh DB, Ziegler W, Parker PJ. Multiple pathways control protein kinase C phosphorylation. EMBO J. 2000;19:496–503. doi: 10.1093/emboj/19.4.496. [DOI] [PMC free article] [PubMed] [Google Scholar]